Abstract
Clonal T cells have been demonstrated in skin lesions of all stages of cutaneous T-cell lymphomas (CTCLs). However, there are conflicting data regarding the CTCL stage at which dissemination of clonal cells into peripheral blood occurs. Although the multifocal occurrence of cutaneous CTCL lesions and T-cell recirculation suggest an early appearance of neoplastic cells in the blood, circulating clonal T cells have only been detected in advanced stages. We investigated their occurrence by a highly sensitive polymerase chain reaction (PCR) assay amplifying T-cell receptor γ rearrangements and subsequent heteroduplex temperature gradient gel electrophoresis (HD-TGGE) of the amplification products. Circulating clonal T cells were found in 26 of 45 patients with mycosis fungoides (MF ), six of seven with Sezary's syndrome (SS), 10 of 13 pleomorphic CTCLs, and three of four unclassified CTCLs. Corresponding skin specimens carried clonal T cells in 29 of 40 MF, three of four SS, 12 of 12 pleomorphic, and two of two unclassified CTCL patients. Except for the blood specimen of a psoriatic patient, all samples of 60 controls (psoriasis vulgaris, atopic dermatitis, and healthy volunteers) revealed polyclonal amplification products. In 30 of 32 CTCL patients carrying a clonal rearrangement in blood and skin, identity of both clones was indicated by HD-TGGE and confirmed by sequencing six of these cases. We found an unexpected high frequency of identical clonal T cells in peripheral blood and skin of CTCL patients, including early stages of MF. This supports the concept of an early systemic disease in CTCL and raises new questions concerning the pathogenesis.
CUTANEOUS T-CELL lymphomas (CTCLs) represent a heterogeneous group of non-Hodgkin's lymphomas clinically originating in the skin and subsequently disseminating into lymph nodes, blood, and other visceral organs.1-3 According to the EORTC classification of primary cutaneous lymphomas, CTCLs are subgrouped into indolent (mycosis fungoides [MF], Sezary's syndrome [SS], pagetoid reticulosis, lymphomatoid papulosis, and large-cell CD30+ CTCL), aggressive (large-cell CD30− CTCL), and some provisional entities.4
Since it is well established that CTCLs are clonal expansions of T cells carrying identical copies of rearranged T-cell receptor (TCR) genes, the demonstration of a predominant T-cell clone in cutaneous infiltrates confirms the diagnosis additional to clinical, histopathologic, and immunophenotypic criteria. Southern blotting displaying TCR-mediated diversity of the restriction fragment length and more sensitive polymerase chain reaction (PCR) assays characterizing the V-(D)-J junction of TCR rearrangements are applied to detect clonality.5-13 Using a sensitive PCR assay, we recently demonstrated clonal disease in skin lesions of early MF.14
In addition to skin biopsy samples, extracutaneous specimens have often been analyzed by molecular biological techniques to investigate an extracutaneous spread of CTCL. Regarding the peripheral blood, the majority of these studies demonstrated circulating clonal T cells only in SS and some cases of advanced stages of other CTCLs. In accordance with the clinical course of these entities, an association of blood involvement with poorer prognosis, lymph node involvement, and an enlarged total body tumor burden was suggested.7,9 15-17
However, the frequent occurrence of multifocal or diffuse cutaneous CTCL lesions and the T-cell nature of the malignant cell emphasize a stage-independent recirculation of the neoplastic cells via the peripheral blood to the skin.1,18 For this reason, circulating clonal T cells should already be detectable in early stages of all CTCL types. Interestingly, early hematogeneous involvement in MF has been supposed by Bunn et al.19 However, the applied analytic techniques including E-rosette cytology, electron microscopy, and cytogenetics possess a low diagnostic specificity and are considered to be of only complementary value in the diagnosis of CTCL.20 With a sensitive PCR-based method, the presence of circulating clonal T cells in early CTCL was demonstrated by Veelken et al21 in two patients with MF stage I. Additionally, Theodorou et al22 demonstrated clonality of blood samples in 47.2% of 37 CTCL cases. Although a high frequency of blood involvement was discussed, this study lacked the differentiation between MF and SS that is well recognized to carry clonal T cells in the peripheral blood23 and the different MF stages, respectively. In conclusion, data concerning the occurrence and significance of blood clonality in CTCL are contradictory so far.
The aim of the present study was to investigate the occurrence of circulating clonal T cells in CTCL by applying a sensitive PCR/HD-TGGE assay to blood and skin samples of a larger cohort of well-classified CTCL. Special attention was paid to the analysis of early stages of MF.
SUBJECTS AND METHODS
Patient samples.Blood specimens were obtained from 129 adult individuals: patients with CTCL (n = 69), atopic dermatitis ([AD] n = 20), psoriasis vulgaris ([PV] n = 20), and healthy volunteers ([HV] n = 20). Additionally, in 98 of 129 patients, a skin biopsy was analyzed (Table 1). No significant differences between the age of the control group (AD, PV, and HV, range 43 to 76 years; median, 61) and that of CTCL patients (range, 43 to 88 years; median, 64) were found by the Mann-Whitney U test. The diagnosis was based on clinical criteria and histologic and immunohistologic assessment of formaldehyde-fixed, paraffin-embedded skin specimens.24 CTCLs were classified according to the revised EORTC classification.4 The TNM classification was applied for further subgrouping of MF cases.24 Four cases of CTCL remained unclassified, since they did not fulfill the criteria of any distinct CTCL entity. The cell lines JM (rearranged Vγ8 and Vγ11) and PEER (rearranged Vγ9) and the peripheral blood of patient with γδ+ T-cell acute lymphatic leukemia ([T-ALL] Vγ10+) served as positive clonal controls.
Sample preparation.Peripheral blood mononuclear cells (PBMC) were prepared from 10 mL heparinized blood by density gradient centrifugation through Ficoll-HyPaque (Pharmacia, Freiburg, Germany). Genomic DNA was prepared from about 1 × 106 PBMC or JM/PEER cells, respectively, by a standard procedure using proteinase K digestion.25 For preparation of genomic DNA from the paraffin-embedded skin specimens, the paraffin of 10 sections per sample (10 μm each) was dissolved with xylene. After centrifugation, the pellet was washed with ethanol and also digested by proteinase K.
TCRγ PCR.TCRγ rearrangements were amplified using primers annealing at the V and J segments, respectively (Table 2). PCR 1 (primers VG1, VG2, VG9, and JG12-a) was applied to all specimens, whereas PCR 2 (primers VG1, VG2, VG9, and JGP12-a) was performed in all control samples and the CTCL specimens appearing polyclonal in PCR 1. A primer for the JP segment was not included because JP is scarcely involved in TCRγ rearrangements.26 27 In addition, a different J segment should be rearranged at the second allele. The reaction mixture included 0.5 to 1 μg (5 μL) genomic DNA, 1.75 U Taq Polymerase, 7.5 μL 10x PCR buffer (Perkin Elmer, Branchburg, NJ), 0.1 mmol/L of each deoxynucleotide triphosphate ([dNTP] Pharmacia, Freiburg, Germany), and 0.6 μmol/L of each primer in a final volume of 75 μL. Amplification was performed on a thermal cycler (Varius-V; Vers, Hannover, Germany) by a 4-minute denaturation step at 95°C, followed by 40 cycles including 1 minute of denaturation at 94°C, 1 minute of annealing at 58°C, and 1 minute of extension at 72°C. Finally, an extension step of 5 minutes at 72°C was added. Six microliters of the PCR products were screened for successful amplification on a 2% agarose gel stained by ethidium bromide.
Determination of clonality.T-cell clonality was established by detection of a dominant TCRγ rearrangement in a heteroduplex-loaded temperature gradient gel electrophoresis (HD-TGGE). Eight microliters of the PCR products were prepared to form heteroduplices (5 minutes of denaturation at 95°C, with gradual cooling to 50°C)28 and separated on the Diagen TGGE-System (Diagen, Hilden, Germany). Electrophoretic assay and subsequent silverstaining were performed according to standard protocols.29 Evaluation of the gradient gels was made blindly by two independent investigators.
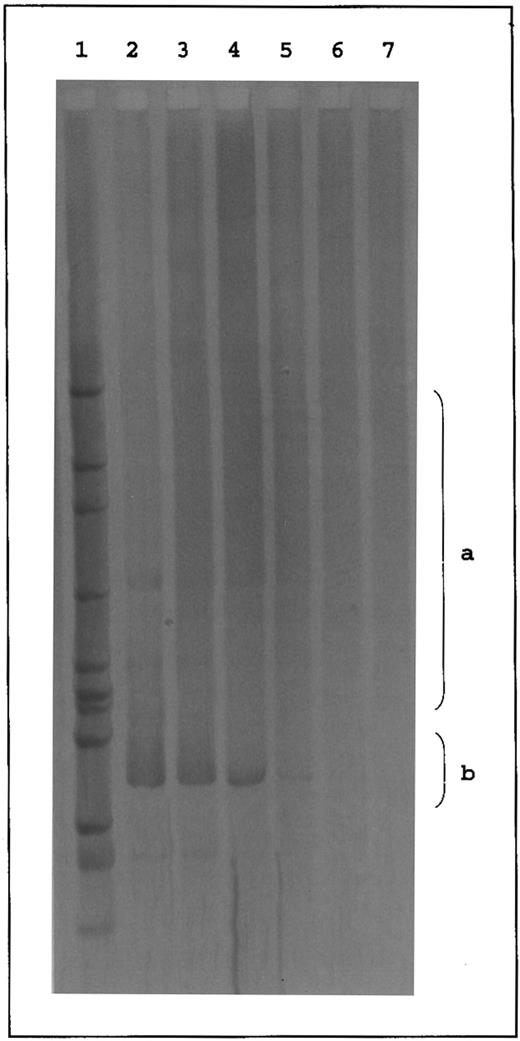
Due to the denaturation-renaturation step, polyclonal (ie, not identical) amplification products form heteroduplices that contain mismatches in the N region. These mismatches decrease the thermal stability of the N region and alter the fragment migration. As a result, a broad smear on the gel is formed in this case. In contrast, clonal (ie, identical) PCR products are expected to produce more stable homoduplices that migrate as sharp bands into the high-temperature range of the gradient gel12 (see Fig 1).
Temperature gradient gel of TCRγ PCR products. Lanes 1 to 5, polyclonal PCR products of PCR 1 using primers VG1, VG2, VG9, and JG12-a (1 to 3) and PCR 2 using primers VG1, VG2, VG9, and JGP12-a (4 and 5) appearing as broad smears in the middle range of the gel (a). Lanes 6 to 9, clonal controls (cell line JM [Vγ8], cell line PEER [Vγ9], T-ALL patient Ra [Vγ10], and cell line JM [Vγ11]) appearing as sharp bands below the middle range of the gel (b). Lanes 10 to 17, clonal PCR products of PCR 1 (11 to 13) and PCR 2 (14 and 15). Lanes 16 and 17, biallelic PCR products of PCR 1. Lane 18, HincII digest of phi X174. S, skin sample; B, blood sample; C, cell line; M, marker.
Temperature gradient gel of TCRγ PCR products. Lanes 1 to 5, polyclonal PCR products of PCR 1 using primers VG1, VG2, VG9, and JG12-a (1 to 3) and PCR 2 using primers VG1, VG2, VG9, and JGP12-a (4 and 5) appearing as broad smears in the middle range of the gel (a). Lanes 6 to 9, clonal controls (cell line JM [Vγ8], cell line PEER [Vγ9], T-ALL patient Ra [Vγ10], and cell line JM [Vγ11]) appearing as sharp bands below the middle range of the gel (b). Lanes 10 to 17, clonal PCR products of PCR 1 (11 to 13) and PCR 2 (14 and 15). Lanes 16 and 17, biallelic PCR products of PCR 1. Lane 18, HincII digest of phi X174. S, skin sample; B, blood sample; C, cell line; M, marker.
Cloning and sequencing of the TCRγ rearrangements.Thirty-two samples of CTCL patients and the clonal controls (JM, PEER, and Ra) were sequenced directly and/or after cloning of the PCR products. For direct sequencing, amplification products were separated by HD-TGGE. The distinct band was cut out and dissolved in 40 μL 1x PCR buffer (Perkin Elmer) overnight. Five microliters of the solution was reamplified under the same conditions described. Primer JG12-i or JGP12-i was applied instead of JG12-a or JGP12-a, respectively (Table 2). The PCR product was purified by the QIAquick PCR purification kit (Qiagen, Hilden, Germany) and sequenced on an automated DNA sequencer (Model 373A; Perkin Elmer Applied Biosystems, Weiterstadt, Germany) by the Taq cycle sequencing method using primers VGseq, JG12-i, or JGP12-i (Table 2). Sequences were aligned to the published germline sequences of the TCRγ V and J segments.30-36 Cloning of the PCR products was performed by applying the TA Cloning Kit (Invitrogen, Fleek, The Netherlands). Plasmids were sequenced using the universal forward-sequencing primer for M 13 by the method mentioned above. For each sample, 12 randomly chosen clones were analyzed. Identical TCRγ sequences repetitively found in the clones of one tissue sample were considered as the predominant T-cell clone.
RESULTS
Validity of the diagnostic system.Ninety-nine of 100 PCR products from PBMC and skin specimens of the control groups (HV, PV, and AD) formed a broad smear in the gradient gel (Table 1 and Fig 1, lanes 1 to 5, range a). In contrast, amplification products from the cell lines and from PBMC of a T-ALL patient (Ra) revealed a clear-cut band below the observed smear (Fig 1, lanes 6 to 9, range b). Accordingly, clonality of a PCR product was considered if a clear-cut band appeared below the middle range of the temperature gradient gel (Fig 1, lanes 10 to 15, range b). Except for the cell lines, these cases revealed a smear of varying intensity above the sharp band representing the polyclonal background. In 10 samples (six blood and four skin specimens), two sharp bands were observed in the gradient gel (Fig 1, lanes 16 and 17, range b) indicating rearrangements of both TCRγ alleles in the T-cell clone.12 These cases were also classified as clonal.
To confirm the specificity of our PCR primers as well as the reliability of the HD-TGGE analysis, 26 clonal and six polyclonal PCR products were sequenced. All clonal amplification products revealed a TCRγ sequence (Table 3). The clonality of the determined sequence was proved by repeated analysis of independent PCR products obtained from a particular specimen (data not shown). In 17 clonal samples analyzed by both sequencing techniques, concordance was observed between direct sequencing and sequencing of multiple clones. The six polyclonal specimens (skin samples of patients Bo, Ti, Cz, and Me and blood samples of patients Ja and La) were sequenced after cloning. Evaluation of at least 12 clones per specimen revealed different TCRγ sequences (data not shown).
The sensitivity of our PCR/HD-TGGE system was determined by dilution of clonal T cells (JM cell line) in polyclonal PBMC of a healthy volunteer. After DNA preparation and amplification with primer VG1 and JG12-a, a distinct electrophoretic band was observed down to a dilution of 103 clonal JM cells in 106 PBMC, corresponding to a detection limit of 0.1% clonal in polyclonal cells (Fig 2, lane 5).
Temperature gradient gel of a dilution experiment. Lane 1, marker (HincII digest of psi 174); lane 2, 100% JM cells; lanes 3 to 6, 10%, 1%, 0.1%, and 0.01% JM cells in PBMC of a healthy volunteer; lane 7, 100% PBMC. Clonality is demonstrated down to 103 JM cells in 106 polyclonal PBMC (0.1%). a, range of polyclonal smears; b, range of clonal bands.
Temperature gradient gel of a dilution experiment. Lane 1, marker (HincII digest of psi 174); lane 2, 100% JM cells; lanes 3 to 6, 10%, 1%, 0.1%, and 0.01% JM cells in PBMC of a healthy volunteer; lane 7, 100% PBMC. Clonality is demonstrated down to 103 JM cells in 106 polyclonal PBMC (0.1%). a, range of polyclonal smears; b, range of clonal bands.
In conclusion, our diagnostic system revealed sufficient specificity and sensitivity.
Analysis of the blood specimens.A T-cell clone was discovered in 45 of 69 blood specimens obtained from CTCL patients (Table 1). Among these samples, the lowest frequency of detected blood clonality was found in MF (57.8%), whereas pleomorphic CTCL revealed clonal PCR products in 76.9% and SS in 85.7% of cases. Three of four unclassified CTCLs also yielded clonal PCR products. Regarding the occurrence of circulating clonal T cells during progression of the disease, in MF stage IA, 46.2% of the cases were found to be clonal, whereas MF stage IB showed clonality in 55.6% of the cases. All blood samples derived from MF stages II to IV showed clonal PCR products. Blood samples of stage I were shown to carry clonal T cells significantly less frequently than the blood specimens of the more advanced stages II to IV characterized by skin tumors or erythroderma and/or involved lymph nodes (P < .05, chi-square).
Among 60 control specimens, the sample of a single psoriatic patient revealed a clonal PCR product (Table 1). Circulating clonal T cells were significantly more frequently detected in CTCL patients than in controls (P < .001, chi-square).
Analysis of the skin specimens.In order to analyze whether the occurrence of circulating clonal T cells is associated with skin clonality, 98 simultaneously obtained skin specimens were analyzed. Clonal PCR products were detected in 79.3% of CTCL patients (Table 1). The highest percentage of clonal skin samples was found in pleomorphic CTCL (100%), whereas SS showed clonality in 75% and MF in 72.5% of the cases. All cutaneous specimens of unclassified CTCL were demonstrated to be clonal. No clonal T cells were detected in skin samples of the 40 controls (Table 1).
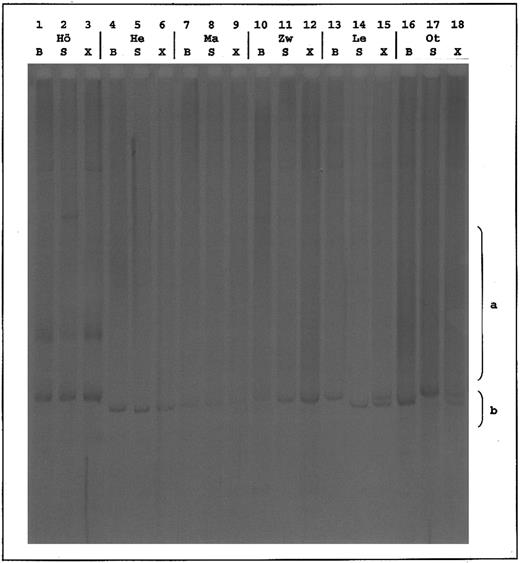
Analysis of corresponding skin and blood samples.In 58 CTCL patients where skin and blood samples were taken simultaneously, clonal T cells in both compartments were found in 32 cases, whereas eight revealed polyclonal rearrangements in blood and skin. Clonality was restricted to the skin in 11 of 40 MF cases and in three of 12 pleomorphic CTCL. In one of four SS patients and in three of 40 MF patients, detection of a T-cell clone was restricted to the blood. However, this phenomenon was not associated with any distinct MF stage (Table 4). To determine the identity of T-cell clones detected in the blood and skin of CTCL patients, PCR fragments from skin and blood samples were mixed, and these mixtures were separated by HD-TGGE (Fig 3). Thirty of the mixtures revealed migration patterns identical to those of the corresponding skin and blood samples. In two cases (patient Le, pleomorphic CTCL; and patient Ot, MF IA), two sharp bands were observed in the mixture lane. Each of these corresponded either to the band of the skin or of the blood sample, indicating different T-cell clones in blood and skin of patients Le and Ot (Fig 3 and Table 4). To confirm the HD-TGGE results, samples from patients Le and Ot and six randomly chosen patients with identical TCRγ rearrangements, as detected by HD-TGGE were sequenced directly and after cloning. In patients Le and Ot, different sequences of the dominating blood and skin rearrangements were determined. For the other six patients (Ma, Ha, Hö, He, Zo, and Zw), identity of the clonal TCRγ rearrangements was verified by sequencing.
Temperature gradient gel of mixed corresponding clonal TCRγ PCR products. S, skin sample; B, blood sample; X, mixture of blood and skin sample (1:1). Patients Hö, He, Ma, and Zw show identical patterns in all 3 lanes; patients Le and Ot have different patterns, whereby lane X appears as summation of B and S. a, range of polyclonal smears; b, range of clonal bands.
Temperature gradient gel of mixed corresponding clonal TCRγ PCR products. S, skin sample; B, blood sample; X, mixture of blood and skin sample (1:1). Patients Hö, He, Ma, and Zw show identical patterns in all 3 lanes; patients Le and Ot have different patterns, whereby lane X appears as summation of B and S. a, range of polyclonal smears; b, range of clonal bands.
In summary, identical T-cell clones were demonstrated in 51.7% of all CTCL patients including 33.3% of MF IA, 41.6% of MF IB, 75% of MF II to IV, SS, pleomorphic CTCL, and all unclassified CTCL cases (Table 4).
DISCUSSION
To evaluate an extracutaneous spread of malignant T cells in CTCL, several groups applied molecular biological techniques. The majority of these studies detected circulating clonal T cells only in SS and some cases of advanced MF or pleomorphic CTCL. Therefore, it was postulated that blood involvement is restricted to advanced cutaneous lymphoma and is associated with poorer prognosis, lymph node involvement, and an enlarged total body tumor burden.7,9,15-17 However, the frequent occurrence of multifocal or diffuse cutaneous CTCL lesions and the recirculational behavior of T cells1,18 supports the hypothesis of an early occurrence of malignant T cells in the peripheral blood. Interestingly, early hematogeneous involvement in MF has been supposed by a few groups.19-22 However, Bunn et al19 used techniques of low diagnostic specificity such as E-rosette cytology, electron microscopy, and cytogenetics,20 whereas the specific PCR-based studies of Veelken et al21 and Theodorou et al22 lacked a sufficient number of analyzed cases and, respectively, a differentiation between MF and SS and a stage-dependent analysis. Using a sensitive TCRγ PCR/HD-TGGE system to investigate a large cohort of well-classified CTCL, we demonstrated circulating clonal T cells in the majority of patients with MF and other CTCL. Surprisingly, this includes high frequencies of detected blood clonality in MF stage I (21 of 40) and pleomorphic CTCL (10 of 13).
PCR assays with subsequent high-resolution electrophoresis are well established for sensitive and specific detection of clonal TCR rearrangements in skin samples of CTCL patients, as well as blood specimens of patients suffering from T-cell leukemia.37-39 However, differences between the rearrangements are minimal and a high separation capacity of the electrophoresis is required.27,40,41 We applied the HD-TGGE technique12 to separate our PCR products. Determining the lower detection limit of our test system, we were able to discover up to 103 clonal in 106 polyclonal T cells (0.1%).
On the other hand, increasing sensitivity might enable detection of minor clones of reactive lymphocytes in skin lesions of nonspecific dermatitis and cutaneous lymphoid hyperplasia proposed as the “clonal dermatitis” concept.42 Moreover, there are some reports describing the detection of clonal T-cell populations in peripheral blood, most notably CD8+ αβ T cells in healthy elderly donors.43 However, our sensitive technique detected no clonality in 40 skin specimens of AD/PV patients, and in blood samples, only one of 60 controls was found to be clonal. Statistical analysis revealed a significantly higher frequency of clonality detection in CTCL patients than in controls, although there was no significant difference regarding proband age.
Moreover, we confirmed the specificity of our PCR/HD-TGGE results by sequencing 26 clonal and six polyclonal amplification products. Applying two different strategies (direct sequencing and cloning with subsequent sequencing), a dominant TCRγ rearrangement could be demonstrated in all clonal cases analyzed, whereas polyclonal samples revealed different sequences after cloning.
Using HD-TGGE and sequencing, in 30 of 32 CTCL patients carrying a clonal rearrangement in the skin and blood compartment, identity of the dominating T-cell clones was demonstrated. This includes 38.9% of all MF stage I cases analyzed and 75% of the investigated patients suffering from MF stage II to IV, SS, and pleomorphic CTCL. Therefore, our findings sufficiently show that CTCL is a systemic and monoclonal disease right from the beginning, even if extracutaneous spread is not yet clinically apparent. With respect to the published data,7,16,17,21 22 this concept is supported by the association between the detection limit of the applied diagnostic method and the frequency of discovered clonality. It can be speculated that increasing sensitivity, ie, clonospecific probes or primers, will enable demonstration of circulating CTCL cells in almost all CTCL cases. Therefore, we believe that the differences still observed in the frequency of blood clonality detection in the distinct CTCL stages are not of qualitative but of quantitative nature.
The detection limit of clones in our assay is about 0.1%. Taking an average of 1010 T cells in the peripheral blood, we would calculate that there are approximately 107 circulating CTCL cells in the 30 CTCL patients. This high quantity could suggest a systemic origin of CTCL. Further support in this direction comes from the findings in four CTCL cases (one SS and three MF stage I/II) with T-cell clones detectable in the peripheral blood but not in skin biopsies. Further analysis in the course of the disease will show whether these clones are the malignant cells responsible for manifestation of the cutaneous lymphoma. It is also conceivable that these findings and the two cases with split clonality in skin and blood and the PV patient already discussed are examples of T-cell clonality occasionally detected in elderly persons,43 or indications of other malignancies such as initial T-cell leukemia.
With respect to a recent data analysis demonstrating a favorable long-term outcome of MF patients with clinical stage IA,44 blood clonality in the early stages as we demonstrated does not seem to be associated with a poorer prognosis. The explanation could be our observation of a high frequency of activated peripheral blood CD8+ T cells, suspected to be cytotoxic T cells, in the majority of early MF patients, indicating a considerable antitumor response.45 Despite the favorable course of MF IA, our results indicating the occurrence of circulating clonal T cells in early MF might support the use of an early systemic treatment, ie, interferon.
In summary, we demonstrated a high frequency of occurrence of identical clonal T cells in peripheral blood and skin of CTCL patients, including early stages of MF. Our findings confirm the evidence for an early systemic disease in CTCL and, with regard to origin and dissemination of the cutaneous lymphoma cell, raise new questions concerning the pathogenesis of the disease. Longitudinal studies should provide further insight into this. Quantification of the circulating clonal cells might be useful as a prognostic parameter for long-term surveillance and as an indicator of therapy response.
ACKNOWLEDGMENT
We thank U. Heiduk and S. Richter for excellent technical assistance and T. Hansen-Hagge for a critical review.
Supported by Grant No. Ste 366/7-1 from the Deutsche Forschungsgemeinschaft.
Address reprint requests to J. Marcus Muche, MD, Department of Dermatology, University Hospital Charité, Humboldt University Berlin, Schumannstraβe 20/21, D-10117 Berlin, Germany.

![Fig. 1. Temperature gradient gel of TCRγ PCR products. Lanes 1 to 5, polyclonal PCR products of PCR 1 using primers VG1, VG2, VG9, and JG12-a (1 to 3) and PCR 2 using primers VG1, VG2, VG9, and JGP12-a (4 and 5) appearing as broad smears in the middle range of the gel (a). Lanes 6 to 9, clonal controls (cell line JM [Vγ8], cell line PEER [Vγ9], T-ALL patient Ra [Vγ10], and cell line JM [Vγ11]) appearing as sharp bands below the middle range of the gel (b). Lanes 10 to 17, clonal PCR products of PCR 1 (11 to 13) and PCR 2 (14 and 15). Lanes 16 and 17, biallelic PCR products of PCR 1. Lane 18, HincII digest of phi X174. S, skin sample; B, blood sample; C, cell line; M, marker.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1636/3/m_bl_0051f1.jpeg?Expires=1765971667&Signature=xlTMv91Li1nM2QGyZ-BUkkQb8qZcW0t2HG~J85FhU7ewkPzIXOiRjD5EZNxWJexNfQAMmWyFwR7FD9QOgENm3l~lF7Wj9GSQABuoET6tXqb1~-ZdkGVsdj-x3RTCtyrJpguR7jopLqqENpTBywQhfxOuN3C4fPU0r1o4wB8kPZ7t0FEguHGtXv3QopHxrj04q~9BQ1ScIfeuu9ASY3g-oAXJAKUtq842S5N6tHddHH4ylXjEc9IP0kRNAcHss-Q4qAPfiLJZAOGSxugKJBcaY5QKc-1KW-RFPHxmaAnsu8cJwyWiyAHhFwCTlwzqnLvz5Urvc2pcz7VQ5psTdRoW9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)