To the Editor:
In the March 15, 1997 issue of Blood, Greenberg et al1 described a new International Prognostic Scoring System (IPSS) for evaluating prognosis in patients with myelodysplastic syndromes (MDS). To determine the effectiveness of the IPSS in patients not used to derive this system, we applied the IPSS to M.D. Anderson patients with MDS. The patients reported by Greenberg et al did not receive AML-type chemotherapy. Because after 1990 our practice was to give such therapy to patients with refractory anemia with blasts in transformation (RAEB-t) and to most patients with RAEB, we limited our analysis to patients seen before 1991. Like Greenberg et al, we included only patients with primary MDS, patients in whom a karyotype was obtained, and chronic myelomonocytic leukemia (CMML) patients only if they presented to us with a white blood cell count (WBC) below 12,000/μL. Such patients (n = 219) comprised 57% of the 381 patients with RA, RA with sideroblasts, RAEB, RAEB-t, or CMML seen here between January 1, 1985 (an arbitrarily selected date) and December 31, 1990.
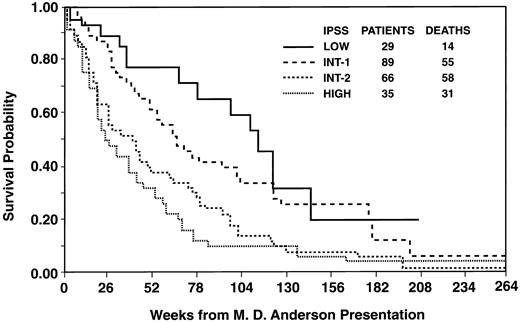
Table 1 compares the 219 M.D. Anderson patients with the 816 patients who formed the basis for the IPSS; the latter patients' characteristics are described in Table 1 of Greenberg et al's report. Our definitions of “cytopenia” and of cytogenetic group (“good,” “intermediate,” “poor”) were chosen to correspond with the definitions of Greenberg et al. The M.D. Anderson patients were younger, but more often had RAEB-t, 2 to 3 cytopenias, and prognostically poor cytogenetics. Given that percent of marrow blasts (21% to 30% least favorable), karyotype, and number of cytopenias (2 to 3 least favorable) determine the IPSS, our patients less often had “low” and “intermediate-1” (INT-1) IPSS scores and more often had INT-2 or “high” scores, the latter associated with the worst prognoses.
Despite the very different nature of the IPSS and the M.D. Anderson patients, the IPSS stratified our patients effectively (Fig 1). However, within the IPSS categories of low, INT-1, and INT-2, probability of survival appeared less in the M.D. Anderson than in the Greenberg et al patients (Table 2); the survival percentiles for the Greenberg et al patients were derived from Fig 6a and Table 4 of their report. Although we did not formally compare our survival data with those of Greenberg et al, the differences appeared noteworthy (eg, median survivals of 2.1 and 1.2 years in our low and INT-1 patients versus 5.7 and 3.5 years in those of Greenberg et al) and unlikely to occur by chance, at least in the INT-1 category in which 89 of our patients were classified. Greenberg et al noted that, independent of the IPSS score, patients over age 60 years had shorter survival than younger patients. However, the survival expectation of our patients in the low, INT-1, and INT-2 categories was worse than the corresponding IPSS patients even if attention was restricted to those IPSS patients over age 60 (or 70) years (Fig 7a, Table 4 of the Greenberg et al report).
Relationship between probability of survival and IPSS in M.D. Anderson patients with myelodysplasia. Log-rank P value < .001.
Relationship between probability of survival and IPSS in M.D. Anderson patients with myelodysplasia. Log-rank P value < .001.
We considered four possible explanations for the discrepant outcomes in M.D. Anderson and IPSS patients. First, our patients may have been referred later in the course of their illness. Factors weighing against this possibility are the relatively short intervals between M.D. Anderson presentation and initial documentation of anemia (median, 4.0 months), thrombopenia (median, 1.0 month), or neutropenia (median, 0.5 months). Second, our therapies may have adversely affected our patients' prognoses. However, the therapies given to our patients are not known to have such effects; these therapies included biologic response modifiers, principally GM-CSF and/or erythropoietin, 52%; transfusions, 30%; and low-dose oral chemotherapy similar to that described by Greenberg et al, 18%. Third, blood counts may have been lower in our patients, as suggested by Table 1. Greenberg et al indicate only the proportions of all 894 patients who had a platelet count below 100,000, a neutrophil count below 1,500, or a hemoglobin level below 10, but do not provide this information separately for the low, INT-1, INT-2, and high categories. Perhaps more important they do not provide information indicating that the relationship between blood count and outcome is best described by dichotomizing blood count as they did, rather than by considering a different relationship, eg, one in which the lower the blood count the worse the outcome. Finally, our patients and those of Greenberg et al may have differed in ways that remain to be defined.
Regardless of the explanation, our results question the applicability of the IPSS. In particular, physicians should not assume a priori that cases of myelodysplasia with a low IPSS score will follow the indolent natural history described by Greenberg et al. As with other prognostic systems, determination of the utility of the IPSS should await further testing of the system in populations other than that from which it was derived.2-4