Abstract
Iron availability regulates ferritin synthesis posttranscriptionally by the interaction between iron-regulatory proteins (IRPs) and an iron responsive element (IRE), a stem-loop sequence located on the 5′ untranslated region of ferritin mRNA. IRPs recognize IREs as a sequence/structure motif, blocking ferritin translation. Recently, we and others independently described families with a combination of hyperferritinemia (serum L-ferritin ≥ 1,000 μg/L, without iron overload) and congenital bilateral cataract, transmitted as an autosomal-dominant trait. The molecular basis were two distinct point mutations in the highly conserved CAGUG(X) hexaloop of L-ferritin IRE on chromosome 19. A new three-generation family with a similar phenotype and a unique genotype is here reported. DNA amplification by polymerase chain reaction and sequence analysis showed a 29-base pair deletion in the L-ferritin IRE, involving the whole 5′ sequence essential to the base pairing of the IRE stem. This deletion is predicted to cause the disruption of IRE stem-loop secondary structure and the nearly complete abolition of the negative control of ferritin synthesis by IRE/IRP binding. Hereditary Hyperferritinemia-Cataract Syndrome (HHCS) appears as a new genetic disorder with a unique phenotype associated with at least four different mutations in the L-ferritin IRE. Hematologists should take into account HHCS in the differential diagnosis of unexplained hyperferritinemia.
IN THE LAST decade important advances have been made in understanding of the regulation of the synthesis of ferritin, the ubiquitous iron-storing protein.1 Whereas cytokines (ie, interleukin-1α and tumor necrosis factor-α) can activate ferritin gene transcription as well as translation, explaining the hyperferritinemia commonly found in cancer and inflammatory diseases,2,3 in absence of these conditions ferritin synthesis is finely regulated at the translational level by iron availability. This mechanism is part of a coordinated control of the expression of other proteins involved in iron homeostasis, such as erythroid δ-aminolevulinate synthase (δ-ALAs) and the transferrin receptor (TfR) (see Fig 1 and reference 4 for a review). It is achieved by the high-affinity interaction between iron-regulatory proteins (IRP 1 and 2) and iron responsive elements (IREs), which are a family of stem-loop sequences located in the untranslated regions (UTR) of the mRNAs.5 A single IRE is located in the 5′ UTR of ferritin and δ-ALAs mRNAs, where binding of IRPs represses translation; on the other hand, five IREs are in the 3′ UTR of the TfR, where binding of IRPs stabilizes the mRNA. Intracellular iron levels modulate the affinity of the IRPs for the IREs. When iron is low, the IRPs/IREs binding prevents iron utilization and storage by blocking the translation of ferritin and δ-ALAs mRNAs, whereas iron uptake is promoted by increasing the synthesis of TfR. Conversely, in iron-repleted cells the affinity of IRPs for IREs is low, so that the synthesis of ferritin and δ-ALAs increases, whereas that of TfR decreases.
Iron-dependent coordinated control of ferritin and transferrin receptor expression at the translational level. IRP changes its binding affinity for the IRE depending on intracellular iron availability. IREs are a family of stem-loop sequences capable of modulating either mRNA translation or stabilization/degradation, according to their location on 5′- or 3′-UTR in the mRNAs. A single IRE is also located in the 5′-UTR of erythroid δ-aminolevulinate synthase (not shown). As for ferritin, IRP blocks mRNA translation when intracellular iron is low, to avoid iron utilization for heme synthesis. See the introduction and reference 4 for a review.
Iron-dependent coordinated control of ferritin and transferrin receptor expression at the translational level. IRP changes its binding affinity for the IRE depending on intracellular iron availability. IREs are a family of stem-loop sequences capable of modulating either mRNA translation or stabilization/degradation, according to their location on 5′- or 3′-UTR in the mRNAs. A single IRE is also located in the 5′-UTR of erythroid δ-aminolevulinate synthase (not shown). As for ferritin, IRP blocks mRNA translation when intracellular iron is low, to avoid iron utilization for heme synthesis. See the introduction and reference 4 for a review.
Ferritin is a multimeric hollow sphere composed of 24 heavy (H, Mr 21,000) and light (L, Mr 19,000) subunits, the genes of which have been assigned to human chromosome 11 and 19, respectively.6 Circulating ferritin, a by-product of intracellular ferritin synthesis,7 generally reflects the size of iron stores, providing a reliable biochemical marker of body iron status. Hereditary hemochromatosis (HH), an autosomal recessive disorder due to a gene close to HLA on chromosome 68 and characterized by parenchymal iron overload, has long been considered the only genetic disease with elevated serum ferritin. Biochemical features of HH are usually an early increase of transferrin saturation followed by a progressive rise of the serum ferritin level, which reflects the increasing iron stores due to excessive iron absorption during several decades.9
In 1995 we first described two Italian families with a new autosomal-dominant genetic disorder, the “Hereditary Hyperferritinemia-Cataract Syndrome” (HHCS),10 clinically characterized by the combination of congenital bilateral cataract and marked elevation of serum ferritin levels (≥1,000 μg/L). The hyperferritinemia was found to be not related to iron overload and entirely due to the increase of the L-subunit, as determined by subunit specific immunoassay. The HHCS is clearly distinguishable from HH because of: (1) the dominant transmission; (2) the lack of any relation with HLA; (3) normal to low serum iron and transferrin saturation, without evidence of parenchymal iron overload assessed by liver and bone marrow biopsy. Typically, if unnecessary phlebotomies are performed, HHCS patients rapidly develop iron-deficient anemia (reversed by adequate iron therapy), despite persistently elevated levels of serum ferritin.10
A few months after the description of HHCS, we identified in the first family the molecular basis of the new disease, consisting of a point mutation in the IRE of ferritin L-subunit gene on chromosome 19 (in the region 19q13.3 → 19qter).11 It was a single base substitution (G to C) in the third nucleotide of the highly conserved CAGUG motif of the IRE loop, essential to the high-affinity interaction between ferritin mRNA and the IRP. Beaumont et al12 also described another three-generation French family with clinical characteristics identical to those of our HHCS patients and carrying an analogue mutation in the L-ferritin IRE, ie, an A to G change in the second nucleotide of the CAGUG loop.12 A third distinct point mutation (G to A change in the IRE bulge) was then found in the second Italian family of our original report.13
We report here a new Italian family with HHCS caused by a 29 base pair (bp) deletion in the 5′ stem of the IRE in the ferritin L-subunit gene, the first described so far, bring to four the number of IRE mutations/deletions observed in HHCS.
MATERIALS AND METHODS
Family history.This three-generation family (Fig 2), living in northern Italy for many generations, was well known to ophthalmologists for the segregation of dominantly inherited congenital bilateral cataract. Visual symptoms became clinically evident during infancy, consisting of glare and slowly progressive loss in visual acuity.
Pedigree of the family with HHCS. Circles = females; squares = males; black symbols = affected members. The arrow indicates the proband. (a) = Age at time of our observation (years or months); (b) age at which the subject underwent surgery for cataract; (c) = serum L-ferritin levels (μg/L); (d) percent of transferrin saturation.
Pedigree of the family with HHCS. Circles = females; squares = males; black symbols = affected members. The arrow indicates the proband. (a) = Age at time of our observation (years or months); (b) age at which the subject underwent surgery for cataract; (c) = serum L-ferritin levels (μg/L); (d) percent of transferrin saturation.
A few years ago, the proband (III-2 Fig 2), a 36-year-old female, underwent a biochemical evaluation of iron status because of slight anemia several months after her first pregnancy. Serum ferritin was 1,753 μg/L whereas serum iron and transferrin saturation were normal (92 μg/mL and 23%, respectively). To evaluate the possible occurrence of HHCS, a family study of biochemical iron parameters was performed. All family members with bilateral cataract also had marked elevation of serum ferritin (>1,000 μg/mL), with no other hematological or biochemical abnormalities, including normal to low serum iron and transferrin saturation. Following inquiry by the proband at the Service of Medical Genetics of the local Hospital, she was directed to our Institute based on a search of a genetic database on the Internet,14 that reported information about our previous description of the HHCS. The age of surgery for cataract and serum L-ferritin levels are shown in Fig 2. A complete family history did not reveal any other clinical symptoms that are apparently linked to the new syndrome.
Molecular analysis of the 5′ UTR of the L-ferritin gene.Genomic DNA was isolated from peripheral blood lymphocytes according to standard protocols. Polymerase chain reaction (PCR) amplification was performed on 300 ng of genomic DNA. Denaturation was at 94°C, annealing at 57°C, and extension at 72°C, each step for 30 seconds for a total number of 30 cycles. The PCR mixture contained in a final volume of 50 μL: 10 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl, 2.5 mmol/L MgCl, 0.01% gelatin, 0.01% Tween 20, 0.01% NP40, 2 U of Taq polymerase, 1% dimethyl sulfoxide (DMSO), 200 mmol/L of each dNTP, 10 pmol of each primer. The primers were: T7F1-TCCTTGCCACCGCAGATTG, to which the T7 tail (TAATACGACTCACTATAGGG) was added; and R1-CCGGATCTGTTCGTCAAAC. Amplified fragments were then sequenced on an automatic sequencer (Applied Biosystem 373A, Foster City, CA), according to manufacturer's protocols.
RESULTS
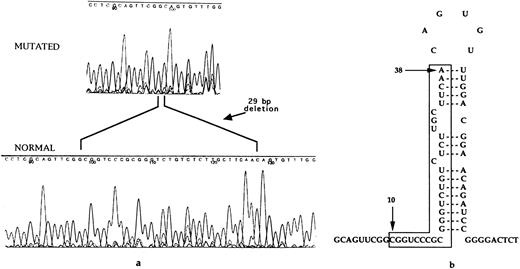
Sequence analysis of the 5′ UTR of the L-ferritin gene permitted the identification of a 29-bp deletion (Fig 3a). The deletion involved residues 10-38 of the normal L-ferritin transcript, corresponding to the 5′ stem of the IRE stem-loop (Fig 3b). The mutation could be easily analyzed by PCR followed by agarose gel electrophoresis counterstained by ethidium bromide. It was found to segregate with the disease within the affected members of the family studied (Fig 4).
(a) DNA automatic sequence, showing in one affected family member the presence of a 29-bp deletion, involving the IRE stem-loop. (b) Representation of the IRE located in the 5′ UTR of L-ferritin. The nucleotides found to be deleted in this HHCS family are marked between lines. They correspond to the whole 5′ sequence involved in base pairing to form the IRE stem. The IRE stem-loop secondary structure is thus predicted to be completely lost, leading to lack of the negative control by sequence/structure specific binding of IRPs.
(a) DNA automatic sequence, showing in one affected family member the presence of a 29-bp deletion, involving the IRE stem-loop. (b) Representation of the IRE located in the 5′ UTR of L-ferritin. The nucleotides found to be deleted in this HHCS family are marked between lines. They correspond to the whole 5′ sequence involved in base pairing to form the IRE stem. The IRE stem-loop secondary structure is thus predicted to be completely lost, leading to lack of the negative control by sequence/structure specific binding of IRPs.
Demonstration of the mutated sequence in affected subjects by PCR using the primers T7F1 and R1 (see text). Lane 1, 1-kB ladder marker (M); lane 2, subject II-1 (affected, see Fig 2); lane 3, subject II-2 (normal); lane 4, subject III-1 (affected); lane 5, subject III-2 (affected); lane 6, normal control. In the presence of the 29-bp deletion an additional band of 150 bp is present.
Demonstration of the mutated sequence in affected subjects by PCR using the primers T7F1 and R1 (see text). Lane 1, 1-kB ladder marker (M); lane 2, subject II-1 (affected, see Fig 2); lane 3, subject II-2 (normal); lane 4, subject III-1 (affected); lane 5, subject III-2 (affected); lane 6, normal control. In the presence of the 29-bp deletion an additional band of 150 bp is present.
DISCUSSION
Clinical and molecular data from this family, carrying a unique deletion in the L-ferritin IRE, give a substantial support to the recent description of the HHCS previously made by us10,11 and, independently, by Beaumont et al.12 In particular, new information about the molecular heterogeneity of the disease is added, as three different single-base substitution in the L-ferritin IRE were so far reported in HHCS families. The IRE(s) are a family of cis-acting stem-loop structures of approximately 30 nucleotides located in mRNAs that encode proteins involved in iron homeostasis.5 Both primary and secondary structures of the IREs are highly conserved during evolution; a hairpin with terminal CAGUG(X) hexaloop is common to all known IREs. This probably reflects the central importance to life of regulating iron metabolism. Depending on the location in the mRNA (5′- or 3′-UTRs) and the specific structure, IREs control either mRNA translation or stabilization. Ferritin mRNA has a single IRE located near the cap of the 5′ UTR. Besides the hairpin structure, distinct features of the ferritin IRE are a G/C-rich stem and an internal UGC bulge, which interrupts the upper stem on the 5′ side (see Fig 3b).5 A series of site-directed mutagenesis experiments have clearly shown that both the sequence and the secondary structure of the IRE stem-loop are essential to the maintenance of the iron-dependent regulation of protein translation.4,15-17 For example, single substitution of nucleotides in the loop or mutations leading to disruption of base pairing in the upper stem decreased the IRE/IRP affinity and/or the capability of regulating translation in response to iron. In keeping with these data, the different single base substitutions in the IRE hexaloop previously found in Italian and French families were shown to be the molecular basis of the HHCS. An in vitro study testing the point mutation in the IRE loop detected by us in the first HHCS family showed that this IRE had a 28-fold lower affinity for IRP than did the native RNA.17 Similarly, the mutation detected in French HHCS patients abolished the binding of IRP to IRE in vitro, and cultured lymphoblastoid cells from the probands exhibited high constitutive, poorly regulated L-ferritin synthesis.12 These data were consistent with the biochemical hallmark of the HHCS, ie, hyperferritinemia as expression of iron-insensitive upregulation of L-ferritin synthesis due to loss of the inhibition of ferritin mRNA translation physiologically operated by the IRE/IRP interaction.
As expected, site-directed mutagenesis studies also showed that even minimal (single base) deletions in the IRE hexaloop reduced IRE/IRP binding more drastically (up to 650-fold) than did substitutions.17 Moreover, the bulge C on the 5′ upper side of the stem was also shown to be critical for the IRE/IRP interaction, as its removal determined a 378-fold reduction of binding affinity with respect to the native IRE.17 In the family here reported, the molecular basis of the iron insensitive upregulation of ferritin expression was represented by a relatively large deletion spanning 29 nucleotides on the L-ferritin IRE. Deleted nucleotides corresponded to the whole 5′ sequence involved in the base pairing essential to form the IRE stem, including the C bulge in the upper stem (Fig 3b). Thus, this deletion is predicted to disrupt the IRE stem-loop secondary structure, leading to the nearly complete abolition of the negative control of ferritin synthesis by IRE/IRP binding.
Despite this unique, apparently severe genotype, the phenotype in this new family is relatively attenuated and quite similar to that previously reported in HHCS families.10 12 The increase of serum L-ferritin was of the same magnitude, serum transferrin saturation tended to be normal to low, and early-onset cataract was the only constantly associated symptom. The lens opacities showed a relatively slow progression during decades from dust-like deposits to starring opacities, so that surgical correction could be delayed to the fourth decade or later.
The mechanism of cataract formation in HHCS remains obscure. The heterogeneity of molecular defects in the L-ferritin IRE leading to the same phenotype, further strengthened by this report, suggests that cataract formation is likely a direct consequence of cellular L-ferritin overproduction. Noteworthy, lens epithelial cells are prone to active ferritin synthesis, especially in response to oxidants.18 An intracellular accumulation of L-rich apoferritin in the lens, analogous to that shown in erythrocytes10 as well as in hepatocytes from HHCS patients,12 may alter the delicate equilibrium between water-soluble proteins in the lens, ultimately leading to loss of transparency. Further studies are needed to clarify this point.
The description of this new family suggests that HHCS, which has an intrinsic interest as an unique human model providing insights into the in vivo regulation of iron homeostasis, may be not so sporadic. Hyperferritinemia is not an uncommon finding in practice. A recent survey from Lee et al19 reported that 122 of 1,826 (6.7%) ferritin determinations in a general hospital population exceeded 1,000 μg/L.19 Differential diagnosis in these circumstances included a broad range of clinical syndromes, such us HH, liver disease, malignancies, systemic infections or inflammations, transfusional iron overload and others. However, in 8 of 95 (8.4%) patients none of the above mentioned conditions could be detected. Hematologists should now consider HHCS in the differential diagnosis of “unexplained” hyperferritinemia. A mistaken diagnosis of HH could lead to unnecessary liver biopsy and/or to the rapid development of phlebotomy-induced iron-deficient anemia.10,11 Similarly, cataract is one of the commonest ocular pathology,20 and serum ferritin measurement should be considered when facing patients with a family history of early-onset cataract. In this way, additional descriptions of HHCS are expected to come, improving the description of the clinical pattern and further elucidating the pathophysiology of the new syndrome.
Supported by Telethon-Italy (Grant no. E.547).
Address reprint requests to Domenico Girelli, MD, PhD, Institute of Medical Pathology, Chair of Internal Medicine, Policlinico Borgo Roma, 37134 Verona, Italy.