Abstract
Primary leukemic cells from patients with acute lymphoblastic leukemia (ALL) can be injected intravenously into mice with severe combined immunodeficiency (SCID) to create a model of human leukemia. Leukemic cells disseminate to murine tissues in a clinicopathologic pattern similar to that seen in humans. Thus far, reports of engraftment of lymphoid leukemia in SCID mice have mainly been from patients with B-cell lineage ALL, for which engraftment occurs more frequently with cells from high-risk patients. There are few data on the engraftment of T-cell lineage ALL in SCID mice. Leukemic cells from 19 patients (16 adult and three pediatric) with T-cell lineage ALL were injected into SCID mice, with overt engraftment of 12 cases (63%). Engraftment of leukemia in SCID mice was associated with earlier death due to leukemia of the patient donors (P < .01, log-rank test). The recently developed non-obese diabetic (NOD)/SCID mouse may expand the uses of the SCID model. Cells from the seven patients with T-cell lineage ALL that failed to cause leukemia in SCID mice were injected into NOD/SCID mice. Overt leukemia engraftment was observed in all seven cases. Thus, growth of human T-cell lineage ALL cells in SCID mice was associated with a high-risk patient group. However, this association was not observed when NOD/SCID mice were used, suggesting that this model would no longer predict patients likely to die early of leukemia, but may provide a more realistic system for studying the biology and treatment of the disease.
THE OUTCOME OF treatment of patients with acute lymphoblastic leukemia (ALL) continues to be unsatisfactory, especially in adults. Complete remission (CR) is achieved in up to 80% of younger adults and children, but the rate is considerably lower (<50%) in those over the age of 60 years. Overall, only 30% of adults and 70% of children are cured.1,2 There is a need for better treatments. Selection of patients in high-risk groups for more intensive therapy or early progenitor cell transplantation may improve response and cure rates.1 2 Increased accuracy in identification of these high-risk patients would have major benefits.
The C.B-17 severe combined immunodeficiency (SCID) mouse is deficient in functional T cells and B cells and unable to reject allogeneic or xenogeneic organ grafts.3 The SCID mouse supports the growth of primary lymphoid leukemic cells without the use of exogenous cytokines. There have been several reports of engraftment of B-cell lineage ALL,4-8 acute myeloid leukemia,9 chronic myelocytic leukemia,10 and chronic lymphocytic leukemia11 in SCID mice, but only a small number of reports of success with T-cell lineage ALL.12-16 Engraftment and proliferation of primary leukemia cells in SCID mice creates an in vivo model of value for investigation of the biology of malignancy and for testing antileukemia therapies.17 However, usually, only a proportion of leukemic samples tested engraft as leukemia in SCID mice. Engraftment of certain subtypes of B-cell lineage ALL in SCID mice has been correlated with a poor outcome of patient donors,8 but no such correlation has been reported for T-cell lineage ALL.
We report the results of a study designed to investigate the engraftment potential of 19 primary human T-cell lineage ALL cell populations. To test the hypothesis that engraftment of samples from patients with T-cell lineage ALL in SCID mice predicts a poor clinical outcome, we correlated overt engraftment of primary leukemic cells in SCID mice with patient death from leukemia. We also investigated the suitability of the more immune-incompetent non-obese diabetic (NOD)/SCID mouse as a model of ALL by inoculating T-cell lineage ALL samples that had not caused leukemia in SCID mice into NOD/SCID mice.
SUBJECTS AND METHODS
Patient materials.Following provision of informed consent, leukemic cells were obtained from 19 patients treated at the Royal Marsden NHS Trust Leukemia Unit. Patient characteristics are detailed in Table 1. All patients were treated between 1987 and 1996 with standard protocols that included anthracyclins, vinca alkaloids, and cyclophosphamide. Samples were cryopreserved bone marrow (BM) or peripheral blood (PB) for 18 patients, and one sample was fresh PB.
Animals.SCID mice (National Institute of Medical Research, Mill Hill, UK) and NOD/SCID mice (Jackson Laboratory, Bar Harbor, ME) were used. The NOD/SCID mouse was developed by back-crossing the SCID mutation onto the NOD/Lt strain. Like SCID mice, NOD/SCID mice exhibit multiple defects in innate immunity and an absence of T-cell and B-cell function, but also lack natural killer cells.18 The progeny are not diabetic and are as robust as conventional SCID mice. All mice were maintained in a specific pathogen-free environment. Female mice aged 6 to 10 weeks received 200 cGy total-body irradiation (60Co source, 60 cGy/min) and within 24 hours were injected intravenously with 1 to 2 × 107 primary human BM or PB cells.
Engraftment of human leukemia in SCID mice and NOD/SCID mice.Mononuclear cells from the samples were separated by Ficoll-Metrizoate density gradient centrifugation (Lymphoprep; Nycomed, Oslo, Norway) and washed in RPMI 1640 medium (GIBCO, Paisley, UK). The cell concentration was adjusted to 1 × 108/mL for injection into mice. Engraftment of human leukemia in mice was monitored by serial tail vein sampling at regular intervals (approximately every 21 days) and then flow cytometric analysis. All mice with continuing weight loss were killed before becoming sick, when PB infiltration or clinical status suggested engraftment. Necropsy was performed, and internal organs were inspected for signs of leukemic infiltration. The spleen, femoral BM, and thymus (if enlarged) were removed, and single-cell suspensions were prepared for flow cytometric analysis. Single-cell suspensions were prepared by passing mouse tissue through a 180-μm wire-mesh filter. Cells were washed in RPMI 1640 medium.
Mice were evaluated for 6 months; if there was still no sign of engraftment, they were killed and examined for signs of disease. This consisted of macroscopic examination of the liver, spleen, and thymus and then flow cytometric analysis of cells from the BM and spleen. Mice with negative results for these investigations were considered nonengrafters.
Flow cytometric analysis of mouse PB, spleen, and BM.The percentage of human cells in murine tissues was measured using fluorescein-conjugated antibody to class I human leukocyte antigen (W6/32; Sera-lab, Crawley Down, UK). PB samples of 20 μL were obtained by tail vein sampling and collected into 30 μL phosphate-buffered saline (PBS) with 50 U/mL preservative-free heparin, and the antibody was added at a concentration of 1:20 for 30 minutes on ice. Two milliliters of lysis solution (0.037 g disodium EDTA, 1.0 g potassium bicarbonate, and 8.3 g ammonium chloride in 1 L) was then added for 10 minutes. After washing twice, using PBS and centrifugation (400g for 4 minutes), samples were resuspended for flow cytometric analysis using an Ortho Cytoron Absolute flow cytometer (Ortho Diagnostic Systems, Raritan, NJ). The proportion of fluorescein-labeled cells was determined within a large gate encompassing all nucleated mouse and human cell populations. Normal SCID mouse blood was included as a negative control with all analyses. Cell suspensions prepared from mice (usually spleen) showing leukemic engraftment with T-cell lineage ALL were also analyzed by flow cytometry using a panel of monoclonal antibodies to human leukocyte cell surface markers. This was to establish the extent to which the primary human immunophenotype was retained with passage through these mice. A panel of mouse monoclonal antibodies for detection of cell markers was used as the primary layer and a fluorescein-conjugated goat anti-mouse antibody (Cappel; Organon Co, Durham, NC) as the secondary layer. Pooled human AB serum was added throughout all steps to avoid nonspecific binding by Fc receptors. The primary monoclonal antibodies used were CD2 (Becton Dickinson, Oxford, UK), CD7 (3A1; Coulter, Luton, UK), CD10 (J5; Coulter), CD19 (B4; Coulter), CD13 (MY7; Coulter), CD33 (MY9; Coulter), and HLA-DR (Becton Dickinson). Normal SCID mouse spleen controls were studied concurrently with each analysis. Samples were analyzed on a Becton Dickinson FACScan flow cytometer using LYSIS II software.
Secondary passage of engrafted human leukemias.Four of the engrafted leukemias were passaged onto fresh, irradiated SCID mice. Spleens were disaggregated as previously described, and then the proportion of human cells was measured by flow cytometry. In all cases, the human cell fraction was more than 95%. Mice were inoculated with 1 to 2 × 107 cells intravenously, and engraftment of human leukemia was monitored as before by serial tail vein sampling at regular intervals.
Screening for Epstein-Barr (EB) virus.To attempt to exclude infection of engrafted SCID mice by EB virus as a potential promoter of xenograft proliferation, spleen cells from three randomly selected SCID mice engrafted with T-cell lineage ALL were screened by immunostaining for EB nuclear antigen. All three SCID samples were negative for the antigen.
Statistical analysis.Kaplan-Meier life tables were constructed for all patients with the following end points: death due to leukemia and time to CR according to standard BM criteria. Patients who died of causes other than leukemia were censored. All other patients are alive. Comparisons of outcome between patient subsets were performed by the log-rank test.
RESULTS
Engraftment in SCID mice.Engraftment occurred in 12 of 19 samples (63%), producing overt disseminated leukemia in mice; the median mouse survival duration was 85 days (range, 28 to 190). All engrafted mice showed marked infiltration of BM and/or spleen by human cells. In most cases, a gradual increase in circulating human cells was seen; however, in some cases, no human cells were detected in PB and evidence of engraftment was obtained at necropsy. All mice lost weight in the first 14 days following irradiation and then steadily gained weight. Regular weighing of mice revealed a second phase of gradual weight loss in mice succumbing to leukemia (data not shown). Two SCID mice with T-cell lineage ALL showed marked thymic enlargement due to leukemic infiltration. The proportion of human cells detected in selected organs was recorded. In this experiment, mice had greater than 10% infiltration in all positive cases (Table 2). In most cases with spleen involvement, liver infiltration was also observed (data not shown). Mouse spleen cell suspensions from eight of 12 T-cell lineage ALL cases showing leukemic engraftment showed strong positivity to monoclonal antibodies to human T-cell lineage epitopes and close homology to primary immunophenotype (Table 3). All four T-cell lineage ALL xenograft populations (T5, T6, T9, and T18) that were passaged into fresh SCID mice engrafted with a pattern of disseminated disease similar to that of implanted primary tissue (Table 2).
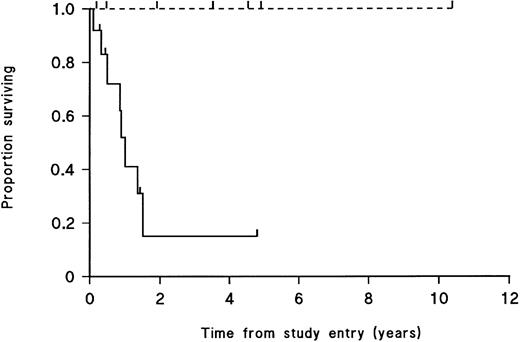
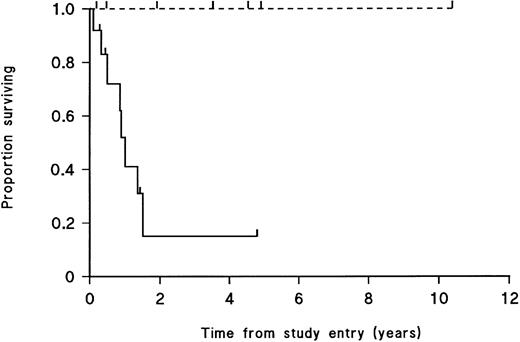
Overt engraftment of leukemic samples in SCID mice is significantly correlated with patient death due to leukemia.Survival curves were constructed plotting patient death due to leukemia for patients whose cells had engrafted in SCID mice and for those whose cells had not engrafted. For patient samples that engrafted, only one of 12 patient donors was alive at follow-up study, versus four of seven patients whose samples failed to engraft (P < .01, log-rank test; Fig 1). The curves for time to CR for patients whose leukemic cells engrafted versus nonengraftment in SCID mice showed no significant difference between the two groups (P ∼ 0.9, log-rank test; data not shown).
Survival from diagnosis to death from leukemia of 19 T-cell lineage ALL patients. ( — ) Patients whose leukemic cell populations engrafted in SCID mice; (- - -) patients for whom there was no engraftment. Patients currently alive and patients who died of causes other than leukemia are indicated by tick marks on the respective curves. Survival is significantly different between the 2 groups (P < .01, log-rank test).
Survival from diagnosis to death from leukemia of 19 T-cell lineage ALL patients. ( — ) Patients whose leukemic cell populations engrafted in SCID mice; (- - -) patients for whom there was no engraftment. Patients currently alive and patients who died of causes other than leukemia are indicated by tick marks on the respective curves. Survival is significantly different between the 2 groups (P < .01, log-rank test).
Engraftment in NOD/SCID mice.The seven T-cell lineage ALL samples that had failed to engraft in SCID mice were injected into irradiated NOD/SCID mice, with engraftment of all seven cases (Table 2); the median mouse survival time was 182 days (range, 62 to 214). As in SCID mice, circulating human cells were detected in murine PB, and necropsy showed leukemia cells disseminated to a variety of tissues, particularly BM, spleen, and liver.
DISCUSSION
We have shown that primary human T-cell lineage ALL can be grown in SCID and NOD/SCID mice. Mice engrafted with T-cell lineage ALL demonstrated disseminated leukemia in a clinicopathologic pattern similar to that of humans, with leukemic cells evident in PB, BM, spleen, thymus, and liver. Patient donors were predominantly adult, and the engraftment rate (12 of 19, 63%) in conventional SCID mice was similar to the rate we have reported for B-cell lineage ALLs.4 6
Engraftment of a patient's T-lineage leukemic cells in SCID mice correlated with death from leukemia of the patient donor. Thus, we have demonstrated from a heterogeneous population of patients with T-cell lineage ALL that the growth of leukemic cells in SCID mice identifies a group of high-risk patients. These data support the findings of Uckun et al,8 where the ability of leukemic cells from newly diagnosed patients with B-cell lineage ALL to cause overt leukemia in SCID mice was also associated with poor prognosis in the patients from whom the cells were obtained. Thus, biologic data generated in the SCID mouse model system may help to predict treatment response in high-risk B-cell lineage and T-cell lineage ALL.8
The clinical status of the patient at the time samples are obtained also seems to be of importance. Palucka et al19 showed that engraftment occurs more rapidly if leukemic cells from patients at relapse rather than presentation are injected. Kamel-Reid et al20 transplanted cells from a small number of patients with relapsed and newly diagnosed B-cell lineage ALL into SCID mice. Cells from patients who had relapsed within 13 months of diagnosis proliferated rapidly in SCID mouse organs, whereas cells obtained at diagnosis from patients who had not yet relapsed were detected in low numbers only. Engraftment of leukemic cells in SCID mice may yield clues to the importance of other biologic factors in leukemogenesis. Stranks et al21 reported an association between deletions of the p16 cell-cycle control gene in B-cell lineage ALL and SCID mouse engraftment. We found no correlation between engraftment of leukemic cells in SCID mice and a longer time to CR in patients. This result reflects the clinical situation in which greater than 80% of ALL patients achieve CR but less than 30% survive long-term,1 2 ie, biologically aggressive leukemias are manifest by relapsing early rather than by failing to remit.
These data show for the largest series of patients reported thus far, that T-cell lineage ALL engrafts in SCID mice, with a similar proportion of cases engrafting as for B-cell lineage disease. Furthermore, T-cell lineage ALL caused disseminated infiltration of murine tissue. In addition, the engraftment pattern of T-cell lineage ALL in SCID mice reported here identified patients at high-risk, as previously shown for B-cell lineage disease.8
We have shown that samples of T-cell lineage ALL that failed to cause leukemia in SCID mice did engraft in NOD/SCID mice. The NOD/SCID mouse is a more receptive host than the SCID mouse for proliferation of primary human leukemia for investigative purposes, but the high engraftment rate of T-cell ALL in NOD/SCID mice suggests that this model will not be useful for identifying a high-risk group. However, our finding that T-cell lineage ALL populations appear to engraft more readily in NOD/SCID mice suggests that this model may be more useful than the SCID mouse for investigating the biology and treatment of T-cell lineage ALL.
ACKNOWLEDGMENT
The authors thank Prof Dorothy Crawford of the London School of Hygiene and Tropical Medicine for EB virus screening, and Clive Lebozer, Sharon Riddler, and Denise Eady for technical assistance.
Supported by The Bud Flanagan Leukaemia Research Fund and The Emma Jenkins Leukaemia Research Fund.
Address reprint requests to John L. Millar, PhD, Academic Department of Haematology and Cytogenetics, Institute of Cancer Research, 15 Cotswold Rd, Belmont, Sutton, Surrey SM2 5NG, UK.