Abstract
High-avidity antibodies against interferon α (IFNα), interleukin-1α (IL-1α), and IL-6 have been demonstrated in preparations of normal human IgG, and in vivo modulation of these cytokines may therefore account for immunomodulatory and anti-inflammatory effects of high-dose intravenous IgG therapy. We have investigated the in vivo recovery and the effect on serum cytokine levels of antibodies to IFNα, IL-1α, and IL-6 infused with IgG preparations. Fifteen treatment series of 0.4 g IgG/kg/d were administered over 3 days to eight patients with autoimmune diseases. All IgG preparations contained variable amounts of antibodies binding to 125I-labeled human IFNα2A, -IL-1α, and -IL-6, and the contents of these molecules correlated with increased levels in serum anticytokine activities after IgG infusion. The infused anti–IL-1α antibody activity was fully recovered, whereas the recovery of anti-IFNα2A antibodies was significantly reduced. Serum antiviral activities were significantly reduced after IgG therapy (before, 0 to 5.6 IU/mL; after, 0 to 0.6 IU/mL). In contrast, enzyme-linked immunosorbent assay (ELISA) showed no significant reduction in the serum levels of IL-6 (before, 1 to 70 pg/mL; after, 2 to 55 pg/mL), and the levels of IL-1α were consistently below the detection limit (<30 pg/mL). In conclusion, increased levels of antibodies to IFNα2A, IL-1α, and IL-6 occurred in patients receiving IgG and this reduced the serum antiviral activity.
INTRAVENOUS ADMINISTRATION of pooled human IgG has been used as a substitution therapy in hypogammaglobulinemia or agammaglobulinemia and in infectious diseases and as an immunomodulatory therapy in disorders such as autoimmune thrombocytopenic purpura, myasthenia gravis, the Guillain-Barré syndrome and other types of polyneuropathies, dermatomyositis, and Kawasaki disease.1-3 Blockade of Fc-receptor function on phagocytes, complement absorption, changes in the distribution and function of T-cell subsets, and antigen-specific immunomodulation through antibodies to microbial components or anti-idiotypic antibodies have been proposed to underlie the effects of IgG therapy.1-4
Pharmaceutically prepared IgG preparations modulate cytokines in vitro and in vivo, and cytokine modulation has been proposed as a possible contributor to the clinical efficacy of IgG therapy, particularly in situations where anti-inflammatory and immunomodulatory effects appear to be a central mechanism of action.5-8
We have previously demonstrated specific, high-avidity and in vitro neutralizing antibodies to interferon α (IFNα), interleukin-1α (IL-1α), and IL-6 in pharmaceutically prepared IgG.8-11 In the present study, we investigated the levels of these naturally occurring anticytokine antibodies in sera from patients with autoimmune diseases before and after IgG infusion. We also tested the effects of the administered anticytokine antibodies on endogenous blood cytokines and antiviral activity in these sera.
MATERIALS AND METHODS
Patients and study design.Eight patients were included in the study (1 man and seven women; median age, 40 years; range, 26 to 47 years). Six patients suffered from systemic lupus erythematosus (SLE), one had polymyositis, and one had mixed connective tissue disease (MCTD), based on the American Rheumatism Association's criteria.12 All patients had previously been treated with IgG (>4 weeks before the study). Four patients received hydroxychloroquin, one received prednisolone, and one received azathioprin. None of the patients had signs of infection before treatment with IgG. All patients had active disease at the time of entering the study.
The IgG preparations were Gammagard (Baxter, Allerød, Denmark) and Sandoglobulin (Sandoz, Basel, Switzerland). All patients received 0.4 g IgG/kg/d over 3 consecutive days, and blood sampling was performed before therapy and 1 hour after the last infusion. This regimen was administered three times to three patients, two times to one patient, and once to four patients. The study was approved by the local ethics committee.
Cytokines and antibody.Human recombinant IFNα2A (Roceron) and monoclonal antibody against IFNα2A were kindly donated by Hoffmann La-Roche (Basel, Switzerland), and leukocyte IFN-α (le-IFNα; Alfanative) by Bionative (Uppsala, Sweden). Human recombinant IL-1–α and IL-6 were generous gifts from Dainippon Pharmaceutical Co (Osaka, Japan), and Sandoz (Basel, Switzerland), respectively. IL-1α and IL-6 were calibrated with the corresponding international reference preparations (National Institute of Biological Standards and Control, Potters Bar, Hertfordshire, UK). IFNα2A was titrated against the international standard GxA01-901-535 (National Institutes of Health [NIH], Bethesda, MD).
Radiolabeling of cytokines and binding assay.The cytokines were radiolabeled by the chloramine T method and handled further as described previously.9,10 After iodination, the radiolabeled cytokines were chromatographed on Sephadex G-75 superfine microcolumns to remove aggregates and salt fractions. 125I–IL-1α and 125I–IL-6 had preserved receptor binding capacity and bioactivities,13 14 and the antiviral activity of 125I-IFNα2A was confirmed using the antiviral bioassay (AVB) described below.
Binding of 125I-labeled cytokines was detected using protein-G affinity chromatography at twofold dilutions of the samples.9 10 Sera, at a maximum concentration of 80% (vol/vol), were incubated with 100 pg 125I-IFNα2A/mL, 400 pg 125I–IL-1α/mL or 200 pg 125I–IL-6/mL, with and without 1,000 times surplus of the respective unlabelled cytokine. After 18 hours at 4°C, duplicate samples of 100 mL were added to protein-G for separation of IgG-bound (B) and free (F ) cytokine molecules. Binding of 125I-labeled cytokine to IgG preparations was assayed up to concentrations of 20 mg/mL IgG.
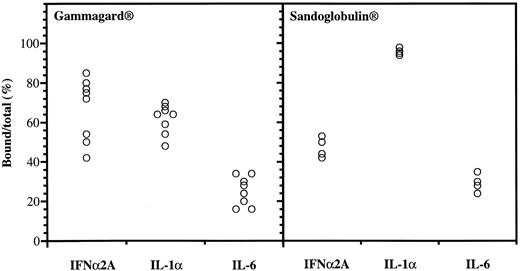
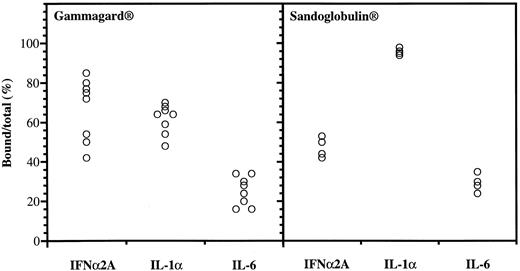
Anticytokine antibodies in different batches of Gammagard and Sandoglobulin. Data were obtained from protein-G affinity chromatography, showing percent bound over total 125I-labeled IFNα, IL-1α, and IL-6.
Anticytokine antibodies in different batches of Gammagard and Sandoglobulin. Data were obtained from protein-G affinity chromatography, showing percent bound over total 125I-labeled IFNα, IL-1α, and IL-6.
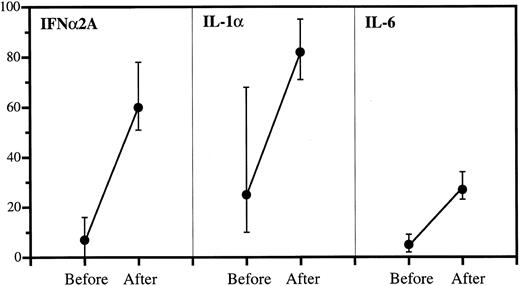
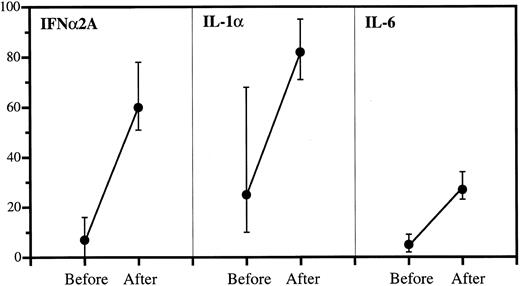
Anticytokine antibodies in patient sera before and after IgG infusions. Data are expressed as percent bound over total (medians and quartiles). The incremental binding was significant in all cases: P < .001 (IFNα2A), P < .001 (IL-1α), and P < .001 (IL-6).
Anticytokine antibodies in patient sera before and after IgG infusions. Data are expressed as percent bound over total (medians and quartiles). The incremental binding was significant in all cases: P < .001 (IFNα2A), P < .001 (IL-1α), and P < .001 (IL-6).
Antiviral bioassay.AVB was performed using encephalomyocarditis (EMC) virus, donated by Dr A.R. Thomsen (Institute of Microbiology and Immunology, University of Copenhagen, Denmark), and a sensitive subclone, SC-3, made by limiting dilution from A549 cells (CCL 185; American Type Culture Collection, Bethesda, MD), as described.10 Briefly, cells were seeded in microtrays at a concentration of 10,000 cells/well and incubated at 37°C in a 5% CO2 humidified air atmosphere. The endogenous antiviral effect was tested on 20 × diluted sera to avoid toxicity; samples used to test for IFNα-neutralizing activity were added 3 IU IFNα/mL. After 24 hours, EMC virus was added at a concentration, which by itself, caused 100% cell death after 24 hours. After another 24 hours, the antiviral effects of sera or IFNα were measured using a 3-(4,5 dimethyl thiazol-2-yl)-2,5-difenyl tetrazolium bromid assay .15 At 10 mg IgG/mL, none of the IgG preparations showed detectable antiviral activity.
Assay for IgG.The IgG contents of serum and IgG preparations were determined by turbidimetry using rabbit antibody to human IgG, code Q 331 (Dako, Copenhagen, Denmark) and HOO-03 reference serum from Janssen Biochemical (Beerse, Belgium).
Assays for immunoreactive IL-1α and IL-6.Enzyme-linked immunosorbent assay (ELISA) for IL-1α was performed using specific rabbit IgG to human IL-1α, as previously detailed.16 The sensitivity limit was 30 pg/mL. This assay does not detect IL-1α in the presence of natural antibody.9 The serum levels of IL-6 were measured by a high sensitivity IL-6 ELISA (R&D Systems, Abingdon, UK) with a detection limit of 0.1 pg/mL. This ELISA detects free IL-6 and, in addition, IL-6 bound to IgG with an efficiency of 30% to 75%.17 None of the IgG preparations contained detectable IL-1α or IL-6 (measured at 10 mg IgG/mL).
Statistics.The Mann Whitney's U-test, the Wilcoxon's matched pairs test, and the Spearmans rank order correlation test were used. P values < .05 were considered significant.
RESULTS
Anticytokine antibodies in different IgG preparations.Variable binding capacities to IFNα2A, IL-1α, and IL-6 were detected in the different batches of IgG (Fig 1). The Gammagard preparations had the highest binding of IFNα2A followed by IL-1α, whereas the opposite was seen in the Sandoglobulin preparations. The anti-IFNα antibodies were specific in that excess IL-1α, IL-6, or IFNγ failed to interfere with the binding to 125I-IFNα2A. The binding to the cytokines was saturable and the anti-IFNα2A antibodies neutralized the antiviral activity of both IFNα2A and le-IFNα in vitro.10 There was a significant positive correlation between the IFNα2A binding capacity and the neutralization of antiviral activity (R = 0.82, P < .00001).
Increased anticytokine binding in sera after IgG administration.As shown in Fig 2, the highest binding capacity in sera collected before IgG therapy was against IL-1α with a median of 25% bound. This increased to 82% after therapy. The corresponding values were 16% to 60% (IFNα2A) and 5% to 27% (IL-6). The incremental bindings afforded by IgG therapy were statistically highly significant in all cases.
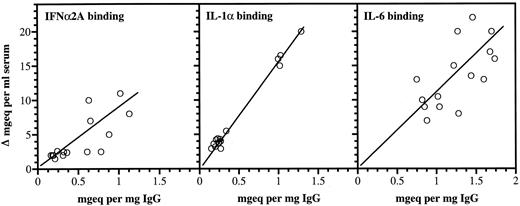
Correlations between anticytokine antibodies in IgG and increases in serum binding of the corresponding cytokines.Significant positive correlations were found between the cytokine binding capacities of the infused IgG preparations and the increased serum binding of the three cytokines (Fig 3). When measured directly for IgG, the mean increase in serum after infusion was 12.9 mg/mL ± 2.3 (standard deviation [SD] n = 15).
Anticytokine antibodies in individual IgG preparations and increased serum cytokine binding capacities after therapy. For each cytokine, the antibody capacities were expressed relative to an arbitrary standard activity of one of the IgG batches. All activities were therefore expressed as mg equivalents/mL serum or mg equivalents/mg IgG of the respective standards. The correlation values were: r: 0.83, P < .001 (IFNα2A), r: 0.77, P < .001 (IL-1α), and r: 0.623, P < .001 (IL-6).
Anticytokine antibodies in individual IgG preparations and increased serum cytokine binding capacities after therapy. For each cytokine, the antibody capacities were expressed relative to an arbitrary standard activity of one of the IgG batches. All activities were therefore expressed as mg equivalents/mL serum or mg equivalents/mg IgG of the respective standards. The correlation values were: r: 0.83, P < .001 (IFNα2A), r: 0.77, P < .001 (IL-1α), and r: 0.623, P < .001 (IL-6).
To compare recoveries of the different anticytokine antibodies, the IgG increments estimated from the binding data (Fig 3) were divided by those measured directly, using the formula:
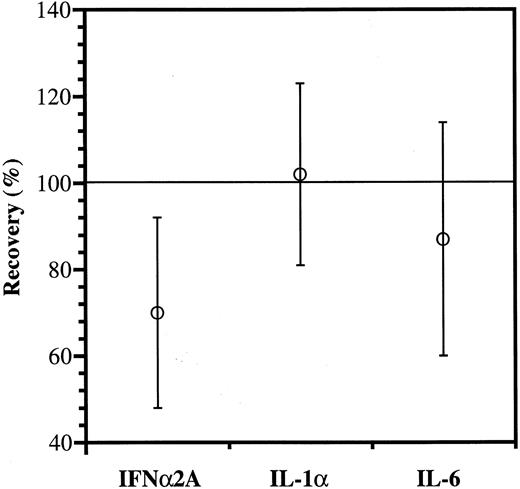
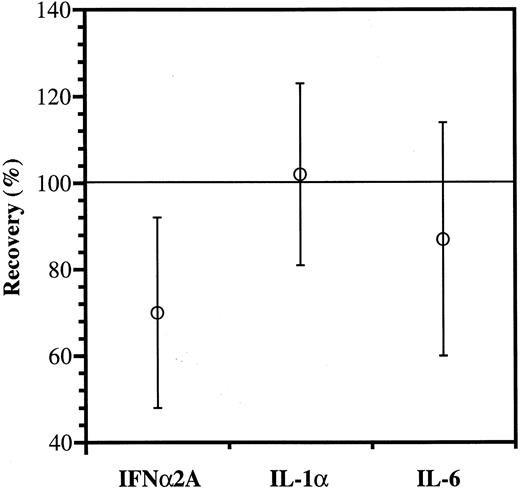
As shown in Fig 4, the recovery of IFNα2A antibody was significantly reduced, whereas IL-1α and IL-6 antibodies appeared to be more fully recovered.
Anticytokine antibody recovery. The IgG increments estimated from the binding data (Fig 3) were divided with those measured directly and shown as mean values ± SD; n = 15. The reduced recovery of IFNα2A antibody is significantly lower than the recoveries of IL-1α and IL-6 antibodies (P < .01 and P = .05, respectively).
Anticytokine antibody recovery. The IgG increments estimated from the binding data (Fig 3) were divided with those measured directly and shown as mean values ± SD; n = 15. The reduced recovery of IFNα2A antibody is significantly lower than the recoveries of IL-1α and IL-6 antibodies (P < .01 and P = .05, respectively).
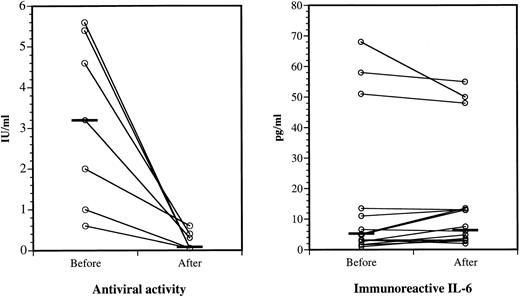
Suppression of IFNα antiviral activity, but not IL-6 immunoreactivity, after IgG administration.All antiviral activity of the seven positive sera was neutralized ex vivo by specific monoclonal antibody to IFNα2A (data not shown). As shown in Fig 5 (left panel), sera exhibited markedly reduced antiviral activities after IgG infusion compared with matched sera collected before therapy. The infused IgG neutralized pretreatment IFN activity in vitro. The IFNα2A binding of the pretreatment sera did not increase after acid treatment, suggesting that they contained few or no IFNα immune complexes. Because none of the sera collected before and after IgG therapy contained detectable levels of IL-1α, we were unable to measure a possible effect of IgG therapy on circulating IL-1α. IL-6 was demonstrated by ELISA in all sera, but the slight differences between the levels before and after IgG infusions were insignificant (Fig 5, right panel).
IFNα antiviral activities and IL-6 immunoreactivities in sera before and after IgG therapy. Left panel, serum antiviral activities before and after IgG administration. Eight of 15 sera did not have detectable antiviral activity before or after IgG (not shown). Horizontal bars show the median values before and after IgG; P < .02. Right panel, Serum IL-6 levels before and after IgG. All sera contained detectable IL-6 when assayed by high-sensitivity ELISA. Horizontal bars show the median values before and after IgG; the difference is not significant.
IFNα antiviral activities and IL-6 immunoreactivities in sera before and after IgG therapy. Left panel, serum antiviral activities before and after IgG administration. Eight of 15 sera did not have detectable antiviral activity before or after IgG (not shown). Horizontal bars show the median values before and after IgG; P < .02. Right panel, Serum IL-6 levels before and after IgG. All sera contained detectable IL-6 when assayed by high-sensitivity ELISA. Horizontal bars show the median values before and after IgG; the difference is not significant.
DISCUSSION
Previously, IgG therapy was intended as a supplement given to patients suffering from hypo- or agammaglobulinemia, or to patients thought to benefit from the provision of specific antibodies to infectious agents. It has now become clear that high-dose IgG therapy is an effective treatment of several autoimmune and inflammatory diseases, including some infectious diseases where infusion of specific antimicrobial antibodies is unlikely to play a major therapeutic role.18-21
Cytokines play a major role in infectious and other immunoinflammatory conditions,22,23 and the recent findings of high-avidity and in vitro neutralizing antibodies to IFNα, IL-1α, and IL-6 in human IgG preparations may explain, at least in part, the immunosuppressive and anti-inflammatory effects of high-dose IgG therapy.7-11 For example, IgG preparations transiently suppress the in vitro production of cytokines such as IL-2, IL-3, IL-4, IL-10, tumor necrosis factor β, and granulocyte-macrophage colony-stimulating factor.5,24 Also, increases in the levels of the IL-1 receptor antagonist have been reported after IgG administration,6,24,25 although this may be an in vitro phenomenon depending on adherence of serum and plasma constituents to plastic.26 The levels of the antibodies to IFNα, IL-1α, and IL-6 have varied significantly between different IgG batches and with preparations from different manufacturers. Very high anti-IFNα antibody levels have been found in some hyperimmune antiviral IgG preparations.10
IL-1α antibodies are the most frequently found anticytokine antibodies in normal sera as well as in human IgG.8 While easily demonstrated in pharmaceutically prepared IgG, natural antibodies to IL-6 and, particularly, to IFNα can be difficult to detect in serum and plasma from healthy individuals. The reason for this is unclear. For example, attempts to separate antigen-antibody complexes have failed to increase the IFN-binding capacity of individual sera (Ross et al, unpublished). This could be because of the very high affinity of these antibodies or the presence of other factors that interfere with the antibodies and/or IFNα-antibody complexes. Another possibility is that very high-tittered IFNα antibodies, present in only a few healthy individuals, may contribute to the anti-IFNα activity found in pooled human IgG.
Endogenous antiviral activity was demonstrated in seven of 15 sera in this study, and this was significantly reduced by the IgG in preinfusion sera and after IgG administration. This indicates direct neutralization of IFNα in the blood by the specific antibodies in the administered IgG preparations. The lack of a significant reduction in the levels of circulating IL-6 could be due to the relatively low levels of anti–IL-6 antibodies in the IgG preparations.
In conclusion, high-dose IgG therapy increased the binding of cytokines to IgG in patients' sera, most likely because of the presence of specific anticytokine antibodies in the IgG preparations. The endogenous antiviral effect in these sera was significantly reduced after the infusion of anti-IFNα antibody positive IgG. While it may prove useful to select IgG preparations with high anticytokine levels for the management of certain immunoinflammatory diseases, it may also be beneficial to administer IgG preparations depleted of anti-IFNα antibodies, particularly if given to patients with known or suspected viral diseases.
ACKNOWLEDGMENT
The expert technical assistance by Merete Tjalve, Susanne Meldgaard, and Ole Christensen is greatly acknowledged.
Supported by the Aage Bang Foundation, the Danish Cancer Society, the Danish Rheumatism Association, the Danish Medical Research Council, and the Danish Biotechnology Program, Copenhagen, Denmark.
Address reprint requests to Prof Klaus Bendtzen, MD, IIR 7411, Rigshospitalet, Tagensvej 20, DK-2200 N, Copenhagen, Denmark.