Abstract
Patients with human immunodeficiency virus-1 (HIV-1) infection often present with bone marrow (BM) failure that may affect all hematopoietic lineages. It is presently unclear whether this failure reflects a direct viral impairment of the CD34+ hematopoietic progenitor cells or whether the virus affects the BM microenvironment. To study the effects of HIV-1 on the BM microenvironment, we examined the stromal cell monolayers in long-term BM culture (LTBMC), which are the in vitro equivalent of the hematopoietic microenvironment. We assessed the hematopoietic support function (HSF ) of human stromal layers by determining the cellular proliferation and colony-forming ability of hematopoietic progenitors from BM cells grown on the stromal layers. We show that the HSF is reduced by in vitro infection of the human stromal cell layer by a monocytotropic isolate of HIV-1 (JR-FL). There is no loss of HSF when the stromal cell layer is resistant to HIV-1 replication, either using murine stromal cell layers that are innately resistant to HIV-1 infection or using human stromal cells genetically modified to express a gene that inhibits HIV-1 replication (an RRE decoy). Decreased HSF was seen using either human or murine hematopoietic cells, if the stromal cells were human cells that were susceptible to HIV-1 infection. These in vitro studies implicate HIV-1 replication in the stroma as the essential component causing decreased hematopoietic cell production in HIV-1 infection.
HUMAN IMMUNODEFICIENCY virus (HIV-1) infection is characterized by the progressive loss of CD4+ T lymphocytes, which may occur through either viral-induced, direct cell death, or through indirect mechanisms, such as induction of apoptosis.1 Recent studies have shown that CD4+ T lymphocyte turnover is extremely rapid in HIV-1 infected subjects.2,3 The contribution of the bone marrow (BM) in replenishing the loss of CD4+ T lymphocytes in HIV-1 infection is currently unclear, since the ability of the adult thymus to govern lymphocyte development is reduced.4 In addition to the hallmark deficiency of CD4+ T lymphocytes, deficiencies of all hematopoietic lineages (anemia, neutropenia, and thrombocytopenia) are observed in some patients with HIV-1 infection, suggesting a generalized impairment of hematopoiesis.5,6 Although hematopoietic suppression in HIV-1 infected patients can be caused by secondary complications of the disease, such as antiviral medication (AZT) and certain opportunistic infections (cytomegalovirus [CMV] and Toxoplasmosis infections),7 HIV-1 itself has been implicated in the impairment of hematopoiesis.
HIV-1 affects the BM in at least one very well-documented way: CD34+ BM progenitor cells isolated from some HIV-1+ individuals are reduced in numbers and impaired in their ability to give rise to colonies.8-10 Theoretically, HIV-1 could exert hematopoietic suppressive effects through direct infection of the stem/progenitor cells or through indirect mechanisms. Direct infection of CD34+ cells has been reported, albeit in a minority of patients.11,12 Because a subset of CD34+ cells also express CD4, they may be subject to the same adverse consequences as CD4+ T lymphocytes after being infected by HIV-1.13 Another hypothesis to explain hematopoietic suppression by HIV-1 postulates a viral-mediated alteration of the BM microenvironment that, in turn, results in dysregulated hematopoietic growth.
This report provides experimental evidence in support of the latter mechanism of hematopoietic suppression by HIV-1, by examining the effects of HIV-1 on the adherent BM cell fraction in long-term BM cultures (LTBMC). The adherent cell fraction, or stromal cell monolayer, is the in vitro equivalent of the BM microenvironment in vivo.14 Stromal cells are a heterogeneous cell pool, consisting of fibroblasts, endothelial cells, adipocytes, macrophages, osteoclasts, and reticular cells. The function of stroma is to regulate hematopoietic preservation and proliferation by providing structure and a complex network of inhibitory and stimulatory cytokines.15-17
As has previously been described in human LTBMC infected with HIV-1ADA ,18 we found that HIV-1 infection of human LTBMC leads to decreased proliferation and colony-forming ability of human BM hematopoietic progenitor cells isolated from seronegative donors. HIV-1JR-FL cells (but not HIVIIIB or HIVADA ) was able to replicate in normal human stroma, at a low level which could be amplified by addition of human monocytic cells. In contrast, HIV-1JR-FL did not replicate in either murine stromal cells or in human stromal cells genetically engineered to express a gene that inhibits HIV-1 replication (an RRE decoy gene). Loss of hematopoietic support function (HSF ) on exposure of stroma to HIV-1 was seen if the stromal cells could be infected by HIV-1, but not with either the murine stroma or with human stroma expressing the RRE decoy. The loss of HSF of stroma on infection by HIV-1 was seen even in chimeric LTBMC, where the proliferation of progenitor cells from murine BM was reduced when grown on HIV-1–infected human stroma. These results show that direct infection of the stromal cells by HIV-1 is responsible for the loss of hematopoietic support function.
MATERIALS AND METHODS
Cells and virus.BM specimens were obtained from surplus cells collected from healthy donors for allogeneic BM transplantation. The use of these cells was approved by the Committee on Clinical Investigations at Children's Hospital Los Angeles.
The amphotropic packaging cell line PA31719 was provided by A.D. Miller and maintained in D-10: Dulbecco's modified Eagle's medium, high glucose (Irvine Scientific, Santa Ana, CA), 10% fetal calf serum (FCS), 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 mmol/L L-glutamine. LTBMC were grown in I-30: Iscove's modified Dulbecco's medium (IMDM), 30% FCS, 1% deionized bovine serum albumin (Sigma, St Louis, MO), 2 mmol/L glutamine, 100 U/mL penicillin, 10 μg/mL streptomycin, 10−6 mol/L hydrocortisone (Sigma), and 10−4 mol/L 2-mercaptoethanol (Sigma). Stromal cell cultures were grown in I-15: IMDM (Irvine Scientific) with 15% FCS, 15% horse serum, 10−4 mol/L 2-mercaptoethanol, 10−6 mol/L hydrocortisone, 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 mmol/L L-glutamine. The S17 murine stromal cell line20 was generously provided by Dr. Kenneth Dorshkind (UC Riverside) and was grown in R-12.5: RPMI (Irvine Scientific), 12.5% FCS, 10−4 mol/L 2-mercaptoethanol, 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 mmol/L L-glutamine. All cells were maintained in humidified incubators in 5% CO2 at 37°C.
Human monocytic cells, used to rescue low levels of virus by cocultivation with stroma, were derived by differentiation of CD34+ hematopoietic cells from normal human BM in LTBMC for 1 week. Murine BM samples were obtained by killing 6- to 8-week-old C57B6/j mice (Jackson Laboratories, Bar Harbor, ME) and harvesting the BM from the femurs.
HIV-1JR-FL viral stock was purchased from Applied Biotechnologies Inc (Columbia, MD) and had a titer of 100 TCID50 /μL as assayed on primary human monocytes. Estimated multiplicities of infection (MOI) were based on this TCID value, divided by the total number of cells exposed to the virus, regardless of the frequency of susceptible cells.
Retroviral vectors.Retroviral vectors used for these studies include the control vector, LN21, which contains the bacterial neomycin resistance gene (neo ), and the L-RRE-neo vector, which contains a small 41 base pair fragment (5′ CAC UAU GGG CGC AGU GUC AUU GAC GCU GAC GGU ACA GGC CA 3′)22 of the rev-response element (RRE) to act as a decoy for REV binding. This sequence was synthesized as an oligonucleotide and subcloned into the Bcl I site of LN, 5′ of the neo gene (L-RRE-neo).23
Both vectors were produced as amphotropic vectors by the PA317 packaging cell line. Vector-containing supernatants were collected as D-10, conditioned for 24 hours at 37°C by subconfluent monolayers of the producer cells. Supernatants were filtered through 0.45-μm filters and immediately frozen at −70°C until needed. Titers of the vectors, assayed by measuring transfer of G418-resistance to 3T3 cells, were: LN 2 × 107/mL and L-RRE-Neo 1 × 106/mL. Vector supernatants were free of amphotropic replication-competent retrovirus, assayed as described.24
Establishment of stromal cell monolayers.Primary stromal cell monolayers were established by overnight adherence of unfractionated human BM cells obtained from the screens used to filter clinical BM harvests. The next day, nonadherent cells were washed off and the cultures were maintained in I-15. Stromal layers were used 7 to 10 days later for transduction by retroviral vectors and support of LTBMC.
Retroviral transduction of primary stromal cell monolayers.After formation of the initial stromal monolayer, the cells were split by trypsinization and 1 × 104 cells were plated into 12.5-cm2 tissue culture flasks. The next day, the medium was removed and replaced by 5 mL of vector-containing supernatant or D-10 (mock transduction), and polybrene (Sigma) was added to a concentration of 4 μg/mL. After a minimum of 2 hours, the medium was replaced with another round of vector supernatant to a total of three cycles. The vector-containing supernatant was replaced with I-15 containing 0.5 mg/mL G418 (with no G418 added to the mock-transduced cultures). When the cells again reached a density of 1 × 104, the cultures were irradiated with 20 Gy in the tissue culture dish. Mock-transduced cultures were irradiated immediately after transduction and not exposed to G418.
HIV-1 infection of nontransduced or vector transduced human stromal cell monolayers.Before HIV-1 infection, the stromal cells were harvested by trypsinization, irradiated with 20 Gy, and 1 × 104 cells were plated in 12.5-cm2 tissue culture flasks in 4 mL of I-15. The stromal cell cultures were infected with HIV-1JR-FL in quadruplicate, over a range of MOI from 0.01 to 1.0 TCID50 /cell. Virus was added in 1 mL of I-15 with 4 μg/mL polybrene. After overnight incubation, the virus was washed out of the cultures with Hanks' Solution and the cultures were maintained in I-15, with biweekly refeeding of fresh medium.
Rescue of low-level HIV-1 viral replication.When required, the low level of HIV-1 viral replication in the stroma was amplified by cocultivation with 1 × 104 human monocytic cells, 8 days postinfection. At the start of cocultivation, all of the cultures were maintained in I-30 with 200 U/mL recombinant human interleukin-3 (IL-3; Immunex Corp, Seattle, WA), 50 U/mL IL-6 (R & D Systems, Minneapolis, MN), and 5 U/mL Steel Factor (SLF-Immunex).
HIV-1 infection of human nonadherent BM cells.1 × 105 monocytic cells, derived from CD34+ cells by culture for 1 week, were pelleted, resuspended in 100 μL I-30, and exposed to HIV-1 JR-FL or tissue culture medium (mock-infection) in the presence of 4 μg/mL polybrene in suspension for 2 hours at an MOI = 0.01. After the virus was washed out twice with R-10 medium, the cells were subcultured onto the various stromal cell layers. LTBMC were grown for an additional 3 weeks and were completely refed fresh medium every 4 days, returning all of the cells back to the LTBMC. HIV-1 gag p24 values and total cell counts from the LTBMC were obtained over this period of time and, after 3 weeks, the colony-forming potentials of these cultures were assayed, as described below.
Establishment of human, murine, or chimeric LTBMC.1 × 104 human CD34+ cells or 1 × 106/mL unfractionated murine BM cells were seeded onto HIV-1 infected or mock-infected monolayers of either human or murine stroma. The cultures were then maintained for 3 weeks, refeeding every 4 days with I-30 medium, supplemented with either human IL-3 (200 U/mL), human IL-6 (50 U/mL), and human SLF (5 U/mL) for LTBMC with human hematopoietic cells or with murine IL-3 (200 U/mL, R&D Systems), human IL-6 (50 U/mL, R&D Systems), and murine SLF (5 ng/mL, Immunex) for cultures with murine hematopoietic cells. After 3 weeks of culture, the numbers of clonogenic progenitors in each culture were assayed.
Colony-forming unit (CFU) assay to determine the numbers of clonogenic progenitors cells in the LTBMC.Cell samples from the LTBMC were grown in semisolid methylcellulose medium to determine the number of clonogenic progenitors measured as CFUs. For measurement of human CFU, methylcellulose was added to I-30 to a final concentration of 1% and supplemented with human IL-3 (200 U/mL), human IL-6 (50 U/mL), human SLF (5 U/mL), human erythropoietin (2 U/mL, AMGEN Inc, Thousand Oaks, CA), and human GM-CSF (50 ng/mL, Immunex). To measure murine CFU, murine IL-3 (200 U/mL) and murine SLF (5 ng/mL) were added to the I-30/1% methylcellulose, instead of human IL-3 and SLF. Samples of 5 × 104 and 1 × 105 cells from each LTBMC were plated in duplicate 35-mm gridded tissue culture dishes in 1.0-mL medium with methylcellulose. CFU were counted after 14 days as colonies greater than 50 cells in size.
P24 antigen enzyme-linked immunosorbent assay (ELISA).HIV-1 p24 Antigen Assays were purchased from Coulter Corp (Hialeah, FL), and performed according to the manufacturer's instruction. The p24 values were either calculated manually or with the aid of the Dynatech MR5000 ELISA plate reader (Dynatech Lab Inc, Chantilly, VA). All culture medium samples were assayed without prior dilution. Samples which gave readings of OD > 1.500 were retested from duplicate aliquots and diluted at 1:10, 1:100, 1:1,000, 1:10,000, and 1:100,000 using LTBMC medium as a diluent. Linear plots of the values were graphed using Cricket Graph on Macintosh (Apple Computers, Cupertino, CA).
Statistical analysis.In the primary data analysis, odds ratios were computed as a function of the cross-products of cell frequencies on infected and uninfected stroma, between various cultures, in human and murine experiments. The standard error of each odds ratio was estimated using formula for comparative prospective studies. Classic chi-square tests were used to test the hypothesis that the odds ratio was equal to one, at alpha equal .05 level of significance. Ninety-five percent confidence intervals were constructed by deriving good first approximations to the lower and upper odds ratio limits of the interval based on log odds ratio. These limits were then adjusted according to the value of the chi-square criterion and the desired chi-square value associated with the test.25 Continuity correction was employed.
RESULTS
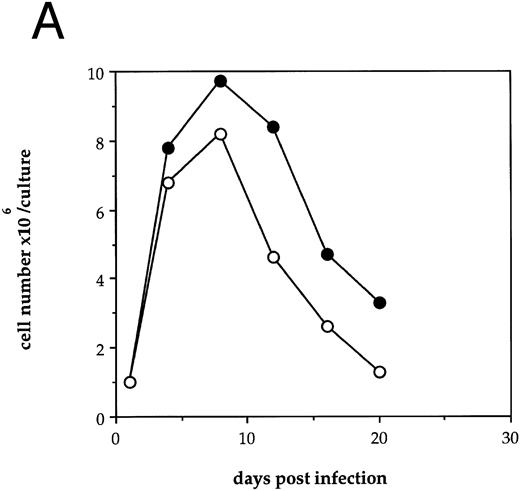
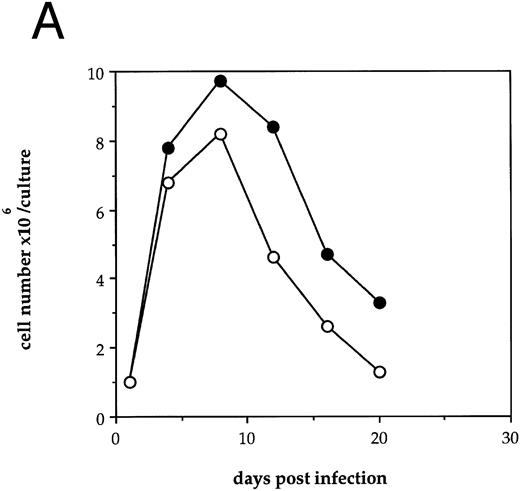
HIV-1 infection of human LTBMC results in decreased hematopoietic cell growth.To examine the effects of HIV-1 on hematopoiesis in vitro, human LTBMC were infected with HIV-1JR-FL or were uninfected. Figure 1A shows the cell counts over 3 weeks from a pair of human LTBMC, one infected with HIV-1JR-FL and one uninfected. Throughout the culture, the numbers of cells produced in the presence of HIV-1 were lower than from the control culture.
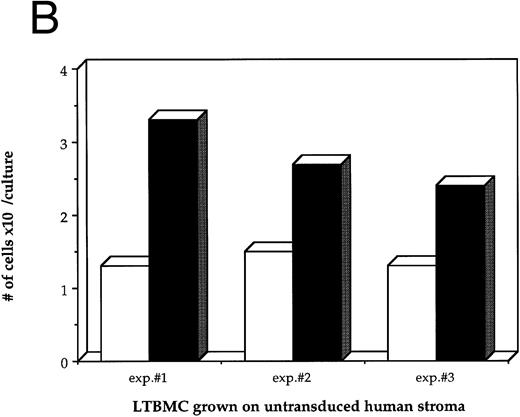
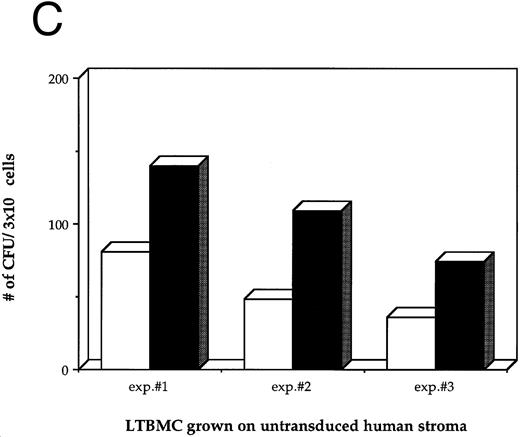
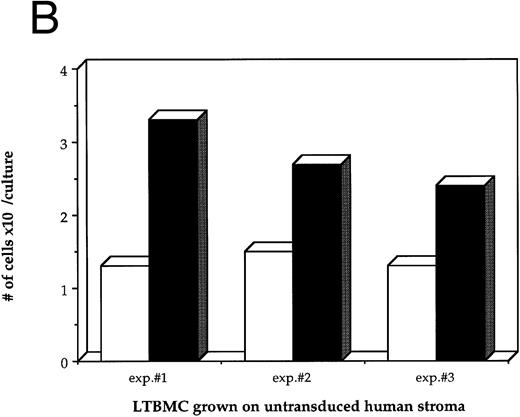
Effects of HIV-1 infection on growth of human LTBMC. (A) Growth curves of HIV-1–infected and –uninfected LTBMC. The total number of cells present in duplicate LTBMC are depicted. (○) Represent the cell numbers for the HIV-1–infected culture, (•) represent the cell numbers for the uninfected culture. (B) Numbers of cells in HIV-1–infected and –uninfected LTBMC. The total numbers of cells present in LTBMC cultured on nontransduced, primary human stroma 3 weeks after infection with HIV-1 (□) or uninfected (▪) were counted. The figure shows results from three independent experiments. (C) Numbers of CFU in HIV-1–infected and –uninfected LTBMC. The total numbers of clonogenic CFU progenitors from the same three sets of LTBMC depicted in (B) were assayed 3 weeks after infection with HIV-1 (□) or uninfected (▪).
Effects of HIV-1 infection on growth of human LTBMC. (A) Growth curves of HIV-1–infected and –uninfected LTBMC. The total number of cells present in duplicate LTBMC are depicted. (○) Represent the cell numbers for the HIV-1–infected culture, (•) represent the cell numbers for the uninfected culture. (B) Numbers of cells in HIV-1–infected and –uninfected LTBMC. The total numbers of cells present in LTBMC cultured on nontransduced, primary human stroma 3 weeks after infection with HIV-1 (□) or uninfected (▪) were counted. The figure shows results from three independent experiments. (C) Numbers of CFU in HIV-1–infected and –uninfected LTBMC. The total numbers of clonogenic CFU progenitors from the same three sets of LTBMC depicted in (B) were assayed 3 weeks after infection with HIV-1 (□) or uninfected (▪).
Figure 1B shows the final cell yields after 21 days of long-term culture from three experiments. The total numbers of cells produced from the HIV-infected LTBMC over 3 weeks of culture in experiments 1, 2, and 3 were reduced by 54.8%, 46.4%, and 48.0%, respectively, compared to the control uninfected cultures.
As a second measure of the effects of HIV-1 on hematopoiesis in the human LTBMC, the colony-forming potential of progenitor cells present in the human LTBMC 3 weeks after infection with HIV-1JR-FL was compared to the colony-forming potential of the uninfected LTBMC. The numbers of CFUs grown from HIV-1–infected and –uninfected cultures in three independent experiments are shown in Fig 1C. In all three experiments, fewer CFU were grown from the HIV-1–infected LTBMC than from the uninfected cultures. The ratios of the numbers of CFU present in the HIV-1–infected cultures to those in the uninfected cultures were 0.58, 0.44, and 0.49, indicating that the numbers of CFU that were present in the HIV-1–infected cultures were reduced by between 42% to 56%. These observations of the numbers of total cells and clonogenic progenitors shows that HIV-1JR-FL adversely affects hematopoiesis in the human LTBMC.
Direct infection of primary human stroma with HIV1JR-FL.LTBMC consist of two distinct cell populations: the hematopoietic cells, which are loosely adherent or nonadherent, and the stromal cell layer, which is tightly adherent to the culture dish. In the previous experiments, addition of HIV-1 to the LTBMC resulted in decreased production of mature hematopoietic cells and declining levels of clonogenic progenitor cells. We performed a series of studies to determine whether it is infection by HIV-1 of the stromal cells or of the hematopoietic cells that is responsible for the adverse effect of HIV-1 on hematopoiesis.
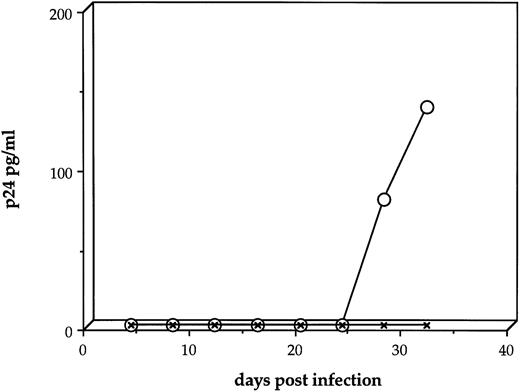
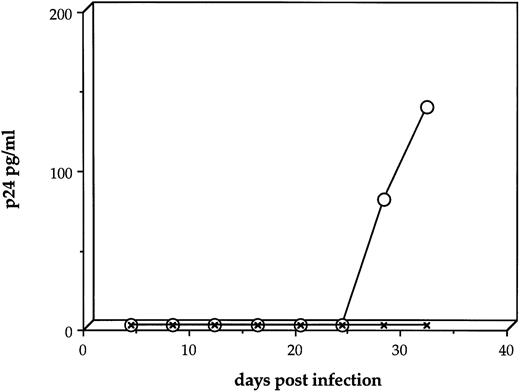
We first sought to determine whether HIV-1JR-FL is capable of directly infecting human stromal cells. Four separate experiments produced essentially the same patterns. Stromal cultures exposed to HIV-1 at an MOI = 1 showed declining p24 levels over the first 2 weeks postinfection, reaching a nadir of 31.3 (±6.8 SEM) pg/mL p24 (Fig 2A). By 3 weeks after inoculation with HIV-1, a productive, although low-level, infection could be measured with 258.2 (±174.2) and 78.4 (±13.4) pg/mL p24 at day 28 and 32 postinfection, respectively (Fig 2A). When duplicate cultures that had been infected with HIV-1 at an MOI of 1.0 were cocultivated with human monocytic cells at day 8 postinfection, the low level of viral replication could be rescued into a vigorously replicating infection, to levels as high as 10,000 pg/mL (Fig 2B).
HIV-1 infection of primary human stromal layers. Primary human stromal layers were infected with HIV-1JR-FL at MOI of 1.0 and 0.1 in duplicate. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 without subsequent addition of monocytic cells (error bars represent the SEM). (B) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 (the duplicates from [A]), with monocytic cells added on day 8. (C) Shows the results of four independent infections of stromal layers at an MOI = 0.1 without subsequent addition of monocytic cells. (D) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 0.1 (the duplicates from [C]) with monocytic cells added on day 8.
HIV-1 infection of primary human stromal layers. Primary human stromal layers were infected with HIV-1JR-FL at MOI of 1.0 and 0.1 in duplicate. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 without subsequent addition of monocytic cells (error bars represent the SEM). (B) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 (the duplicates from [A]), with monocytic cells added on day 8. (C) Shows the results of four independent infections of stromal layers at an MOI = 0.1 without subsequent addition of monocytic cells. (D) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 0.1 (the duplicates from [C]) with monocytic cells added on day 8.
Human stromal cultures infected with HIV-1JR-FL at an MOI = 0.1 produced low but detectable levels of p24 in three of four experiments (19.5 ± 7.5 pg/mL p24) (Fig 2C); however, the low or sometimes undetectable level of viral replication in the stroma could be amplified in every experiment by cocultivation with human monocytic cells, to a mean level of 903.1 ± 315.3 pg/mL p24 (Fig 2D). Stromal cultures infected with HIV-1 at an MOI = 0.01 never produced a productive infection, nor could the virus be rescued from these cultures (data not shown). These findings show that human BM stromal cell layers can be infected in vitro by a monocytotropic clinical isolate of HIV-1 using a high MOI, although the level of virus production is low in the absence of monocytic cells.
To investigate potential mechanisms of HIV-1–mediated growth suppression in vitro, the levels of tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β) in uninfected and HIV-1–infected stromal cultures were measured by ELISA. No differences were observed for the levels of either of the cytokines between stromal cultures infected with HIV-1 and uninfected cultures (data not shown).
HIV-1 replication in stroma is necessary for virus recovery by cocultivation.While the rescue of HIV-1 from stromal layers by cocultivation with monocytic cells suggests replication of the input HIV-1 in the stromal cells, potentially, the monocytic cells could become infected by residual input HIV-1, which remained infectious without actually replicating in the stromal cells. To determine whether input HIV-1 can remain infectious under the culture conditions without replication in target cells, we examined the potential to rescue HIV-1 from cultures in which the stromal cells should be incapable of supporting HIV-1 replication.
We compared the ability to rescue HIV-1 from cultures of normal human stroma to the rescue of HIV-1 from cultures comprised of stromal cells from murine BM (the S17 stromal line). Murine cells are incapable of supporting replication of HIV-1,26 27 so that recovery of HIV-1 from the murine cultures would suggest that input HIV-1 can be recovered by cocultivation with human monocytic cells. As illustrated in Fig 3, HIV-1 could not be recovered by cocultivation of human monocytic cells on the murine stromal layer 8 days after addition of HIV-1 at an MOI = 1.0. As before, HIV-1 could be rescued from human stromal cell layers by addition of human monocytic cells. This result suggests that rescue of HIV-1 by monocytic cells reflects active HIV-1 infection of the human stromal cells.
Amplification of HIV-1 from viral-exposed human and murine stromal layers. Primary human stromal layers (○) or layers of the murine S17 stromal cell line (X's) were infected with HIV-1JR-FL at MOI = 1.0. On day 8 after infection, human monocytic cells were added to each culture to amplify low levels of HIV-1 present. Samples of the culture supernatants were then sampled daily and analyzed for p24 gag protein by ELISA.
Amplification of HIV-1 from viral-exposed human and murine stromal layers. Primary human stromal layers (○) or layers of the murine S17 stromal cell line (X's) were infected with HIV-1JR-FL at MOI = 1.0. On day 8 after infection, human monocytic cells were added to each culture to amplify low levels of HIV-1 present. Samples of the culture supernatants were then sampled daily and analyzed for p24 gag protein by ELISA.
Human marrow stroma transduced by an RRE decoy gene does not support HIV-1 replication.As an alternative method to examine stromal cells incapable of supporting HIV-1 replication, we used a retroviral vector which carries an “RRE decoy gene” (L-RRE-neo) to transduce primary human stromal cells. L-RRE-neo has been shown to inhibit replication of HIV-1 in highly permissive cells, including human T lymphocytes and monocytic cells.23 Primary human stromal cells were transduced with the L-RRE-neo vector or the control vector, LN, which carries only the bacterial neo gene, selected in G418 to obtain populations of stromal cells that were uniformly transduced, and exposed to HIV-1 at an MOI = 0.5.
As before, a low level of HIV-1 replication was seen in the control primary human stroma, but not in the murine S17 stroma (Fig 4A). A high level of HIV-1 (2,619 pg/mL) could be rescued from the normal human stroma by cocultivation with human monocytic cells, but not from the S17 stroma (Fig 4B). The primary human stroma transduced by the L-RRE-neo vector did not produce detectable levels of HIV-1 p24 (Fig 4A), nor could HIV-1 be rescued from the human stroma transduced with L-RRE-neo (Fig 4B). This observation supports the previous finding that HIV-1 must be able to replicate in the stroma for its rescue by cocultivation with monocytic cells. The stromal cells transduced by the control vector LN produced HIV-1 at levels similar to those seen with nontransduced human stroma, suggesting that the process of retroviral vector transduction and G418 selection, per se, does not interfere with the ability of human stroma to support HIV-1 replication. Therefore, we conclude that HIV-1 can directly infect human stroma and that the HIV-1 that is rescued from normal human stromal cultures (or LN-transduced human stroma) by cocultivation with monocytic cells results from active infection and replication in the stromal cells, rather than from passive recovery of residual input HIV-1. Introduction of the RRE decoy into the human stromal cells prevents HIV-1 infection of the stroma and subsequent rescue of virus by monocytic cells.
HIV-1 infection of human stromal cultures transduced by retroviral vectors. Duplicate cultures of primary, nontransduced human stroma (•), human stroma transduced with the L-RRE-Neo vector (▴), human stroma transduced with the LN vector (▪), and murine S17 stroma (X's) were infected with HIV-1JR-FL at an MOI = 0.5. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) shows the results from the stromal layers infected with HIV-1, without subsequent addition of monocytic cells. (B) Shows the results from the stromal layers infected with HIV-1 (the duplicates from [A]), with monocytic cells added on day 8.
HIV-1 infection of human stromal cultures transduced by retroviral vectors. Duplicate cultures of primary, nontransduced human stroma (•), human stroma transduced with the L-RRE-Neo vector (▴), human stroma transduced with the LN vector (▪), and murine S17 stroma (X's) were infected with HIV-1JR-FL at an MOI = 0.5. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) shows the results from the stromal layers infected with HIV-1, without subsequent addition of monocytic cells. (B) Shows the results from the stromal layers infected with HIV-1 (the duplicates from [A]), with monocytic cells added on day 8.
Hematopoiesis is not decreased by HIV-1 infection of LTBMC containing stroma that does not support replication of HIV-1.To determine if infection of the stroma is the direct cause of impaired hematopoiesis in the human LTBMC, we compared the HSF of LTBMC in which the stroma is unable to support active HIV-1 replication to those in which the stroma is able to support HIV-1 growth. Human hematopoietic cells that were either infected by HIV-1JR-FL or uninfected were subcultured on either of three different types of stroma: (1) the murine stromal cell line S17, (2) primary human stroma transduced with the retroviral vector L-RRE-neo, and (3) primary human stroma transduced with the retroviral vector LN, or were grown in the absence of stroma. Three weeks after HIV-1 infection and initiation of the LTBMC, the numbers of total cells produced and the numbers of clonogenic progenitor cells in each culture were assayed.
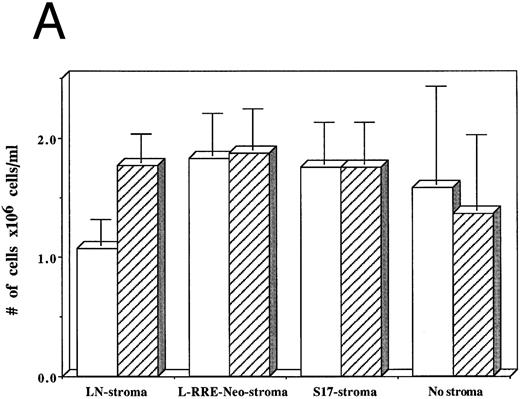
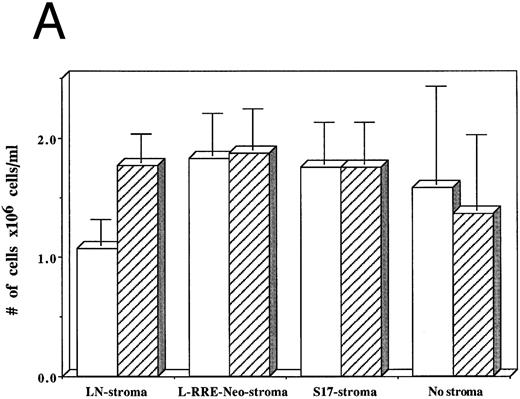
From among three separate experiments, HIV-1–infected hematopoietic cells grown on human stromal cells transduced by LN showed a 40% decrease in the numbers of cells produced after 3 weeks of culture compared to hematopoietic cells that were not infected by HIV-1 (Fig 5A). In contrast, HIV-1–infected hematopoietic cells grown on either human stromal cells transduced by L-RRE-neo, on murine S17 stromal cells, or in the absence of stroma showed no decrease in the numbers of cells produced compared to cultures initiated with hematopoietic cells not infected with HIV-1.
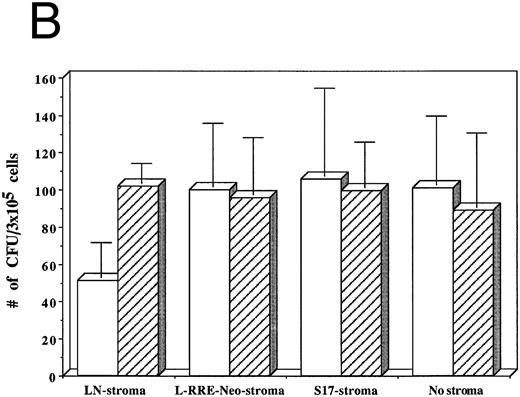
Effects of HIV-1 infection on growth of human LTBMC. (A) Numbers of cells in HIV-1–infected and –uninfected LTBMC. The total numbers of cells present in LTBMC were counted 3 weeks after infection with HIV-1 (□) or uninfected (▨) cultured on: human stroma transduced with the LN vector (LN-stroma), human stroma transduced with the L-RRE-neo vector (L-RRE-Neo-stroma), the murine S17 stromal line (S17), or without any stromal layer (no stroma). The figure shows results from three independent experiments (error bars represent the SEM). (B) Numbers of CFU in HIV-1–infected and –uninfected LTBMC. The total numbers of clonogenic CFU progenitors from the same three sets of LTBMC depicted in (B) were assayed 3 weeks after infection with HIV-1 (□) or uninfected (▨).
Effects of HIV-1 infection on growth of human LTBMC. (A) Numbers of cells in HIV-1–infected and –uninfected LTBMC. The total numbers of cells present in LTBMC were counted 3 weeks after infection with HIV-1 (□) or uninfected (▨) cultured on: human stroma transduced with the LN vector (LN-stroma), human stroma transduced with the L-RRE-neo vector (L-RRE-Neo-stroma), the murine S17 stromal line (S17), or without any stromal layer (no stroma). The figure shows results from three independent experiments (error bars represent the SEM). (B) Numbers of CFU in HIV-1–infected and –uninfected LTBMC. The total numbers of clonogenic CFU progenitors from the same three sets of LTBMC depicted in (B) were assayed 3 weeks after infection with HIV-1 (□) or uninfected (▨).
The numbers of clonogenic progenitors present in the same cultures after three weeks were also determined (Fig 5B). The CFU content of the HIV-1–infected LTBMC grown on human stroma transduced with the control LN vector, previously shown to allow HIV-1 replication, was reduced by 41.5% to 62.4% when compared to the CFU content of the uninfected LTBMC. When cells were grown on either human stroma transduced by L-RRE-neo, or on the murine S17 stromal line no decreases in the numbers of CFU occurred in the cultures infected by HIV-1. Additionally, growth of the hematopoietic cells in the absence of any stromal layer also showed no adverse effects from HIV-1 infection. These results strongly suggest that HIV-1 infection of the stromal cells is required for the decreased hematopoietic support function of LTBMC. Conversely, HIV-1JR-FL does not cause a loss of hematopoietic support function when the stromal cells are protected from HIV-1 replication in vitro, either innately with murine cells, genetically with expression of the RRE decoy, or by the simple absence of stroma.
HIV-1 infection of chimeric LTBMC shows that infection of the hematopoietic cells is not necessary for the decreased hematopoiesis produced by HIV-1.Although the experiments described above strongly implicate the stromal layer as the key cell component which must be infected by HIV-1 for the decrease in hematopoiesis from the LTBMC, it is possible that the human hematopoietic cells may also play a role by amplifying the low amounts of HIV-1 virus present in the stroma and reinfecting additional stromal cells. To determine whether active infection of the hematopoietic cell component of the LTBMC is necessary for the observed decrease in hematopoiesis produced by HIV-1, we compared the results using human hematopoietic cells, which should be infectable by HIV-1, to those using murine hematopoietic cells, which should be incapable of supporting HIV-1 replication. Murine BM hematopoietic cells can proliferate on human stroma when the medium is supplemented with murine IL-3 and murine SLF.
Chimeric LTBMC were produced, using either human or murine BM hematopoietic cells on one of four different stromal support monolayers: (1) primary human stroma (nontransduced), (2) human stroma transduced by the L-RRE-Neo retroviral vector carrying the anti-HIV-1 RRE decoy sequences, (3) human stroma transduced by the control LN retroviral vector, and (4) the murine stromal cell line S17. The cultures of each type of stromal cells were established in quadruplicates as previously described. Two of each were infected with HIV-1JR-FL at an MOI = 0.5 and two were left uninfected. Eight days post-infection with HIV-1JR-FL , both HIV-1–infected and –uninfected stromal layers were used to establish human and murine LTBMC by addition of either human or murine hematopoietic cells. After 3 weeks, the hematopoietic function of the cultures was measured by counting the total number of cells that had been produced in each culture, as well as the numbers of clonogenic progenitors.
As before, growth of human hematopoietic cells showed an approximately 50% decrease in the presence of HIV-1 when grown on either normal (nontransduced) human stroma (42.2%) or human stroma transduced by the LN vector (46.3%), but no decrease when grown on either human stroma transduced by L-RRE-neo or on murine S17 stroma (Table 1). A similar pattern was seen when murine hematopoietic cells were used for the LTBMC, with decreased numbers of total cells and progenitors in the presence of HIV-1 when grown on stroma capable of supporting HIV-1 replication (nontransduced human stroma and LN-transduced human stroma), but not when grown on stroma incapable of supporting HIV-1 replication (human stroma transduced by L-RRE-neo and murine S17 stroma). These findings show that active infection of the hematopoietic cell component of the LTBMC is not necessary for the observed decrease in hematopoiesis produced by HIV-1.
To assess the statistical significance of the degree of suppression of CFU formation caused by HIV-1 infection of stroma, we determined the odds ratios for the frequencies of CFU formed between various cultures. If infection of stroma by HIV-1 does not lead to loss of hematopoietic support function, then the ratios of the numbers of CFU present in cultures infected by HIV-1 to those not infected by HIV-1 would be equal to one. The ratios of the numbers of CFU formed between HIV-1–infected and –uninfected cultures can be compared between the cultures with different stromal conditions by calculating the odds ratio. If the value for the odds ratio is significantly greater than 1, then HIV-1 infection had a suppressive effect in one of the cultures.
The odds ratios for the numbers of CFU from cultures of human and murine hematopoietic cells grown on L-RRE-neo stroma compared to those grown on nontransduced primary stroma are 1.605 and 1.789 leading to rejection with >95% confidence of the null hypothesis that HIV-infection had equal effects on both sets of cultures (Table 2). Similarly, the odds ratios for the cultures of human and murine hematopoietic cells on L-RRE-neo stroma to LN stroma are 1.637 and 1.676, again rejecting the hypothesis that the effects of HIV-1 on these cultures are equal with >95% confidence. Thus, HIV-1 does adversely affect the numbers of CFU in cultures of either human or murine hematopoietic cells maintained on HIV-1 susceptible stroma (primary nontransduced or LN-transduced), compared to L-RRE-neo, but shows no difference for cells on L-RRE-neo stroma and S17 stroma.
In contrast, the odds ratios for the numbers of CFU grown from cultures of human or murine hematopoietic cells grown on L-RRE-neo stroma to S17 stroma are close to one (1.031 and 1.007, respectively), supporting the null hypothesis that HIV-infection had equal effects on both sets of cultures. Similarly, the odds ratios for the cultures of human and murine hematopoietic cells on LN stroma to nontransduced stroma are 0.980 and 1.067, again showing that the effects of HIV-1 on these cultures are equal with >95% confidence. Thus, HIV-1 does adversely affect the numbers of CFU in cultures of either human or murine hematopoietic cells maintained on HIV-1 susceptible stroma (primary nontransduced or LN-transduced), compared to L-RRE-neo, but shows no difference for cells on L-RRE-neo stroma and S17 stroma.
Finally, we measured the level of HIV-1 replication which occurred in the chimeric LTBMC. Samples of the culture medium were collected at the completion of the experiments and assayed for HIV-1 p24 by ELISA (Table 3). Detectable levels of HIV-1 were seen only in the LTBMC which had permissive human stroma (nontransduced or LN-transduced) and human hematopoietic cells which could amplify the virus. No detectable HIV-1 was produced in the chimeric LTBMC with murine hematopoietic cells, despite the loss of hematopoietic suppression seen in the presence of permissive stroma (nontransduced or LN-transduced). Thus, loss of hematopoietic support function from HIV-1 infection of stroma may occur even in the absence of significant levels of HIV-1 replication in the hematopoietic cells.
DISCUSSION
This report investigates the mechanisms of HIV-1–mediated suppression of hematopoiesis by distinguishing the in vitro effects of HIV-1 infection on the hematopoietic cells from the effects on the stromal support layer. Our findings strongly indicate that the decrease in hematopoietic cell production following HIV-1 infection results solely from viral replication in the stromal cells and not from infection of the hematopoietic cells themselves.
Several studies have shown that the numbers of colonies formed in vitro from BM samples from HIV-1+ patients are reduced compared to control marrow samples.10,12,28-33 It is difficult to distinguish whether the reduced numbers of colonies formed is due to decreased numbers or function of the progenitor cells. Histochemical analysis of 96 BM biopsy samples from HIV-1–infected subjects showed reduced numbers of CD34+ cells, compared to normal values.34 However, other studies have found that the numbers of colonies formed from marrow HIV-1+ patients are indistinguishable from the control samples.8,9,35,36 We have recently examined the content of clonogenic progenitor cells in marrow samples from 21 HIV-1–infected donors and found extensive variability in the numbers of CD34+ cells and their colony-forming capacity.37 In our series, most samples from asymptomatic HIV-1 infected donors were similar to those from uninfected control marrow samples; however, most patients with severely reduced numbers of peripheral blood CD4 T lymphocytes (<200/mm3 ) had greatly reduced numbers and growth of marrow progenitor cells. Although other studies have also suggested that reduced clonogenicity of hematopoietic precursors was related to the extent of disease progression,12 30 discrepancies among the different studies are difficult to fully evaluate, since potentially important clinical information is often not reported, such as the stage of disease, presence of coinfections with microorganisms such as CMV and toxoplasmosis, or concomitant treatment with myelotoxic antiretroviral drug inhibitors.
Numerous studies have shown that the majority of CD34+ cells purified from HIV-1–infected patients are not infected with HIV-1 (negative for HIV gag or env DNA sequence by PCR).11,12,30,31,38,39 Most studies could not detect any deleterious effect from direct HIV-1 infection of human hematopoietic progenitors.8,40-42 However, other studies found that in vitro infection of CD34+ cells with HIV-1 or simple exposure of CD34+ cells to HIV-1 env gp120 led to decreased viability and diminished cell function.43-45
Since infection of CD34+ cells does not appear to be a common finding associated with hematopoietic suppression, several studies investigated the role of changes in the hematopoietic microenvironment. Serum or accessory cells, such as T lymphocytes and macrophages, from HIV-1–infected patients have been added to marrow cells in vitro to measure the effects on CFU growth. Serum from HIV-1–infected patients was found to contain factors which inhibited hematopoiesis, which were identified as either antibodies to gp120,8 or an 87-kD glycoprotein of unknown function.46 Perplexingly, addition of HIV-1–infected T lymphocytes to human marrow cells in CFU assays has been reported to result in either stimulation47 or suppression9 of CFU growth. Conditioned medium from tat-stimulated macrophages was found to contain TGF-β, which was able to suppress colony formation.31 Another study found that antisense oligonucleotides to tat and nef transcripts restored the colony-forming ability of BM mononuclear cells, but not of purified CD34+ cells, suggesting that viral replication in an accessory cell contributed to hematopoietic suppression.
The BM stroma is the major component of the microenvironment which regulates hematopoietic cell activity. Stroma is a heterogeneous mixture of cells, including fibroblasts, macrophages, endothelial cells, and adipocytes; operationally, stromal cells in vitro consist of the adherent cells that grow from marrow samples. Direct infection by HIV-1 of some of these stromal cell types may impair the ability of the stroma to support hematopoietic cell survival or proliferation. Histological examination of BM biopsies from acquired immune deficiency syndrome (AIDS) patients showed the presence of HIV-1 gag protein in BM reticular cells34 and ultrastructural analysis revealed multiple abnormalities in stromal cells.48
Primary human stroma appears to be susceptible to in vitro infection by HIV-1 when monocytotropic strains such as HIVADA , HIVBa-L , and HIVJR-FL are used, but not with the lymphocytotropic strains HIVLAV and HIVIIIB .18,49-51 HIVIIIB has been reported to be able to infect primary stroma, when it was mainly mesenchymal in origin,52 as well as the BS-1 stromal cell line, also of mesenchymal origin.53 Gill et al51 identified stromal macrophages as the predominant target of HIV-1 infection in vitro. Furthermore, a recent study by Moses et al showed that a major component of stroma, the microvascular endothelial cells, was consistently found to be infected by HIV-1 when isolated from patients.54
We showed that primary human marrow stromal cells can be infected by HIV-1JR-FL at high MOI. Replication of virus occurs at a low level in cultures containing only stromal cells, but can be amplified by addition of susceptible hematopoietic cells. Fluorescence-activated cell sorter analysis of three different stromal cultures showed 1.8% to 2.1% endothelial cells (by immunofluorescent staining for von Willebrand's factor) and 1.8% to 2.6% monocyte/macrophages (CD13+), either of which may be infectable by HIV-1. However, the level of expression of HIV-1 p24 gag and gp120 env protein were too low to detect in infected stroma, despite the detection of low levels of p24 release by the more sensitive ELISA assay (data not shown).
In contrast, production of virus cannot be detected, even with amplification, from stroma in which the virus cannot replicate, either in innately resistant murine stroma (S17) or in human stroma genetically manipulated by introduction of a gene (an RRE decoy) which inhibits HIV-1–gene expression.
Four groups have attempted to show that HIV-1–infected stroma is diminished in its capacity to support hematopoiesis. When the BS-1 human stromal cell line was infected with HIVIIIB , its ability to support murine hematopoiesis was suppressed.53 Two recent studies were contradictory in their findings on the ability of stroma infected by HIVADA to induce hematopoietic suppression. While Schwartz et al18 found an approximately 50% decrease in the numbers of CFU-GM produced in infected cultures compared to controls, Marandin et al50 found no differences in the numbers of colonies from cultures infected by either HIVADA or HIVBa-L compared to controls. A possible explanation for this discrepancy may be the conditions under which the stromal cells were grown in vitro, which may lead to losses of specific cell types, such as macrophages or endothelial cells, during prolonged culture. The differences seen by various investigators may also relate to specific properties of the HIV-1 isolates which were used, as observed by Cen et al.45
To determine whether replication of HIV-1 in stroma contributes to the decrease in hematopoiesis, we compared the proliferation of human hematopoietic progenitor cells infected by HIV-1 using stromal cells that were either susceptible or resistant to HIV-1 replication. HIV-1–infected hematopoietic cells grown for 3 weeks on HIV-1–susceptible stroma produced diminished numbers of mature cells and clonogenic progenitors, when compared to the uninfected control. HIV-1–infected cells grown on HIV-1–resistant stroma (murine S17 or primary human stroma transduced with the RRE decoy) displayed proliferation and colony-forming abilities, which were indistinguishable from the uninfected controls. Thus, replication of HIV-1 in the stromal cells was necessary for the decrease in hematopoiesis seen in the HIV-1–infected cultures.
In fact, HIV-1 infection of the nonadherent hematopoietic cell fraction of the long-term cultures appeared to be irrelevant in causing the decreased hematopoiesis. This observation was unequivocally shown in experiments with chimeric cultures in which murine hematopoietic cells were grown on human stroma. Under these conditions, the murine hematopoietic cells are dependent on the hematopoietic support function of the stroma but are resistant to direct infection with HIV-1. When murine hematopoietic cells were grown on HIV-1 infected human stroma, their ability to give rise to colonies was significantly diminished. Growth on stroma which was protected from direct HIV-1 infection allowed normal production of mature cells and progenitor cells, independently of whether the hematopoietic cells were murine cells, incapable of being infected by HIV-1, or human cells, which allowed some HIV-1 replication to occur.
The use of chimeric murine/human cultures and genetically modified human stromal cells to show the primary role of stromal infection by HIV-1 on the loss of the hematopoietic support function extends the prior observations by Schwartz et al.18 Our observations show that the loss of hematopoietic function in vitro is solely mediated by HIV-1 infection of the stroma and that replication of HIV-1 in the hematopoietic cells is neither necessary nor sufficient for decreased hematopoiesis. Thus, in vitro HIV-1 infection does not directly impair the proliferative capacity of the hematopoietic cells, but rather causes the loss of the HSF of the stroma.
The biological mechanisms by which infected stroma leads to the loss of HSF are unclear. Conceptually, two different basic mechanisms may be involved: diminished production of a factor which stimulates hematopoiesis or increased production of a factor which inhibits hematopoiesis. Data have been presented supporting both of these mechanisms. Schwartz et al55 found that addition of antibodies, which neutralize the inhibitory cytokines TNF-α and IL-455 or addition of recombinant IL-3, granulocyte colony-stimulating factor (G-CSF ), and c-kit ligand to HIV-1–infected marrow cultures improved hematopoiesis.18 In one study, macrophages exposed to recombinant tat protein produced elevated levels of TGF-β, which suppressed CFU growth.56 But, elevated levels of TGF-β or TNF-α were not detected in HIV-1–infected human marrow cultures by either Marandin et al50 or in our present study. Addition of exogenous TGF-β or TNF-α did not alter the growth of human colony-forming unit granulocyte macrophage (CFU-GM) from nonadherent marrow mononuclear cells in the presence of exogenous IL-3 or c-kit ligand.57 Furthermore, since stroma is a heterogeneous cell pool, altered cytokine expression in an infected cell may exert its effect on an uninfected cell, making the operant mechanisms complex and difficult to dissect.
The therapeutic implications of these findings are presently unclear. Certainly, a better understanding of the mechanisms by which HIV-1 impairs hematopoiesis may be used to guide clinical interventions for HIV-1 infected patients with hematopoietic defects. Diminished production of necessary stimulatory factors could be overcome by administration of the appropriate recombinant factor and overproduction of an inhibitory factor may be counteracted with neutralizing molecules, such as recombinant soluble receptors. Our findings suggest the possibility of improving hematopoiesis in HIV-1–infected patients by genetically modifying some of their marrow stromal cells to be resistant to infection by HIV-1, with subsequent re-engraftment of the stroma into the patient's marrow in vivo. However, there are conflicting findings in the literature as to whether stromal cells from transplanted marrow can engraft after BM transplantation.58-60 Further studies in animal models or possibly by transplantation of gene-marked autologous marrow stromal cells may reveal the extent to which stromal replacement may be achieved.
Supported by grants from the National Institute of Allergy and Infectious Diseases, Division of AIDS Treatment (SPIRAT Award #AI36606); and a grant from the T.J. Martell Foundation for Leukemia, Cancer, and AIDS Research (New York, NY). D.B.K. is the recipient of an Elizabeth Glaser Scientist Award from the Pediatric AIDS Foundation (Santa Monica, CA).
Address reprint requests to Donald B. Kohn, MD, Division of Research Immunology/BMT, Mailstop #62, Children's Hospital Los Angeles, Los Angeles, CA 90027.

![Fig. 2. HIV-1 infection of primary human stromal layers. Primary human stromal layers were infected with HIV-1JR-FL at MOI of 1.0 and 0.1 in duplicate. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 without subsequent addition of monocytic cells (error bars represent the SEM). (B) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 (the duplicates from [A]), with monocytic cells added on day 8. (C) Shows the results of four independent infections of stromal layers at an MOI = 0.1 without subsequent addition of monocytic cells. (D) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 0.1 (the duplicates from [C]) with monocytic cells added on day 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1787/4/m_bl_0040f2a.jpeg?Expires=1769102304&Signature=3BT6NgfFmOPC2DlYodpxq9L6JsXwjJxXhDz3Zvu9zqryPM74P~3PCNJXvYBCmcLHYTMB9Dvo4XPXLB1qEZziiT~AarxRJbLj0ABoXpuXyQOmgKL~m5ba~7TKHhLMidiQT494KVUqmjeSh~icNw2vCzqCuCCC6XQk~j4u5uDUQjh3s6egT4vF2sMNOvapsdqL1w4Ua2h4WiCo4VSKlLJWPw651Ouzj2M2GTDezyxwv2GUxRQbBzIVdVIZnEbvOzHjVMcSF3EIIwiGcZ1zhCmvfUrmQPRFk7lpFUiLkv12r9wbEhhDX0~-XIamOaNm9Es3OvTcqueVqBf38I6RdLTtXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. HIV-1 infection of primary human stromal layers. Primary human stromal layers were infected with HIV-1JR-FL at MOI of 1.0 and 0.1 in duplicate. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 without subsequent addition of monocytic cells (error bars represent the SEM). (B) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 (the duplicates from [A]), with monocytic cells added on day 8. (C) Shows the results of four independent infections of stromal layers at an MOI = 0.1 without subsequent addition of monocytic cells. (D) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 0.1 (the duplicates from [C]) with monocytic cells added on day 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1787/4/m_bl_0040f2b.jpeg?Expires=1769102304&Signature=PdL60cqxvRK3Dh8TU7R2Y8Kut9Cue~TxuvwBOmWVUAPxnj~VOSwm2uebvTyOrzwj2tMNuPG3a9zv65lIdjPD8rdmz752qDoe6sNZTPhUic217t75ZUgAp2dtGkiHDuEPs7Q-FOU6m3TuuPs8XPC6P1OC2NENCg27sj6UlnSGu1rtNmqIgT5VAYqSgnkeEOLnG2OAgan68sVUqK2-hwwGFXrF4CE3pJnuSoRO1JWbgjTbHNg4zi~eVR1hhxAa4BKmIX-23KpUBcLFDiIpgnWU61~43GbltLrlIkFcV4kEiN5nvstq7cZGKSiN6V83MNgyi5A2kdMrsObiyaaPLK9PRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. HIV-1 infection of primary human stromal layers. Primary human stromal layers were infected with HIV-1JR-FL at MOI of 1.0 and 0.1 in duplicate. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 without subsequent addition of monocytic cells (error bars represent the SEM). (B) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 (the duplicates from [A]), with monocytic cells added on day 8. (C) Shows the results of four independent infections of stromal layers at an MOI = 0.1 without subsequent addition of monocytic cells. (D) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 0.1 (the duplicates from [C]) with monocytic cells added on day 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1787/4/m_bl_0040f2c.jpeg?Expires=1769102304&Signature=fPr0d-r9BZvcQWWIm7ciwwMcI~oRyX-Zi3EXaM3Ri-ugMfu2jlF412zsE7V8eg4vj6i443faiPhz~AUYJFPj70Dh7tbr2I7QwgB8NMGj-U3CXPyCOXlzmUFjcLBVxfHgxN~prQpPwaZ8awDfu-d3K-qLcV9mWuyDbPGTm5kGS2axU5RGS8FE4WBFn6VkaXo~xtEDq9t9VMzQ6MMqqE0mnH2HbG7ZwS1TslSqccYUFHsmXG6nbYQLV4QTOnKfabdNt4EW8rVX9j6Lt9Vaw1OakXrDVjtEXaV~H885ChHca2-RBHRUaqqO0t50Jmy8GEwHKf87Nje5IJdoUiz01AT3kA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. HIV-1 infection of primary human stromal layers. Primary human stromal layers were infected with HIV-1JR-FL at MOI of 1.0 and 0.1 in duplicate. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 without subsequent addition of monocytic cells (error bars represent the SEM). (B) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 (the duplicates from [A]), with monocytic cells added on day 8. (C) Shows the results of four independent infections of stromal layers at an MOI = 0.1 without subsequent addition of monocytic cells. (D) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 0.1 (the duplicates from [C]) with monocytic cells added on day 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1787/4/m_bl_0040f2d.jpeg?Expires=1769102304&Signature=I1L7RCBPu97pj2pzPgFaCCfcPjRl4fr2rY1CQL4P72DHI00F-goE2NJezbqTFoaTYNH1CprFAxnzc1t7K9dmMbAPRxXkik9UDqG7DAFRKAoT9oOoZIysg3SOEE~OAv3vq27TiQjlxDrBDBC-EcMWZ7b~pCym0~rgpRU9dSveUX2XHRTmjPBOEoIq8ehpXJOuqPgqeCAqh0cuKBSOxBO5NwjhVfo8Gl2wY5pW7Jv62oi7qavhLcNRbAbKQQ2Se43bYHV8zGxlx1U1iKKG7rSoBZ657wDFqYLJoISYaOHXA6H42~IGccj39qMXH9eR7mpqMYQCEKzqnR5eAUDBclayXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. HIV-1 infection of human stromal cultures transduced by retroviral vectors. Duplicate cultures of primary, nontransduced human stroma (•), human stroma transduced with the L-RRE-Neo vector (▴), human stroma transduced with the LN vector (▪), and murine S17 stroma (X's) were infected with HIV-1JR-FL at an MOI = 0.5. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) shows the results from the stromal layers infected with HIV-1, without subsequent addition of monocytic cells. (B) Shows the results from the stromal layers infected with HIV-1 (the duplicates from [A]), with monocytic cells added on day 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1787/4/m_bl_0040f4a.jpeg?Expires=1769102304&Signature=NVtTEosVFuu~ck-LLJR0jsvqkpXlcL8RfGPyfWkP4OoWuUFJEFsz5-y7sO7QIqqFxqPI2r92jWkIQiw6zl1yM7c5J9v18CiCrSEaZS7JRkpGp6yUQqJqpQ1gjR70~UiPqwOZC9~HK0cncjrgbN4CsbKfYswvRaGUwwQ477598vWkd2zn8PHVOxwS~Zu4-a~SRgIls2~HIPRf6fs9V8kRfFk0zOOLWEznjRVHLvGPpEvaoBSY9KOhXGjCUK0Ssq4N7GLmKkuLqFrL-vmENn6B0tkX3dgicbe~wT8tGQQbyjNwOe4h2eLSR0IjeGB3-rqPt~kAuKGYlnxF8fvaQP5b0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. HIV-1 infection of human stromal cultures transduced by retroviral vectors. Duplicate cultures of primary, nontransduced human stroma (•), human stroma transduced with the L-RRE-Neo vector (▴), human stroma transduced with the LN vector (▪), and murine S17 stroma (X's) were infected with HIV-1JR-FL at an MOI = 0.5. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) shows the results from the stromal layers infected with HIV-1, without subsequent addition of monocytic cells. (B) Shows the results from the stromal layers infected with HIV-1 (the duplicates from [A]), with monocytic cells added on day 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1787/4/m_bl_0040f4b.jpeg?Expires=1769102304&Signature=fTA19~xIfMJg~dWHqfDepx2HNeijXvcSFVCHKdeGjoje~yGyA4yL86X3lwLY01GAk-Bp8mtOyVKHQr65YA2mYS5Mz073CZYDlX10wdTn2ryRWTrnlMDp6omEBIDzf3p6YV0kBe1BKNIp94nxHjFG42ZPf8HvILG~JZJdPeoISwf4Cvrh4gItPydaWzqSq0VYOEE527x9YQoeKUUxUsMi2-aBVsRc9mCF3rq6odFfepRh3GMaTodCfD9fbSigHow~C0Uv6DkbtBkqbLhBeKegkJlmnN-BxPwXpKTlGJnqqGrBcGs31A3fulcS-0fMjsISbgCTa9NyFz9nXGlvFj5CwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 2. HIV-1 infection of primary human stromal layers. Primary human stromal layers were infected with HIV-1JR-FL at MOI of 1.0 and 0.1 in duplicate. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 without subsequent addition of monocytic cells (error bars represent the SEM). (B) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 (the duplicates from [A]), with monocytic cells added on day 8. (C) Shows the results of four independent infections of stromal layers at an MOI = 0.1 without subsequent addition of monocytic cells. (D) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 0.1 (the duplicates from [C]) with monocytic cells added on day 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1787/4/m_bl_0040f2a.jpeg?Expires=1769542128&Signature=2dtaRzQybKscu1pqsA7EjaeUy2Vmkt47l-5m4UPjkg2Ovy~4fOPqNcPlfFXmZKB4PAVXJ7krarggmxJDQQvO4zw-FfNGv~7XmUjNcws6kaXGaNeph~Mi3kFZDujadnOU5zpW0JdyDSiJ4zPOpeH8cedkXD41Q~au~HHxl-k3aoc6YOeKTBADfW~xgmDmGaX0KSKhRXQcmMGy6v1N7xN~uVfYdSVwmzog~v1tSMQKuPsNTtrMQ~ltHiyu30bJBL5DCWUsgP9YmbbIhaOKBufOiLr8YkWI43zCZI4Vrzy9a0ZAnI5pBO~IopZEppqqivs7LXmaWylraqphYbt41b~XPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. HIV-1 infection of primary human stromal layers. Primary human stromal layers were infected with HIV-1JR-FL at MOI of 1.0 and 0.1 in duplicate. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 without subsequent addition of monocytic cells (error bars represent the SEM). (B) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 (the duplicates from [A]), with monocytic cells added on day 8. (C) Shows the results of four independent infections of stromal layers at an MOI = 0.1 without subsequent addition of monocytic cells. (D) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 0.1 (the duplicates from [C]) with monocytic cells added on day 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1787/4/m_bl_0040f2b.jpeg?Expires=1769542128&Signature=MA6jKybCEwCIpUGqbl-IQ9DJKskEEYi-6cyp5Hn8DgSu2RBP0SUAwOtuuoa9vzSU7EiTOO4J-EQ3iOm00fwnOAsZsr6zM8jxbxDSVTIWhQNYR94LusyurduSagDIYlV~f5Pcmay7Bv8A4ctpN6agwRMMnzthoHdiT8DpDDC-cplqsNquxrDjy8JjqLqU8GWNu5rGtsMxyXQdn6v89GPXjtVQ4muWUODMRhedgd1Er1gPqOUrGn6PVNvynyu6ps-UMrTZcCwT3J9i4CYFrXazgjW177uOrgw-qNW3mftEJjF7chaUbtzhPVD17SGDZIoCXsm8uZ7ZT7ht1gUgMUXgLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. HIV-1 infection of primary human stromal layers. Primary human stromal layers were infected with HIV-1JR-FL at MOI of 1.0 and 0.1 in duplicate. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 without subsequent addition of monocytic cells (error bars represent the SEM). (B) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 (the duplicates from [A]), with monocytic cells added on day 8. (C) Shows the results of four independent infections of stromal layers at an MOI = 0.1 without subsequent addition of monocytic cells. (D) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 0.1 (the duplicates from [C]) with monocytic cells added on day 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1787/4/m_bl_0040f2c.jpeg?Expires=1769542128&Signature=T1HGVa6hfzxbnTWkD5Yr1MhI0PX4BFbw3Gfh9uxsw8Epyn9Pp~3so4J1NEWniu-srPkg1WdwK7K2S7Pw~CwH9SbsoyKWOk9efUAhx~ExNurf7A-In6TtTKiOkPRNmZKqDtQpU8VngJgfFIoYt2eEoCjGeKZEg5Zs3ZUaJz6U-gkEM9WeaDXvIxVt5oFLiKrSoLxKQ8VzkKMYPCSycjtYmBQcMC7nI0iCCp0R~zneeZmX3twpUIuQ8rAdKbeXwHjlH7lyqds4tkC5TB~XoCxfStmW29F8yBOMLO1TeLOOQiTYTpQyVTbd8jWFtEXktkoyjOipsWBw1fN6LyeB60ORXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. HIV-1 infection of primary human stromal layers. Primary human stromal layers were infected with HIV-1JR-FL at MOI of 1.0 and 0.1 in duplicate. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 without subsequent addition of monocytic cells (error bars represent the SEM). (B) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 1 (the duplicates from [A]), with monocytic cells added on day 8. (C) Shows the results of four independent infections of stromal layers at an MOI = 0.1 without subsequent addition of monocytic cells. (D) Shows the results of four independent infections of stromal layers with HIV-1 at an MOI = 0.1 (the duplicates from [C]) with monocytic cells added on day 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1787/4/m_bl_0040f2d.jpeg?Expires=1769542128&Signature=2EXFl7VUn4ww6ccst4pN5kVGTG0hpCv60OJFn3hvzCxBhAMManhoA2AJaUjjd1TU3IcHtwbqT1w2i-9zrjEZe~fgWNDUJ1ZDLRN4~4XZNpxPubKNRbpPr2nLyiqCTff41ZvhlkGYQk1jztnxUpgcR2QPIzm5FwfJG-Ue3bNWmrSZD1cCl4YZXmNRn6vtjSlIJ9mv8JYKxHQK2X6uLUw~8hZVNykwDxRtLVTt4xZeUXUsz2wNUlgQ4oN64R4BkEit00dXjBkC25p7KIWAycJFfePZOqmffWwXS-B3EyW7jhodx6YMZUWtGrxkwJ5HbHhvNEPPB34vsm0UTjUQztnMfg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. HIV-1 infection of human stromal cultures transduced by retroviral vectors. Duplicate cultures of primary, nontransduced human stroma (•), human stroma transduced with the L-RRE-Neo vector (▴), human stroma transduced with the LN vector (▪), and murine S17 stroma (X's) were infected with HIV-1JR-FL at an MOI = 0.5. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) shows the results from the stromal layers infected with HIV-1, without subsequent addition of monocytic cells. (B) Shows the results from the stromal layers infected with HIV-1 (the duplicates from [A]), with monocytic cells added on day 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1787/4/m_bl_0040f4a.jpeg?Expires=1769542128&Signature=RoJw2uOzW0FMOg2bQHHwpfdyMm9nIeSdWI-0RNF2H8HPoDTGBTUlMuim2P0ECh1ppCKTV5oQwTI0TPBmYViainLdILu6oCwXbC--3RnOkPPO~j1Meb8MB3dNLYSW5Fd2Whzxc3bHJ0bZenkLoGyUV~InqvZsIZGMA3hUS0DI6hsCWfUGQhti-S3pGtqmVLMtvXYEalM4tVtDgoEITPKDQJh7aa7bC3qUnb~YaXUOPospL3lF4nJJv~8SzQblaYeTkcR6f3ekiMPj4iCTbU6j690uYcPEqpDBzY0I81Rc~BooaI-riZpQR8EJEQ~nqxuUyt4RNYcy8o-vtRCZYwBLeQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. HIV-1 infection of human stromal cultures transduced by retroviral vectors. Duplicate cultures of primary, nontransduced human stroma (•), human stroma transduced with the L-RRE-Neo vector (▴), human stroma transduced with the LN vector (▪), and murine S17 stroma (X's) were infected with HIV-1JR-FL at an MOI = 0.5. On day 8 after infection, human monocytic cells were added to one of each pair of cultures to amplify low levels of HIV-1 present. Samples of the culture supernatants were sampled every 4 days and analyzed for p24 gag protein by ELISA. (A) shows the results from the stromal layers infected with HIV-1, without subsequent addition of monocytic cells. (B) Shows the results from the stromal layers infected with HIV-1 (the duplicates from [A]), with monocytic cells added on day 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/5/10.1182_blood.v90.5.1787/4/m_bl_0040f4b.jpeg?Expires=1769542128&Signature=hwM2UyUJYDADPsrXyhGGcf2~wKYrHiCzPgTrPCcIZc7Q1kz1L5bX~6h1aWAlgw~9aLEXVQ4lqWvRunMODjjrsDpYJwRCeL4xDJgdDOBa6fKPwrWuAb8pyBscfhsfSlGy7YY4kF1VIQDoS5XwJvrgWBcVyUatNAlsJVyKvCE7tPAfQ1wOuyLhqWLMJ4UD~fL-ARCj94fwimMDFFRN9gqDOGx0zEgwCQlzZ2kBXfnoiUwTgLSJ9kbo9bTySjtZ-xKtlzXKWX2Pa79oEtpoyjzf6t5sn1q~5ZDwSwlh38Q0CCNW4vsr7fYNCwWzJgHJtnrecLUPgac1KQqx7-b5H8JcOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)