Abstract
Stem cell factor (SCF ) has a major role in hematopoiesis and in the regulation of mast cell development and function. For example, recombinant human SCF (rhSCF ) can induce the development of human mast cells from precursor cells in vitro, stimulate mediator release from human skin mast cells in vitro, and promote both the development and functional activation of human skin mast cells in vivo. In the present study, we used a new ultrastructural enzyme-affinity method, employing diamine oxidase (DAO)-conjugated gold particles (DAO-gold), to detect histamine in skin biopsies obtained from patients with breast carcinomas who were receiving daily subcutaneous (SC) injections of rhSCF in a phase I study of this cytokine. We examined control biopsies obtained at sites remote from rhSCF injection as well as biopsies of rhSCF-injected skin that were obtained within 2 hours and 30 minutes of the SC injection of rhSCF at that site. The rhSCF-injected sites (which clinically exhibited a wheal-and-flare response), but not the control sites, contained mast cells undergoing regulated secretion by granule extrusion. The DAO-gold-affinity method detected histamine in electron-dense granules of mast cells in control and injected skin biopsies; however, the altered matrix of membrane-free, extruded mast cell granules was largely unreactive with DAO-gold. Notably, DAO-gold bound strongly to fibrin deposits and collagen fibers that were adjacent to degranulated mast cells. These findings represent the first morphologic evidence of histamine secretion by classical granule exocytosis in human mast cells in vivo.
STEM CELL FACTOR (SCF ), also known as mast cell growth factor, kit ligand, and Steel factor, is the ligand for SCF receptor (SCFR), a member of the receptor tyrosine kinase III family of receptors that is encoded by the c-kit proto-oncogene.1-4 SCFR is expressed on mast cells and SCF is critical for the development and survival of these cells.1-7 SCF also has major actions in hematopoiesis and in promoting the differentiation, proliferation, and survival of early hematopoietic progenitor cells.1-4 8
In light of the possibility that recombinant human SCF (rhSCF ) may have clinical utility, for example, in promoting hematopoiesis or enhancing the recovery of circulating hematopoietic progenitor cells,4,8 phase I studies were conducted to begin to assess the possible effects of this cytokine in human subjects in vivo. Because it had already been shown that recombinant rat SCF can induce the c-kit–dependent release of mediators and cytokines from mouse mast cells in vivo9,10 or in vitro10-12 and that rhSCF can promote mediator release from partially purified populations of human skin mast cells in vitro,13 one of the objectives of such studies was to assess whether rhSCF might induce human mast cells to secrete mediators in vivo.
We previously reported that the subcutaneous (SC) administration of rhSCF daily for 14 days to patients with breast cancer induced a modest increase in numbers of dermal mast cells at sites that had not been directly injected with the cytokine.6 In addition, all of the subjects enrolled in this phase I study developed a cutaneous wheal-and-flare response at each rhSCF-injected site, a reaction that was associated with extensive, anaphylactic-type mast cell degranulation, as assessed by routine transmission electron microscopy.6,14 Sites that had been directly injected with rhSCF also exhibited extracellular edema, fibrin deposition, and recruitment of acute inflammatory cells.14 By contrast, control biopsies, obtained at sites that had not been directly injected with rhSCF, showed no evidence of mast cell secretory activity, extracellular edema, fibrin deposits, or inflammatory cell infiltrates.14
In the present study, we took advantage of the extensive mast cell degranulation that was present at sites of rhSCF injection to assess the ultrastructural localization of histamine in human mast cells that are undergoing classical anaphylactic degranulation in vivo. Although there is no question that mast cells store histamine in their granules and that this biogenic amine can be released by mast cells in response to agents that induce the cells to degranulate,15 until recently it has not been possible to study this process morphologically at the ultrastructural level. We therefore developed a diamine oxidase-gold (DAO-gold) ultrastructural enzyme-affinity technique to localize histamine.16 This method, which can be used in conventionally prepared ultrastructural samples, is based on the affinity of the enzyme, diamine oxidase (histaminase), for its substrate, histamine.16 Using this approach, we have previously localized histamine to the secretory granules of human16,17 and mouse18 mast cells and human basophils19,20 and examined the distribution of histamine during stimulated secretion of human basophils and mast cells ex vivo.19-21 We have also used the method to characterize the release of histamine by piecemeal degranulation of mast cells, during inflammation in the eyelids of interleukin-4 transgenic mice18 and in human tissues at sites of inflammatory bowel disease.22 Yet, until this study, we have not been able to investigate the ultrastructural localization of histamine in human mast cells that are undergoing classical anaphylactic degranulation in vivo.
MATERIALS AND METHODS
The skin biopsies were obtained (after receiving signed informed consent forms which had been approved by the Institutional Review Board for Human Studies) from three women with advanced breast carcinoma (stage IIIB or IV) who had volunteered to participate in a phase I trial of recombinant methionyl-human SCF (rhSCF ).6 The selection criteria for the subjects and a description of the protocol for daily subcutaneous dosing with rhSCF are described in detail in Costa et al.6 Briefly, in the subjects studied in this report, rhSCF was administered before the initiation of chemotherapy. Also, all of the subjects were premedicated with H1 (50 mg diphenhydramine administered orally every 6 hours) and H2 (150 mg ranitidine administered orally every 12 hours) antihistamines to minimize the pruritus associated with the reactions to subcutaneous rhSCF.6 Two patients (nos. 1 and 2) received daily SC injections of rhSCF (5 μg/kg/d) in the anterior thigh over a period of 13 days. One patient (no. 3 in this study and no. 185 in Costa et al6 ) received only 5 daily SC injections of 25 μg/kg of rhSCF in the abdominal wall; she was then withdrawn from the study because of the development of a systemic adverse event.6 After local intradermal injection (7E0.5 mL) of a 1.0% solution of lidocaine within approximately 1 to 2 cm of the planned biopsy site, punch biopsies (4 to 6 mm) of skin were obtained from the indurated wheal and flare lesion that developed within approximately 60 minutes of injection of rhSCF at each injection site; the biopsies were obtained between 1 hour 40 minutes and 2 hours 30 minutes after administering the rhSCF into the site. Identically prepared, lidocaine-injected, contralateral control sites (ie, sites not directly injected with rhSCF ) on the opposite thigh or side of the abdomen of the same patients were also biopsied. The control biopsies for patients no. 1 and 2 were obtained at the same time as the biopsies of the rhSCF injection sites (ie, on rhSCF dosing days 1 and 13 or 6 and 13, respectively), whereas, for patient no. 3, the rhSCF injection site was biopsied on day 1 of dosing (ie, the first rhSCF injection site in this subject was biopsied) and a control biopsy was obtained 11 days after the cessation of dosing with rhSCF. Biopsies were immediately immersed in a pool of freshly prepared fixative (2.0% paraformaldehyde, 2.5% glutaraldehyde, and 0.025% CaCl2 in 0.1 mol/L sodium cacodylate buffer, pH 7.4) at room temperature, trimmed into small blocks while immersed, and then fixed and processed for transmission electron microscopy as previously described.23
For enzyme-affinity staining to detect histamine, DAO-gold was prepared as described.16 For cytochemical labeling, section-containing grids were inverted and floated on drops of phosphate-buffered saline for 5 minutes, followed by incubation on drops of DAO-gold at 37°C for 60 minutes. The grids were vigorously washed in distilled water and stained with dilute lead citrate for 10 minutes before viewing in a Philips 300 or 400 electron microscope (Philips North America, Mahwah, NJ). Controls to assess the specificity of cytochemical labeling of human mast cell granules with DAO-gold were performed as described.16 These controls included (1) labeling of grids with DAO-gold that had been passed over Pasteur pipette columns packed with histamine-agarose beads; (2) incubation of grids for 60 minutes at 37°C in DAO (to oxidize histamine), washing, and labeling with DAO-gold; and (3) staining of grids for 60 minutes at 37°C in colloidal gold alone.
RESULTS AND DISCUSSION
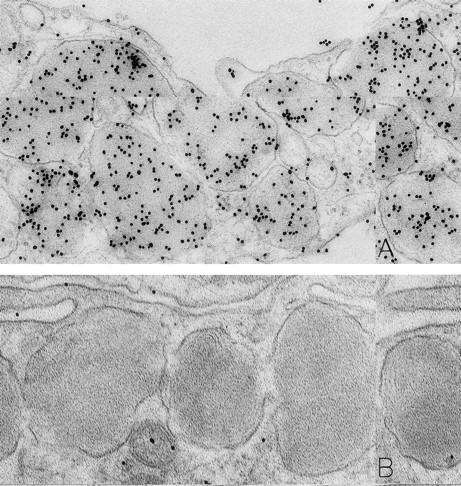
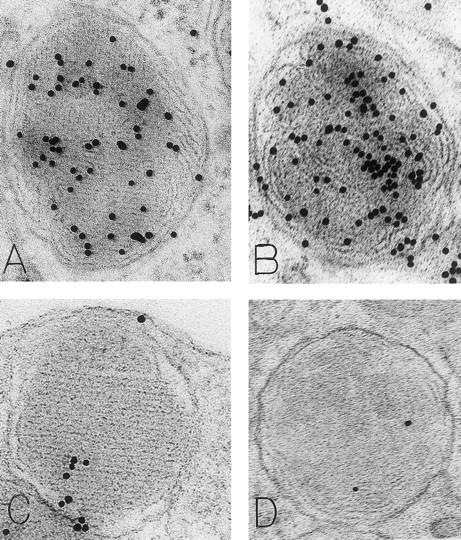
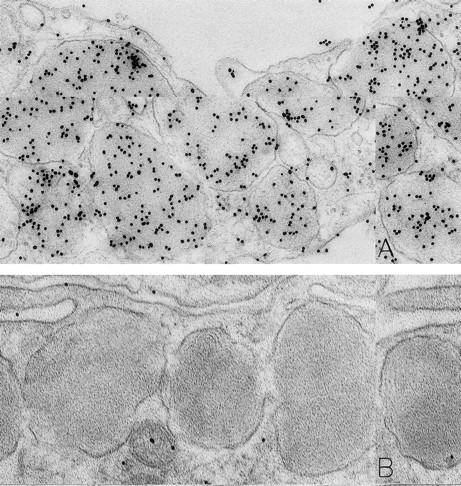
As noted in our previous reports,6 14 ultrastructural evidence of anaphylactic-type degranulation, including the extracellular extrusion of membrane-free cytoplasmic granules, was observed only in biopsies from sites that had been directly injected with rhSCF. Using the DAO-gold enzyme-affinity method, reactivity (gold label) was very strong in the granules of nonsecretory mast cells (in biopsies of either control or rhSCF-injected sites) as well as in any electron-dense cytoplasmic granules remaining in the cytoplasm of skin mast cells that were undergoing secretion (Figs 1, 2, and 3). Moreover, retention of DAO-gold labeling (indicating the presence of histamine) was evident in cytoplasmic granules that exhibited all of the substructural patterns that can be observed in human skin mast cells (Fig 3). Such patterns include granules with crystal (Fig 3A), scroll (Fig 3B), or mixed patterns.
Mast cells in skin biopsies obtained 1 hour 40 minutes (A) or 2 hours (B) after the SC injection of 25 or 5 μg/kg rhSCF, respectively, in patients no. 3 (A) and no. 1 (B), who had received daily SC injections of rhSCF for 13 days. Reactivity for histamine is indicated by DAO-gold labeling in the cytoplasmic granules of a mast cell that exhibits no morphologic evidence of secretory activity (A); DAO-gold label is absent when the grid containing a section of the specimen was digested with DAO before staining with DAO-gold (B). Original magnifications: (A) ×67,500; and (B) ×75,000.
Mast cells in skin biopsies obtained 1 hour 40 minutes (A) or 2 hours (B) after the SC injection of 25 or 5 μg/kg rhSCF, respectively, in patients no. 3 (A) and no. 1 (B), who had received daily SC injections of rhSCF for 13 days. Reactivity for histamine is indicated by DAO-gold labeling in the cytoplasmic granules of a mast cell that exhibits no morphologic evidence of secretory activity (A); DAO-gold label is absent when the grid containing a section of the specimen was digested with DAO before staining with DAO-gold (B). Original magnifications: (A) ×67,500; and (B) ×75,000.
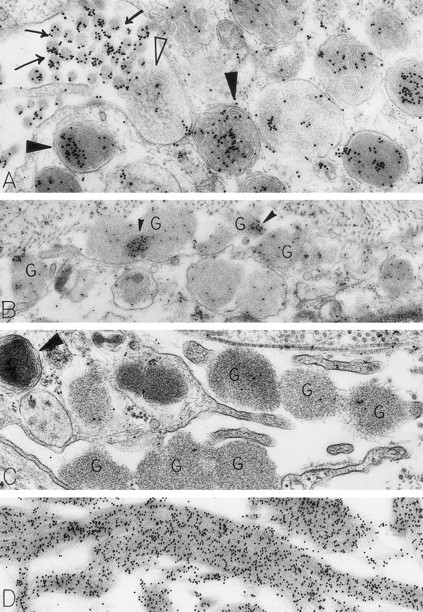
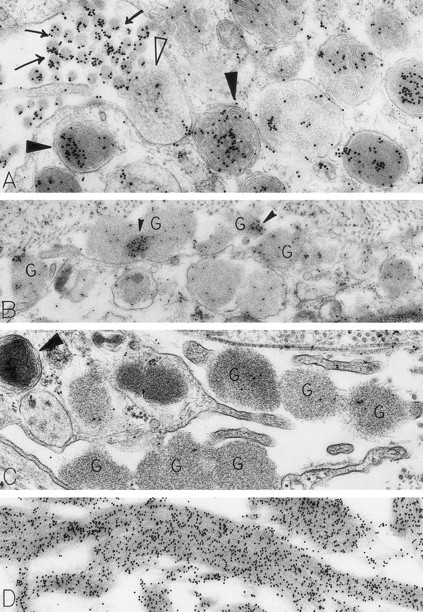
Skin biopsies from sites that had been injected with rhSCF, obtained from patients no. 2 and no. 3 who had received rhSCF for 13 days (A) or 1 day (B through D), respectively. The biopsy in (A) was obtained 1 hour 45 minutes after the SC injection of 5 μg/kg of rhSCF, whereas the biopsy in (B), (C), and (D) was obtained 1 hour 40 minutes after the SC injection of 25 μg/kg of rhSCF. A large amount of DAO-gold label is present on the electron-dense contents of cytoplasmic granules in the mast cell in (A; solid arrowhead); the cell exhibits evidence of secretory activity, and an extruded, non–membrane-bound granule with altered matrix (which is located external to the plasma membrane) retains very little DAO-gold label for histamine (open arrowhead). However, DAO-gold heavily labels adjacent collagen fibers, which are seen in cross-section (arrows). In (B), multiple extruded, membrane-free mast cell granules (G), which exhibit altered matrix materials, are present in the interstitium near the degranulating mast cell; these extruded granules exhibit relatively little DAO-gold labeling for histamine, except in regions of the granules that contain the most electron-dense matrix material (solid arrowheads). In (C), the DAO-gold was absorbed with solid-phase histamine before being used to stain the specimen. Nearly all DAO-gold staining of intracellular mast cell granules (arrowhead), as well as of the extruded, membrane-free granules (G), was abolished by this specificity control. (D) shows that the extensive fibrin deposits in the extracellular space in this biopsy are heavily labeled with DAO-gold. Original magnifications: (A) ×52,500; (B) ×28,000; (C) ×46,500; and (D) ×45,000.
Skin biopsies from sites that had been injected with rhSCF, obtained from patients no. 2 and no. 3 who had received rhSCF for 13 days (A) or 1 day (B through D), respectively. The biopsy in (A) was obtained 1 hour 45 minutes after the SC injection of 5 μg/kg of rhSCF, whereas the biopsy in (B), (C), and (D) was obtained 1 hour 40 minutes after the SC injection of 25 μg/kg of rhSCF. A large amount of DAO-gold label is present on the electron-dense contents of cytoplasmic granules in the mast cell in (A; solid arrowhead); the cell exhibits evidence of secretory activity, and an extruded, non–membrane-bound granule with altered matrix (which is located external to the plasma membrane) retains very little DAO-gold label for histamine (open arrowhead). However, DAO-gold heavily labels adjacent collagen fibers, which are seen in cross-section (arrows). In (B), multiple extruded, membrane-free mast cell granules (G), which exhibit altered matrix materials, are present in the interstitium near the degranulating mast cell; these extruded granules exhibit relatively little DAO-gold labeling for histamine, except in regions of the granules that contain the most electron-dense matrix material (solid arrowheads). In (C), the DAO-gold was absorbed with solid-phase histamine before being used to stain the specimen. Nearly all DAO-gold staining of intracellular mast cell granules (arrowhead), as well as of the extruded, membrane-free granules (G), was abolished by this specificity control. (D) shows that the extensive fibrin deposits in the extracellular space in this biopsy are heavily labeled with DAO-gold. Original magnifications: (A) ×52,500; (B) ×28,000; (C) ×46,500; and (D) ×45,000.
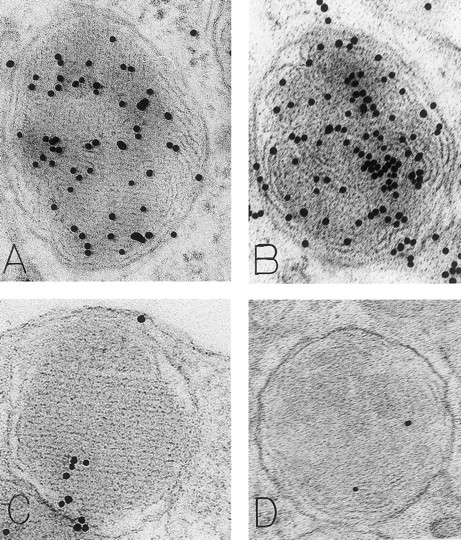
Mast cell granules in skin biopsies of rhSCF-injected sites from patients who had received rhSCF for 6 days (A and B; obtained 2.5 hours after the SC injection of 5 μg/kg of rhSCF in patient no. 2) or 13 days (C and D, obtained 2 hours after the SC injection of 5 μg/kg of rhSCF in patient no. 1). The photomicrographs illustrate the ultrastructure of electron-dense granules in nonsecretory mast cells. Parallel crystalline arrays, oriented either in several directions (A) or all in one direction (C), are typical for the cytoplasmic granules of human skin mast cells. The DAO-gold staining (A) was largely abrogated by absorption of the DAO-gold with solid-phase histamine before staining (C). The granule in (B) is filled with a membranous scroll pattern that is also heavily labeled with the DAO-gold. The DAO-gold staining is not seen in the scroll granule in (D) from a section that was digested on the grid with DAO before DAO-gold staining. Original magnifications: (A) ×110,000; (B) ×136,500; (C) ×93,000; and (D) ×72,000.
Mast cell granules in skin biopsies of rhSCF-injected sites from patients who had received rhSCF for 6 days (A and B; obtained 2.5 hours after the SC injection of 5 μg/kg of rhSCF in patient no. 2) or 13 days (C and D, obtained 2 hours after the SC injection of 5 μg/kg of rhSCF in patient no. 1). The photomicrographs illustrate the ultrastructure of electron-dense granules in nonsecretory mast cells. Parallel crystalline arrays, oriented either in several directions (A) or all in one direction (C), are typical for the cytoplasmic granules of human skin mast cells. The DAO-gold staining (A) was largely abrogated by absorption of the DAO-gold with solid-phase histamine before staining (C). The granule in (B) is filled with a membranous scroll pattern that is also heavily labeled with the DAO-gold. The DAO-gold staining is not seen in the scroll granule in (D) from a section that was digested on the grid with DAO before DAO-gold staining. Original magnifications: (A) ×110,000; (B) ×136,500; (C) ×93,000; and (D) ×72,000.
Specificity controls for the histaminase-gold-based affinity technique, including absorption of the DAO-gold reagent by solid-phase histamine before sample staining (Figs 2C and 3C) and digestion of the section with histaminase before DAO-gold staining (Figs 1B and 3D), showed a marked reduction of label or a complete absence of reactivity. In every case tested, there was no staining of sections with colloidal gold alone (data not shown).
Many of the dermal mast cells in the rhSCF-injected sites were undergoing secretion, as indicated by the extrusion of membrane-free, altered granules through multiple openings in the plasma membrane (Fig 2A through C). Such findings are very similar to those observed in isolated human skin mast cells that had been stimulated with anti-IgE in vitro.24 We found that the extruded, membrane-free mast cell granules persisted near mast cell surfaces, as well as enmeshed among adjacent collagen fibers, for up to 2.5 hours after rhSCF injection (the latest interval after injection that we studied). Notably, these membrane-free, swollen, rounded granules with altered matrices generally contained merely trace amounts of DAO-gold labeling for the presence of histamine (Fig 2A and B). Rarely, we observed mast cells with intracytoplasmic degranulation channels containing altered, swollen granules; these structures and the contained membrane-free granules also were generally devoid of histamine labeling. The specificity controls resulted in no gold labeling of the extruded granules, indicating that the weak level of DAO-gold labeling of these structures indeed represented residual histamine associated with the matrices of the released granules (Fig 2C).
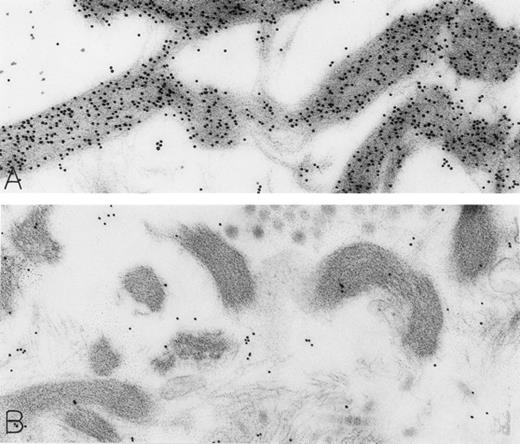
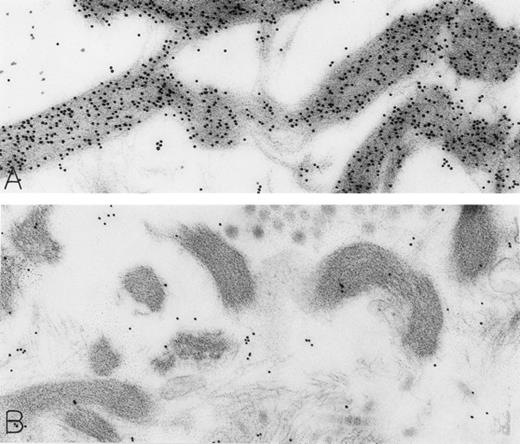
The rhSCF-injected skin sites had extensive deposits of extracellular fibrin. Much of this fibrin was bound to interstitial collagen, whereas some fibrin deposits occurred near secretory mast cells and their extruded granules. There was striking labeling of these masses of interstitial fibrin with DAO-gold (Figs 2D and 4A). By contrast, no fibrin was detectable in the control skin biopsies. Specificity controls for the enzyme-affinity method for histamine resulted in no labeling of fibrin (Fig 4B). Bundles of extracellular interstitial collagen in rhSCF-injected sites also stained with the DAO-gold reagent (Fig 5A). Again, specificity controls resulted in no labeling of the collagen at such sites (Fig 4B). In many areas, secretory mast cells, with DAO-gold-negative, extruded, membrane-free granules attached to their surfaces, were intimately associated with gold-labeled collagen fibrils (Fig 2A). However, collagen in the basal lamina (whether present beneath the basal layer of the epidermis and skin adnexae, surrounding Schwann cells in dermal nerves, or underlying vascular endothelial cells and pericytes) did not stain with the DAO-gold enzyme-affinity method (data not shown).
A skin biopsy from an rhSCF-injected test site (obtained 1 hour 40 minutes after the SC injection of 25 μg/kg of rhSCF ) from patient no. 3, who had received a single dose of rhSCF (day 1 of dosing), shows extensive DAO-gold-labeled fibrin (A) that is not seen if the section was digested with DAO before staining with DAO-gold (B). Original magnifications: (A) ×56,000; and (B) ×52,000.
A skin biopsy from an rhSCF-injected test site (obtained 1 hour 40 minutes after the SC injection of 25 μg/kg of rhSCF ) from patient no. 3, who had received a single dose of rhSCF (day 1 of dosing), shows extensive DAO-gold-labeled fibrin (A) that is not seen if the section was digested with DAO before staining with DAO-gold (B). Original magnifications: (A) ×56,000; and (B) ×52,000.
Skin biopsies of rhSCF-injected test sites from patients who had received rhSCF for 1 day (A; obtained 1 hour 40 minutes after the SC injection of 5 μg/kg of rhSCF in patient no. 2) or 6 days (B; obtained 2.5 hours after the SC injection of 25 μg/kg of rhSCF in patient no. 3) show DAO-gold staining of collagen (A) that is largely abrogated by absorption of the DAO-gold with histamine before staining (B). Original magnifications (A) ×31,000; and (B) ×38,500.
Skin biopsies of rhSCF-injected test sites from patients who had received rhSCF for 1 day (A; obtained 1 hour 40 minutes after the SC injection of 5 μg/kg of rhSCF in patient no. 2) or 6 days (B; obtained 2.5 hours after the SC injection of 25 μg/kg of rhSCF in patient no. 3) show DAO-gold staining of collagen (A) that is largely abrogated by absorption of the DAO-gold with histamine before staining (B). Original magnifications (A) ×31,000; and (B) ×38,500.
In summary, we have used a DAO-gold enzyme-affinity method to show (1) intracellular cytoplasmic granule stores of histamine in human skin mast cells, (2) the depletion of these stores in association with rhSCF-induced anaphylactic degranulation, and (3) potential extracellular histamine binding sites in the vicinity of degranulated mast cells. This represents the first demonstration of histamine secretion by regulated granule extrusion in vivo in mast cells of any species.
The principal findings of this study are as follows: (1) all electron-dense secretory granules in mast cells, of whatever substructural types and within either secretory or nonsecretory mast cells, contained histamine; (2i) non–membrane-bound mast cell granules with altered matrices, whether present within intracellular degranulation channels (a rare event in stimulated human skin mast cells24 ), in the process of being extruded to the cells' exterior, or bound to the extracellular surfaces of cells or enmeshed among interstitial collagen fibers and/or fibrin deposits, showed a marked reduction in, or a complete absence of, DAO-gold reactivity; (3) DAO-gold labeled interstitial collagen fibers and fibrin deposits in areas adjacent to actively secreting mast cells; (4) basal lamina collagen and areas of interstitial edema were devoid of DAO-gold label; and (5) specificity controls for histamine-labeled mast cell granules, as well as DAO-gold reactivity associated with interstitial collagen fibers or fibrin, were negative.
The most straightforward interpretation of these findings is that the electron-dense granules of human skin mast cells in vivo contain histamine and that rhSCF induced anaphylactic degranulation, leading to an exteriorization of the granule matrices and resulting in the rapid loss of histamine from these non–membrane-bound granules; some of the histamine then became associated with interstitial collagen fibrils or fibrin deposits. We previously noted a similar binding of the DAO-gold reagent to collagen fibrils in gut biopsies of patients with inflammatory bowel disease.22 However, the mast cells in these gut biopsies typically exhibited piecemeal degranulation, not anaphylactic degranulation, and these specimens did not contain fibrin deposits.
We believe that the various ultrastructural localizations of the DAO-gold label in this study, other than nuclear localization, reflect the presence of histamine and not polyamines (other substrates of DAO). Because cellular polyamines are generally present within nuclei, it is most likely that the nuclear label that we noted in these and similar preparations reflects the presence of polyamines.16 However, extracellular levels of polyamines are thought to be quite low.25 The extracellular sites of DAO-gold label reported here were associated with active secretion of mast cell granule histamine stores, and mast cell granules are not known to contain polyamines.
However, we do not know whether the DAO-gold reactivity associated with fibrin deposits or collagen represents histamine that has been noncovalently associated with these structures or the product of the covalent attachment of histamine to these substrates by transglutaminases.26-30 Transglutaminases are calcium ion-dependent enzymes that catalyze an acyl transfer reaction via a protein-protein cross-linkage, resulting in ε-(γ-glutamyl)-lysine bonds.30 Alternatively, the primary amino group of histamine (or a polyamine) can form an ε-(γ-glutamyl)-lysine or (γ-glutamyl) bond to proteins.31
Transglutaminases are involved in a variety of biologic events associated with tissue stabilization,32-35 such as clot stabilization by circulating factor XIIIa.29,32 Moreover, immunohistochemical studies have colocalized both serum (factor XIIIa) and tissue transglutaminases to the extracellular matrix of damaged tissues in Crohn's disease,36 a site at which we have identified collagen fibril-associated DAO-gold reactivity and piecemeal degranulation of mast cells,22 and, in mouse mast cells, degranulation is associated with the activation of transglutaminase and the production of protein-bound γ-glutamyl histamine.37 Taken together, these findings raise the possibility that either tissue or mast cell-derived transglutaminases can catalyze the covalent attachment of histamine to collagen or fibrin at sites of mast cell degranulation.
ACKNOWLEDGMENT
The authors thank Peter K. Gardner for editorial assistance in the preparation of the manuscript.
Supported by National Institutes of Health Grants No. AI-33372 and PO1-HL-56383 (Project 4) and by Amgen Inc.
Address reprint requests to Ann M. Dvorak, MD, Department of Pathology/East Campus, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215.