Abstract
In the current study, we investigated the repercussions of the interaction between tumor cells (LSA) and the tumor-specific cytotoxic T lymphocyte (CTL) (PE-9) when both expressed Fas and Fas ligand (FasL). The CTL clone, PE-9, expressed high levels of Fas and FasL upon activation through the T-cell receptor (TCR). Furthermore, the activated PE-9 cells used both perforin- and FasL-based pathways to kill Fas-positive (Fas+) LSA tumor cells. Interestingly, LSA tumor cells also constitutively expressed FasL but not perforin, and killed Fas+ PE-9 CTLs and Fas+ but not Fas-negative (Fas−) activated T cells and thymocytes, as detected using the JAM test. PE-9 CTLs, cultured for 24 hours in the presence of cell lysates of FasL-bearing LSA cells but not FasL-deficient P815 cells, exhibited significant apoptosis as detected using the TUNEL method. Moreover, another FasL+ T-cell lymphoma line, EL-4, induced apoptosis in Fas+ but not in Fas− T cells in a similar fashion. The current study demonstrates for the first time that not only can the tumor-specific CTL mediate Fas-based killing of tumor cells, but FasL+ tumor cells can kill the Fas+ tumor-specific CTL. Thus, the survival of the tumor or the host may depend on which cell can accomplish this task more efficiently. The current study also suggests that FasL-based killing of CTLs by specific tumor cells may constitute a major limiting factor in successful immunotherapy.
RECENT STUDIES have demonstrated that cytotoxic T lymphocytes (CTLs) can kill target cells using two distinct lytic pathways: first, the degranulation pathway that uses perforin, possibly in combination with granzymes, and second, the Fas-based pathway in which the interaction between Fas ligand (FasL) expressed on the CTL and Fas on target cells triggers apoptosis and target cell death.1 It is believed that cross-linking of Fas by an appropriate ligand is sufficient to trigger cell death.2 3 This raises the interesting question of whether tumor targets that express FasL can kill a Fas-positive (Fas+) tumor-specific CTL following interaction between these two cells.
FasL is known to be expressed in both membrane-bound or soluble form. Recently, FasL was shown to be expressed by melanomas, hepatocellular carcinomas, and colon cancer, and furthermore, such cells were found to induce apoptosis in Fas-sensitive but not in Fas-resistant lymphoma cells.4-6 These data suggested that FasL expression by tumor cells may contribute toward creating an immune privileged site and immunosuppression. Despite such recent reports, whether FasL-bearing tumor cells can directly kill tumor-specific CTLs has not been previously tested. CTLs are relatively more resistant than tumor targets in their susceptibility to cell-mediated cytotoxicity.7 Also, not all Fas+ target cells are susceptible to FasL-based killing.6 Thus, it is necessary to directly test whether FasL-bearing tumor cells can kill tumor-specific CTLs.
To this end, we used a T-cell lymphoma line, LSA, and the tumor-specific CTL clone, PE-9, previously characterized in our laboratory.8 9 The current study demonstrates for the first time that tumor cells that express FasL can kill the specific effector CTL and other activated T cells that express Fas. Such a mechanism may help the tumor cells evade the specific immune surveillance and induce immunosuppression in the host.
MATERIALS AND METHODS
Mice.Adult female C57BL/6 (+/+) and C57BL/6 lpr/lpr mice were purchased from Jackson Laboratories (West Grove, PA) and maintained in our animal facility.10
Tumor cell lines.LSA is a radiation leukemia virus-induced T-cell lymphoma, and EL-4 is a chemically induced T-cell lymphoma; both are syngeneic to the C57BL/6 strain. L1210 Fas+ and L1210 Fas− cell lines represent murine lymphoma lines derived from the DBA/2 strain, transfected with sense and antisense Fas cDNA.11 AutoD1.4T is a T-cell line that underwent spontaneous transformation in vitro.12 P815 is a mastocytoma syngeneic to the DBA/2 mouse. All tumor cell lines were grown in syngeneic mice or maintained in culture with RPMI 1640 medium (GIBCO, Grand Island, NY) supplemented with 2 mmol/L glutamine, 50 μmol/L 2-mercaptoethanol, 10 mmol/L HEPES, 1 mmol/L glutamine, 40 μg/mL gentamicin sulfate, and 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA).
CTL line.PE-9 is a CD8+ αβT-cell receptor–positive (TCR+) CTL clone isolated from C57BL/6 mice rejecting the LSA tumor.8,9 PE-9 CTLs mediate antigen-specific killing of LSA tumor cells, but not other syngeneic or allogeneic targets.8 9 PE-9 cells were maintained in culture by supplementing the medium with 50 U/mL rIL-2 and occasionally stimulating them with irradiated (2,500 rads) or mitomycin C–treated LSA cells.
Activation of PE-9 T cells through the TCR.Before studying the expression of Fas or FasL or the cytotoxicity, PE-9 cells were cultured with irradiated LSA tumor cells for 48 hours, and the viable cells were purified by centrifugation on Ficoll-Hypaque (Sigma Diagnostics, St Louis, MO).9 In addition, in some experiments PE-9 cells were stimulated with immobilized anti-CD3 or anti-αβTCR monoclonal antibodies (MoAbs) for 48 hours.13
Antibodies.Anti-Fas (Jo2) and anti-mouse FasL MoAbs were obtained from Pharmingen (San Diego, CA). Fluorescein isothiocyanate (FITC)-labeled F(ab′)2 fragments of anti-hamster IgG and anti-mouse IgG were purchased from Jackson Immunoresearch (Baltimore, MD). The hybridomas 145.2C11 (anti-CD3 MoAb, hamster IgG) and H57-597 (anti-αβTCR, hamster IgG) were grown in our laboratory and purified as described elsewhere.10
Detection of Fas, Fas L, and perforin expression using reverse transcriptase–polymerase chain reaction.Reverse transcriptase–polymerase chain reaction (RT-PCR) was used to detect membrane-bound Fas, FasL, and perforin gene expression in a similar way as previously described.10 The primers used were as follows: Fas sense primer, 5′-GCACAGAAGGGAAGGAGTAC-3′; Fas antisense primer, 5′ GTCTTCAGCAATTC-TCGG GA-3′ (amplified fragment, 455 bp); FasL sense primer, 5′-GAGAAGGAAA-CCCTTTCCTG-3′; FasL antisense primer, 5′-ATATTCCTGGTGCCCATGAT-3′ (amplified fragment, 940 bp); perforin sense primer, 5′-GGTCAGAATGCAAGCAGA-AGCACAA-3′; perforin antisense primer, 5′-TTGGAGGTGAGGTGGAACTGAAGTT-3′ (amplified fragment, 499 bp); mouse β-actin sense primer, 5′-ATCCTGACCCTGAACTACCCCATT-3′; and β-actin antisense primer, 5′-GCACTGTAGTTTCTCTTCGACACGA-3′ (amplified fragment, 464 bp). PCR amplification products were visualized in 1.5% agarose gels after staining with ethidium bromide.
Flow cytometric analysis of Fas and FasL.Activated PE-9 cells, thymocytes, or various tumor cells were incubated with antibodies against Fas or FasL, washed, and incubated with FITC-conjugated secondary antibody. The cells were analyzed for fluorescence using a Coulter (Hialeah, FL) Epics V flow cytometer.9
Specific killing using 51Cr-release assay.51Cr-release assay was performed to study the killing of tumor cells by CTLs as described elsewhere.5,6 Briefly, tumor targets or LPS blasts9 were labeled with 100 μCi 51Cr by incubating at 37°C for 60 minutes. Various ratios of effector to target cells in triplicate were mixed in 96-well round-bottom plates (Costar, Cambridge, MA) and incubated at 37°C for 4 hours. Spontaneous release was measured by incubating 51Cr-labeled cells alone, and total release was determined by incubating target cells with 0.1% sodium dodecyl sulfate. After 4 hours incubation, the supernatants were harvested and radioactivity was measured using a gamma counter (TmAnalytical, Elk Grove Village, IL). Cytotoxicity was calculated as follows: % specific killing = E − S/T − S × 100, where E is experimental release, S is spontaneous release, and T is total release.
Use of concanamycin A to inhibit perforin-based cytotoxicity.In some assays, concanamycin A was used to study the relative role played by perforin and FasL expressed by T cells in the cytotoxicity of various targets as described by Kataoka et al.14 Cytotoxicity testing was performed using the 51Cr-release assay as already described, except that the effector cells were preincubated with concanamycin A (ICN Pharmaceuticals Inc, Costa Mesa, CA) at a concentration of 100 nmol/L for 2 hours in a final volume of 100 μL in microtiter plates, followed by addition of labeled target cells.
DNA fragmentation and specific killing using the JAM test.JAM test was used to study the ability of tumor cells to kill T cells. DNA fragmentation was assessed by labeling the target T cells with 3H-thymidine as described by Matzinger13 and modified in our laboratory.15 Briefly, target cells consisting of PE-9 cells, con A–activated spleen cells,8 or thymocytes were labeled with 5 μCi/mL 3H-thymidine (ICN, Irvine, CA) by incubating in a humidified atmosphere with 5% CO2 at 37°C for 6 hours. Excess 3H-thymidine was washed off, and 5 × 103 target cells were mixed with varying numbers of effector tumor cells in 96-well plates (Costar, Cambridge, MA). The plates were incubated at 37°C for 2.5 hours, and the cells were harvested onto glass-fiber filters using a semiautomatic cell harvester. The filters were dried and added to liquid scintillation fluid, and radioactivity was counted in a beta counter (Tm analytical). The mean percentage of DNA fragmentation was calculated from triplicate culture using the following formula: % DNA fragmentation = (S − E)/S × 100, where S is retained DNA in the absence of killers (spontaneous) and E is experimentally retained DNA in the presence of killers.
Detection of apoptosis using the TUNEL method.Apoptosis in the CTL clone, PE-9, was detected using terminal deoxynucleotidyl transferase (TdT) and FITC-dUTP, referred to as TdT-mediated nick end-labeling, or the TUNEL technique,16 for labeling DNA strand breaks (Boehringer Mannheim, Indianapolis, IN). PE-9 cells (1 × 106) were cultured in tissue culture plates with cell lysates of (1 × 106) LSA, EL-4, or P815 cells in 2 mL medium. Twenty-four hours later, the PE-9 cells were washed twice with medium containing phosphate-buffered saline (PBS) and fixed with 4% p-formaldehyde for 30 minutes at room temperature. The cells were next washed with PBS, permeabilized on ice for 2 minutes, and incubated with FITC-dUTP for 1 hour in the incubator. Fluorescence of the cells was measured by flow cytometry as previously described.16 The analysis was performed by a Coulter Epics V flow cytometer. Five thousand cells were analyzed per sample.
RESULTS
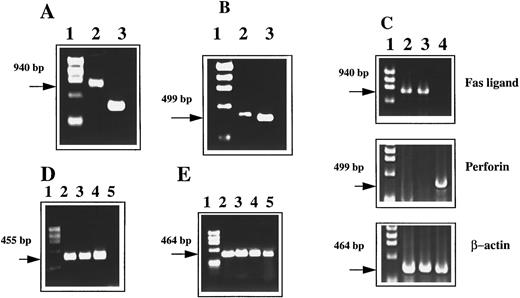
Expression of Fas and FasL by PE-9 CTLs and LSA tumor cells.PE-9 is a CTL clone isolated from C57BL/6 mice rejecting syngeneic LSA tumor. PE-9 cells are CD8+ and αβTCR+ and mediate tumor-specific MHC-restricted lysis of LSA.9 To further investigate whether Fas-FasL interactions play a role in the cytotoxicity mediated by PE-9 cells against LSA, we first analyzed this cell line for expression of Fas and FasL mRNA. The PCR data shown in Fig 1 suggest that PE-9 cells stimulated through the TCR expressed FasL gene (Fig 1A) and perforin (Fig 1B). Interestingly, when tumor cell lines were screened for expression of these molecules, LSA and EL-4 but not AutoD1.4T cells constitutively expressed FasL (Fig 1C). Also, AutoD1.4T cells but not LSA or EL-4 were found to constitutively express the perforin gene (Fig 1C). TCR-activated PE-9 cells and LSA tumor cells were also found to express Fas (Fig 1D).
Detection of Fas, FasL, and perforin mRNA using RT-PCR. PE-9 cells were stimulated with immobilized anti-CD3 MoAbs before PCR analysis. (A) PE-9 cells analyzed for FasL expression: lane 1, a molecular marker; lane 2, FasL; lane 3, β-actin (as a control). (B) PE-9 cells analyzed for perforin expression: lane 1, a molecular marker; lane 2, perforin; lane 3, β-actin. (C) FasL and perforin expression by tumor cell lines: lane 1, a molecular marker; lane 2, LSA tumor; lane 3, EL-4; lane 4, AutoD1.4T tumor. (D) Expression of Fas: lane 1, a molecular marker; lane 2, PE-9 cells; lane 3, LSA; lane 4, C57BL/6 thymocytes; lane 5, L1210 Fas− cell line. (E) β-Actin controls for Fig 1D (lanes identical to those depicted in D).
Detection of Fas, FasL, and perforin mRNA using RT-PCR. PE-9 cells were stimulated with immobilized anti-CD3 MoAbs before PCR analysis. (A) PE-9 cells analyzed for FasL expression: lane 1, a molecular marker; lane 2, FasL; lane 3, β-actin (as a control). (B) PE-9 cells analyzed for perforin expression: lane 1, a molecular marker; lane 2, perforin; lane 3, β-actin. (C) FasL and perforin expression by tumor cell lines: lane 1, a molecular marker; lane 2, LSA tumor; lane 3, EL-4; lane 4, AutoD1.4T tumor. (D) Expression of Fas: lane 1, a molecular marker; lane 2, PE-9 cells; lane 3, LSA; lane 4, C57BL/6 thymocytes; lane 5, L1210 Fas− cell line. (E) β-Actin controls for Fig 1D (lanes identical to those depicted in D).
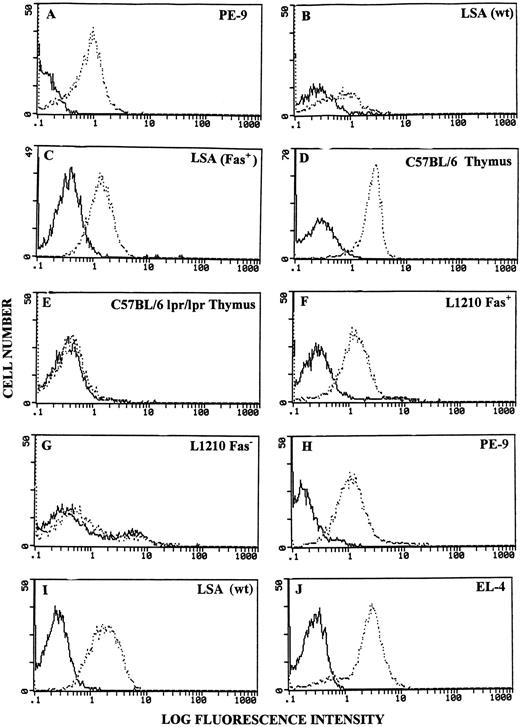
Surface expression of Fas and FasL on PE-9 and LSA cells was further corroborated using flow cytometry. PE-9 cells maintained in culture with irradiated LSA cells and IL-2 were found to express significant levels of Fas (Fig 2A). Wild-type LSA tumor cells expressed significant but lower levels of Fas (Fig 2B). However, by cloning, we were able to isolate a stable cell line that exhibited higher levels of Fas, designated the LSA (Fas+) cell line (Fig 2C). Fas expression by this and the PE-9 cell line was comparable to that exhibited by thymocytes from C57BL/6 (+/+) mice (Fig 2D) and the L1210 Fas+ cell line (Fig 2F ). Also, as expected, thymocytes from C57BL/6 (lpr/lpr) mice and the L1210 Fas− cell line failed to exhibit significant levels of Fas (Fig 2E and G).
Detection of Fas and FasL using flow cytometry. Various cells were stained for expression of Fas or FasL. A to G, Fas expression; H to J, FasL expression. Bold histograms represent cells incubated with FITC-conjugated secondary Abs only; broken histograms depict cells stained with anti-Fas or anti-FasL Abs + FITC-conjugated secondary Abs.
Detection of Fas and FasL using flow cytometry. Various cells were stained for expression of Fas or FasL. A to G, Fas expression; H to J, FasL expression. Bold histograms represent cells incubated with FITC-conjugated secondary Abs only; broken histograms depict cells stained with anti-Fas or anti-FasL Abs + FITC-conjugated secondary Abs.
When FasL expression was studied with flow cytometry, PE-9 cells demonstrated low levels of expression (data not shown); however, upon activation using either LSA or MoAbs against the TCR, they expressed higher levels of FasL (Fig 2H), consistent with recent studies demonstrating that FasL is upregulated following activation through the TCR.17 In addition, LSA and EL-4 tumor cells were found to express high levels of FasL constitutively (Fig 2I and J). Also, AutoD1.4T and P815 tumor cells failed to exhibit FasL when analyzed with flow cytometry (data not shown).
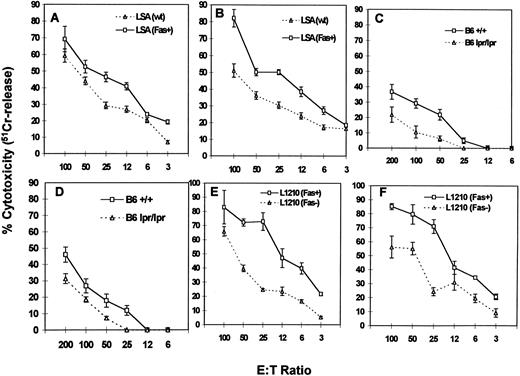
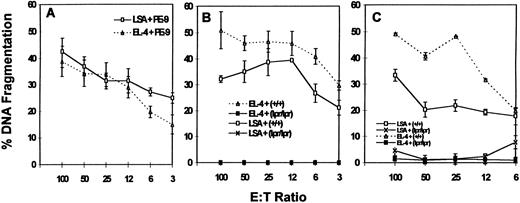
PE-9 CTLs can mediate FasL-based cytotoxicity.We next addressed whether PE-9 cells use FasL-dependent cytotoxicity to kill the specific LSA targets. The data shown in Fig 3 demonstrate that activated PE-9 CTLs mediated the increased cytotoxicity of mutant LSA Fas+ cells that expressed higher levels of Fas compared with wild-type LSA cells (Fig 3A and B). Furthermore, addition of MoAbs against Fas (Jo2) caused significant inhibition in the cytotoxicity of LSA tumor cells (data not shown). When LPS-activated B-cell blasts from C57BL/6 (+/+) and C57BL/6 (lpr/lpr) mice were used as targets in a redirected cytotoxicity assay in the presence of MoAbs against the αβTCR or anti-CD3 MoAbs,9 Fas+ targets were found to be more susceptible to lysis compared with Fas− targets (Fig 3C and D). Furthermore, identical results were obtained when Fas+ L1210 targets were compared with Fas− L1210 targets (Fig 3E and F ). These data together demonstrated that PE-9 cells, when activated, were using the FasL-dependent pathway to kill Fas+ target cells. However, the fact that Fas− targets were also killed by PE-9 cells suggested that perforin also played a significant role.
Cytotoxicity mediated by PE-9 cells against LSA and other targets. PE-9 cells were tested for lytic activity against various 51Cr-labeled targets. PE-9 CTLs were activated with MoAbs against the TCR before cytotoxicity assay, or the MoAbs against the TCR were added while performing cytotoxicity assays to facilitate activation of T cells and redirected lysis. The MoAbs used were against αβTCR in A, C, and E and against CD3 in B, D, and F. In A and B, LSA wild-type (wt) and a clone expressing higher levels of Fas (LSA Fas+) were used as targets. In C and D, LPS blasts from the spleens of +/+ and lpr/lpr mice were used. In E and F, L1210 Fas+ and Fas− transfectants were used as targets.
Cytotoxicity mediated by PE-9 cells against LSA and other targets. PE-9 cells were tested for lytic activity against various 51Cr-labeled targets. PE-9 CTLs were activated with MoAbs against the TCR before cytotoxicity assay, or the MoAbs against the TCR were added while performing cytotoxicity assays to facilitate activation of T cells and redirected lysis. The MoAbs used were against αβTCR in A, C, and E and against CD3 in B, D, and F. In A and B, LSA wild-type (wt) and a clone expressing higher levels of Fas (LSA Fas+) were used as targets. In C and D, LPS blasts from the spleens of +/+ and lpr/lpr mice were used. In E and F, L1210 Fas+ and Fas− transfectants were used as targets.
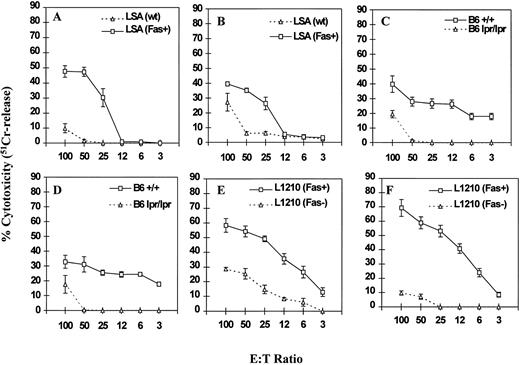
To further address the relative contributions of perforin and FasL, the ability of PE-9 cells to kill various target cells was studied in the presence of concanamycin A, which has been shown to inhibit perforin-based but not FasL-based cytotoxicity.14 Cytotoxicity was studied using various targets as described in Fig 3. The data shown in Fig 4 indicate that concanamycin A markedly inhibited the cytotoxicity mediated by PE-9 cells against most Fas− targets, whereas significant cytotoxicity was still demonstrable against Fas+ targets. It should be noted that the inhibitory effect of concanamycin A against Fas− targets was more pronounced at lower E:T ratios, consistent with a prior study.14 Also, differences in the susceptibility of Fas+ and Fas− targets to PE-9 cell-mediated killing were more striking in the presence of concanamycin A (Fig 3v Fig 4), which together suggested that PE-9 cells were using both Fas-based and perforin-based lytic pathways. It should be noted that several recent studies have demonstrated that in a short-term cytotoxicity assay, lysis of target cells can be attributed completely to perforin and FasL,18 thereby ruling out the possible involvement of other factors.
Effect of concanamycin A on cytotoxicity mediated by PE-9 cells against Fas+ and Fas− targets. PE-9 cells were tested for lytic activity against various 51Cr-labeled targets exactly as described in Fig 3, except for the addition of concanamycin A. The cytotoxicity assay depicted in A to F was performed in a similar fashion as described for the respective panels in Fig 3.
Effect of concanamycin A on cytotoxicity mediated by PE-9 cells against Fas+ and Fas− targets. PE-9 cells were tested for lytic activity against various 51Cr-labeled targets exactly as described in Fig 3, except for the addition of concanamycin A. The cytotoxicity assay depicted in A to F was performed in a similar fashion as described for the respective panels in Fig 3.
LSA tumor cells can kill tumor-specific CTLs in a FasL-dependent manner.Inasmuch as LSA tumor cells also expressed FasL, we next investigated whether these tumor cells could kill the tumor-specific Fas+ PE-9 CTL, as well as other Fas+ T cells. To study this cytotoxicity, we used the JAM test for several reasons: first, unlike tumor cells, T cells and thymocytes do not label well with 51Cr; secondly, the JAM test is more sensitive and detects DNA fragmentation that occurs before membrane damage.19 Moreover, when tumor cells and PE-9 cells were mixed, one would expect not only the tumor cells to kill the PE-9 cells but also vice versa. Thus, a sensitive assay in which DNA fragmentation can be detected within 2 hours clearly provided an advantage.
The data depicted in Fig 5A demonstrate that LSA and EL-4 tumor cells mediated efficient killing of the tumor-specific CTL, PE-9. Furthermore, LSA and EL-4 tumor cells induced DNA fragmentation in +/+ but not in lpr/lpr thymocytes (Fig 5B) and Con A blasts (Fig 5C). It should be noted that LSA and EL-4 cells failed to express perforin (Fig 1), thereby ruling out the possible involvement of perforin. Also, the AutoD1.4T tumor cell line that expressed perforin but not FasL failed to kill any of the T cells screened (data not shown).
LSA and EL-4 tumor cells can mediate DNA fragmentation in Fas+ PE-9 and other T cells. PE-9 T cells (A), thymocytes (B), or con A–activated T-cell blasts (C) were labeled with 3H-thymidine and used as targets to study the ability of LSA and EL-4 tumor cells to mediate cell death.
LSA and EL-4 tumor cells can mediate DNA fragmentation in Fas+ PE-9 and other T cells. PE-9 T cells (A), thymocytes (B), or con A–activated T-cell blasts (C) were labeled with 3H-thymidine and used as targets to study the ability of LSA and EL-4 tumor cells to mediate cell death.
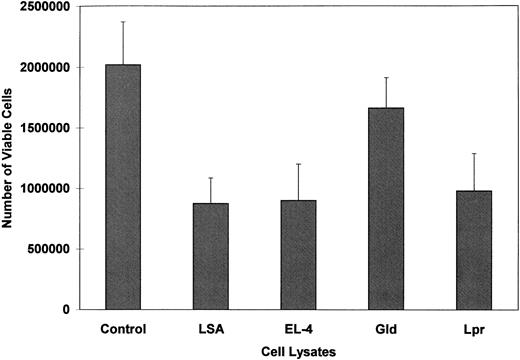
Cell lysates of FasL-bearing tumors can induce apoptosis in PE-9 CTLs.Fas+ PE-9 cells were cultured in vitro in the presence of cell lysates from FasL+ LSA or EL-4 cells for 24 hours, and cell viability was measured using a trypan blue dye exclusion assay. The data shown in Fig 6 suggest that within 24 hours there was approximately a 50% decrease in the number of viable cells compared with the medium controls. In these studies, we used lysates of lymph nodes from 4-month-old gld/gld or lpr/lpr mice, which have been previously shown to express massive amounts of FasL.20 However, FasL from gld/gld mice is functionally defective.20 Lysates from lpr but not from gld cells could induce a significant decrease in the viability of PE-9 cells (Fig 6).
PE-9 CTLs cultured in the presence of lysates of LSA or EL-4 tumor cells exhibit decreased cell viability. Fas+ PE-9 cells were cultured in vitro for 24 hours in the presence of medium alone (control) or lysates of LSA, EL-4, or lymph node cells from gld or lpr mice. Cell viability was measured by trypan blue dye exclusion. Vertical bars represent mean viable cell counts in triplicate cultures ±SD.
PE-9 CTLs cultured in the presence of lysates of LSA or EL-4 tumor cells exhibit decreased cell viability. Fas+ PE-9 cells were cultured in vitro for 24 hours in the presence of medium alone (control) or lysates of LSA, EL-4, or lymph node cells from gld or lpr mice. Cell viability was measured by trypan blue dye exclusion. Vertical bars represent mean viable cell counts in triplicate cultures ±SD.
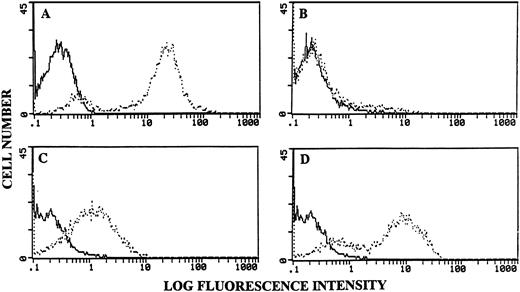
To further corroborate the finding that FasL-bearing LSA and EL-4 cells induce apoptosis in PE-9 CTLs, the cells cultured in a similar fashion as described earlier were stained with TdT and FITC-dUTP. The data shown in Fig 7 indicate that PE-9 cells cultured with lysates of LSA or EL-4 induced marked apoptosis (Fig 7C and 7D), whereas similar concentrations of lysates from FasL-deficient P815 tumor cells failed to induce significant apoptosis (Fig 7B). In these experiments, thymocytes subjected to radiation (2,000 rads) and cultured for 24 hours in vitro served as a positive control (Fig 7A).
PE-9 CTLs cultured with lysates of LSA or EL-4 cells exhibit apoptosis. PE-9 cells were cultured in the presence of cell lysates as described in Fig 6. They were stained with TdT + FITC-dUTP and analyzed using a flow cytometer. (A) Irradiated thymocytes as a positive control for apoptosis. PE-9 cells cultured with lysates from FasL-deficient P815 (B) or FasL+ LSA (C) or EL-4 (D) cells are depicted. Bold histograms represent negative controls consisting of cells cultured in the presence of medium; broken histograms show cells irradiated (A) or cultured with cell lysates (B, C, and D).
PE-9 CTLs cultured with lysates of LSA or EL-4 cells exhibit apoptosis. PE-9 cells were cultured in the presence of cell lysates as described in Fig 6. They were stained with TdT + FITC-dUTP and analyzed using a flow cytometer. (A) Irradiated thymocytes as a positive control for apoptosis. PE-9 cells cultured with lysates from FasL-deficient P815 (B) or FasL+ LSA (C) or EL-4 (D) cells are depicted. Bold histograms represent negative controls consisting of cells cultured in the presence of medium; broken histograms show cells irradiated (A) or cultured with cell lysates (B, C, and D).
DISCUSSION
In the current study, we demonstrated using a tumor cell line and a tumor-specific CTL clone that when both effector and target cells express Fas and FasL, they can induce apoptosis in each other. The ability of FasL+ tumor cells to kill tumor-specific CTLs is particularly interesting and suggests that such tumor cells may evade the action of CTLs by their ability to induce apoptosis in CTLs. Such a mechanism may explain why immunotherapy with tumor-specific CTL clones may fail to eradicate the tumor. This is consistent with our previous observation that PE-9 cells can afford protection only in 50% of LSA tumor–bearing mice and that IL-2 administration does not further improve the efficacy.8
FasL was originally thought to be expressed only on CTLs and natural killer cells. However, recent studies demonstrated that FasL may be expressed by immunologically privileged tissues such as stroma cells of the eye and Sertoli cells of the testis.21-23 Furthermore, a variety of cancers such as large granular lymphocytic leukemias, colon cancer, hepatocellular carcinomas, and melanomas were found to express FasL.24 Such FasL-bearing tumor cells have been shown to kill other tumor target cells bearing Fas,4-6 thereby suggesting that FasL is functional in cancer cells and that such a mechanism can explain how some tumor cells can evade immune attack by CTLs and natural killer cells. The data from the present study provide direct evidence to this effect.
In the current study, we noted that not only LSA but also a nonspecific tumor such as EL-4 could kill PE-9 cells, consistent with the observation that ligation of Fas is enough to trigger apoptosis.2 3 The ability of EL-4 cells to kill PE-9 cells or that of LSA and EL-4 cells to kill a variety of nonspecific Fas+ targets can be explained by the fact that effector and target cells may be brought in contact with each other during the in vitro cytolytic assay, thereby facilitating Fas-FasL interactions. Alternatively, the tumor cells produced soluble FasL that triggered cell death. However, it is important to note that the killing of PE-9 cells by LSA cells can occur naturally following antigen-specific interaction between PE-9 and LSA cells.
Several studies have shown that engagement of Fas by its ligand is sufficient to trigger cell death.2,3 If this is true, culture of a cell line that expresses both Fas and FasL should trigger cell death. This may indeed explain why in vitro cultured LSA tumor cells rapidly die after attaining higher cell densities. On the other hand, Fas-FasL interactions may not always be sufficient to induce cell death.6,18 For example, in a previous study, FasL+ EL-4 cells failed to kill Fas+ target cells.18 The reason for the discrepancy in this finding versus the current study is not clear. The investigators speculated that the EL-4 cell line used in their study may have expressed a mutant or nonfunctional form of FasL or, alternatively, may lack an accessory molecule critical for induction of lysis. Other molecules by which tumor cells escape from self-inflicted injury may include acquisition of an intracellular mechanism that renders the cells resistant to Fas-induced apoptosis.24
Some types of tumors have also been shown to produce soluble FasL. A clinical case of nasal lymphoma was accompanied by high serum levels of soluble FasL.25 These levels correlated with marked liver damage and pancytopenia. Such data suggested that FasL expression by tumor cells may also lead to the systemic toxicity and multiorgan failure often seen in cancer patients.
In summary, the current study demonstrates that when tumor cells and antigen-specific CTLs express both Fas and FasL, there can be a bidirectional interaction and killing in both cell types. The outcome may depend on the stage of activation of cells and degree of expression of signaling molecules and cytolytic factors. On the other hand, this may constitute an important mechanism by which tumor cells evade the destroying forces of the host immune system, and may explain why LSA tumor–bearing mice fail to exhibit tumor-specific CTL activity and do so only following chemotherapy that eliminates a majority of the tumor cells.26 27 Expression of FasL by tumor cells may constitute a major obstacle for successful immunotherapy using tumor-specific CTLs.
Supported by grants from the American Cancer Society (IM747) and National Institutes of Health (NIH AI01392) and in part by an Independent Scientist award from the NIH (to M.N.).
Presented at the Fourth International Union of Biochemistry and Molecular Biology Conference on Life and Death of the Cell, Edinburgh, UK, July 14-17, 1996.
Address reprint requests to Prakash S. Nagarkatti, PhD, Department of Biology, Virginia Tech, Blacksburg, VA 24061.