Abstract
The classification of factor VIII deficiency, generally used based on plasma levels of factor VIII, consists of severe (<1% normal factor VIII activity), moderate (1% to 4% factor VIII activity), or mild (5% to 25% factor VIII activity). A recent communication described four individuals bearing identical factor VIII mutations. This resulted in a severe bleeding disorder in two patients who carried a normal factor V gene, whereas the two patients who did not display severe hemophilia were heterozygous for the factor VLEIDEN mutation, which leads to the substitution of Arg506 → Gln mutation in the factor V molecule. Based on the factor VIII level measured using factor VIII–deficient plasma, these two patients were classified as mild/moderate hemophiliacs. We studied the condition of moderate to severe hemophilia A combined with the factor VLEIDEN mutation in vitro in a reconstituted model of the tissue factor pathway to thrombin. In the model, thrombin generation was initiated by relipidated tissue factor and factor VIIa in the presence of the coagulation factors X, IX, II, V, and VIII and the inhibitors tissue factor pathway inhibitor, antithrombin-III, and protein C. At 5 pmol/L initiating factor VIIa⋅tissue factor, a 10-fold higher peak level of thrombin formation (350 nmol/L), was observed in the system in the presence of plasma levels of factor VIII compared with reactions without factor VIII. Significant increase in thrombin formation was observed at factor VIII concentrations less than 42 pmol/L (∼6% of the normal factor VIII plasma concentration). In reactions without factor VIII, in which thrombin generation was downregulated by the addition of protein C and thrombomodulin, an increase of thrombin formation was observed with the factor VLEIDEN mutation. The level of increase in thrombin generation in the hemophilia A situation was found to be dependent on the factor VLEIDEN concentration. When the factor VLEIDEN concentration was varied from 50% to 150% of the normal plasma concentration, the increase in thrombin generation ranged from threefold to sevenfold. The data suggested that the analysis of the factor V genotype should be accompanied by a quantitative analysis of the plasma factor VLEIDEN level to understand the effect of factor VLEIDEN in hemophilia A patients. The presented data support the hypothesis that the factor VLEIDEN mutation can increase thrombin formation in severe hemophilia A.
IN NORMAL HOMEOSTASIS, the procoagulant system is in balance with the anticoagulant and fibrinolytic systems. The procoagulant reaction starts with the binding of plasma factor VIIa to tissue factor (TF ). The membrane-bound factor VIIa⋅TF enzyme complex activates the zymogens factor X and factor IX by limited proteolysis.1 Factor IXa combines with factor VIIIa on a phospholipid surface, forming the intrinsic tenase that generates additional factor Xa. Factor Xa associates with factor Va on a phospholipid membrane surface to form prothrombinase, which activates prothrombin to thrombin. (For reviews on blood coagulation and membrane-dependent reactions in blood coagulation see, respectively, Davie et al2 and Mann et al.3 ) The anticoagulant system consists of several stoichiometric protease inhibitors including the tissue factor pathway inhibitor (TFPI),4 antithrombin-III (AT-III),5 heparin cofactor-II,6 and the dynamic protein C pathway, which involves thrombin, protein C, protein S, and thrombomodulin. The negative feedback mechanism provided by the protein C pathway is initiated by the binding of thrombin to thrombomodulin and the activation of protein C by the thrombin/thrombomodulin complex. (For a review on thrombomodulin and protein C see Esmon.7 ) Activated protein C (APC) is thought to exert its inhibitory effect on coagulation by proteolytically inactivating the cofactors of prothrombinase and intrinsic tenase, factor V(a) and factor VIII(a), respectively.8-14
Defects in the procoagulant or anticoagulant systems by a single point mutation may result in a bleeding disorder or thrombosis, respectively. In these cases variability in the phenotypic expression (ie, the presence or the degree of the disease) among individuals carrying identical genetic mutations is frequently observed. In some cases such variability may be ascribed to the coinheritance of another mutation in the same or another gene. For example, coinheritance of both factor XI and factor VIII deficiencies results in a more severe bleeding tendency than that associated with either deficiency alone.15 Examples of increased thrombotic risk by the combination of heterozygous inheritance are the factor VLEIDEN mutation with either protein C16 or protein S17 deficiency, which explains some of the variation observed in thrombotic risk associated with the heterozygous deficiencies. We have recently analyzed in an in vitro model the consequences of the coexistence of factor VLEIDEN with protein CVermont.18 This resulted in a stable factor Va molecule, which would predict excessive thrombin generation in individuals bearing those two mutations. Conversely we speculated that factor VLEIDEN might be beneficial in the case of hemophilia A by prolonging thrombin formation.18 Subsequently, Nichols et al19 described hemophilic patients who displayed the phenotype of moderate hemophilia A with a factor VIII genotype, which is ordinarily associated with severe hemophilia. Two individuals with less severe disease carried the G to A mutation in the factor V gene,19 which is responsible for the Arg506 → Gln substitution, which results in the APC-resistant factor VLEIDEN.20-22 Hence, the factor VLEIDEN mutation seems to have the potential to compensate for a defect in the factor VIII molecule in vivo. In this study we recreate, in vitro, the condition of moderate to severe hemophilia A combined with the factor VLEIDEN mutation in a reconstituted model of the TF pathway to thrombin.23-25 The data obtained with this model provided experimental biochemical support for the hypothesis that the factor VLEIDEN mutation can increase thrombin formation in hemophilia A.
MATERIALS AND METHODS
Reagents. Phosphatidylserine (PS) from bovine brain, phosphatidylcholine (PC) from egg yolk, and HEPES were purchased from Sigma (St Louis, MO). D-phenylalanyl-L-arginine chloromethyl ketone (FPR-ck) was a gift from Dr R. Jenny (Haematologic Technologies Inc, Essex Jct, VT). Spectrozyme TH was purchased from American Diagnostica (Greenwich, CT).
Proteins. Human coagulation factors X and IX and prothrombin were isolated from fresh frozen plasma using the general methods of Bajaj et al26 and were depleted from contaminants and traces of active enzymes as described elsewhere.24 Human protein C was purified and treated with FPR-ck as described.25 Human factor V was isolated from freshly frozen human plasma by using the method of Nesheim et al.27 Human factor VLEIDEN was isolated as described22 from citrated plasma of patients, which were found to be homozygous for the mutation by DNA analysis.21 Human AT-III was purified as described by Griffith et al.28 Recombinant factor VIII and recombinant TF (residues 1-242) were provided as gifts from Drs Shu Len Liu and Roger Lundblad (Hyland division, Baxter Healthcare Corp, Duarte, CA). Recombinant human coagulation factor VIIa was purchased from NOVO Pharmaceuticals. Recombinant soluble thrombomodulin (Solulin) was provided as a gift from Dr J. Morser (Berlex, Richmond, CA). Recombinant full-length TFPI produced in Escherichia coli was provided as a gift from Dr K. Johnson (Chiron Corp, Emeryville, CA).
Coagulation activation experiments. Thrombin generation initiated by factor VIIa⋅TF in a reconstituted procoagulant model using mean plasma protein concentrations was studied as described previously.23 TF was relipidated into 400 μmol/L phospholipid vesicles, composed of 75% PC and 25% PS, for 30 minutes at 37°C in 20 mmol/L HEPES, 150 mmol/L NaCl, 2 mmol/L CaCl2 pH 7.4 (HEPES/Ca2+). The relipidated TF was incubated with factor VIIa for 20 minutes at 37°C to allow factor VIIa⋅TF complex formation. Factor V and factor VIII were added in microliter amounts of concentrated stock solutions to the equilibrated factor VIIa⋅TF mixture (total volume of addition not exceeding 0.25% of the final reaction volume), and immediately thereafter the reaction was started by the addition of a solution containing factor X, factor IX, and prothrombin, which was prepared in HEPES/Ca2+. The zymogen solution was preheated at 37°C for 3 minutes before addition to the factor VIIa⋅TF, factor V, and factor VIII mixture. When TFPI, AT-III, or protein C were included, they were added to the factor X, factor IX, and prothrombin mixture. Thrombomodulin was added to the factor VIIa⋅TF mixture.
The final concentrations of the proteins in the reaction chosen to represent mean plasma values are 160 nmol/L factor X, 90 nmol/L factor IX, 0.7 nmol/L factor VIII, 20 nmol/L factor V, 1.4 μmol/L prothrombin, 2.5 nmol/L TFPI, 3.4 μmol/L AT-III, and 65 nmol/L protein C. The TF concentration was varied to change the concentration of the initiator complex while factor VIIa was kept constant and in substantial excess at 0.1 nmol/L. Final phospholipid concentration was 200 μmol/L. After initiation of the reaction, aliquots were withdrawn from the reaction mixture and quenched in 20 mmol/L EDTA, 20 mmol/L Tris, 150 mmol/L NaCl, pH 7.4, containing 0.4 mmol/L Spectrozyme TH and assayed immediately for thrombin activity. The hydrolysis of the substrate was monitored by the change in absorbance at 405 nm using a Vmax spectrophotometer (Molecular Devices Corp, Menlo Park, CA). Thrombin generation was calculated from a standard curve prepared by serial dilutions of α-thrombin.
RESULTS
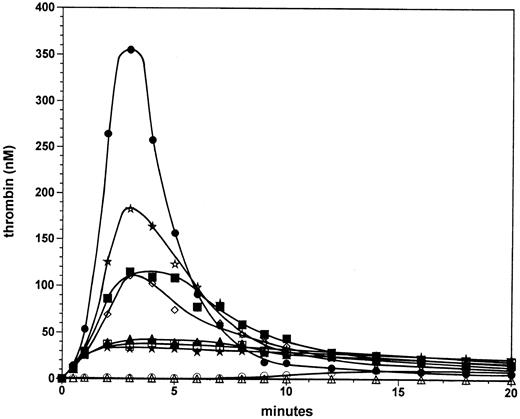
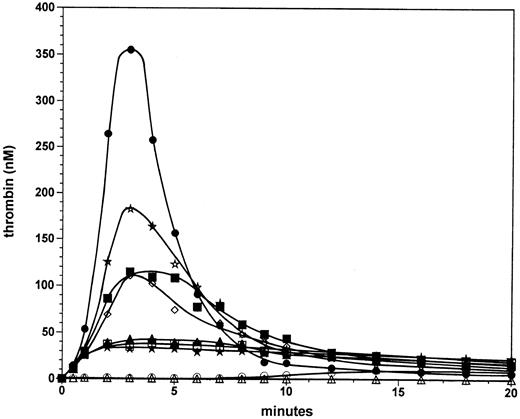
Factor VIII titration at 5 pmol/L factor VIIa⋅TF. A factor VIII titration was performed in the reconstituted procoagulant model with only the stoichiometric inhibitors TFPI and AT-III present at their respective plasma concentration. Previously we found that in the presence of this combination of inhibitors the explosive propagation phase of thrombin generation becomes a threshold-limited event with respect to the initiating factor VIIa⋅TF concentration.24 Figure 1 displays thrombin generation in mixtures containing varying amounts of factor VIII initiated by the addition of 1 pmol/L or 5 pmol/L factor VIIa⋅TF. At 1 pmol/L VIIa⋅TF in the presence of 0.7 nmol/L factor VIII (100%; open circles), significant thrombin generation was not observed for up to 8 minutes in the reaction. After 8 minutes, thrombin generation occurred slowly and reached a level of 10 nmol/L at 14 minutes, a level that was observed to remain constant until 20 minutes into the reaction. At 1 pmol/L factor VIIa⋅TF in the absence of factor VIII (open triangles), no significant thrombin was generated in the 20-minute period evaluated. In marked contrast, at 5 pmol/L factor VIIa⋅TF and 0.7 nmol/L factor VIII (solid circles), significant thrombin generation occurs. After the first 30 seconds, in which 12 nmol/L enzyme is formed, thrombin generation proceeds with a maximal rate of ∼210 nmol/L/min. The maximum level (350 nmol/L) is reached at 3 minutes in the reaction. Subsequently the thrombin level decreases with a t1/2 of ∼1.5 minutes, resulting in 18 nmol/L thrombin at 9 minutes. These low levels of thrombin persist up to 20 minutes. The dramatic change in the maximal rate of thrombin generation with only a fivefold change in the initiator concentration from 1 to 5 pmol/L illustrates the threshold for explosive thrombin generation under these experimental conditions.
Factor VIII titration at 5 pmol/L factor VIIa⋅TF. Thrombin formation was initiated by 5 pmol/L factor VIIa⋅TF in the presence of 0% (solid stars), 3.125% (open squares), 6.25% (solid triangles), 12.5% (open diamonds), 25% (solid squares), 50% (open stars), and 100% (solid circles) of the normal plasma concentration of factor VIII. Thrombin generation is also shown for reactions initiated by 1 pmol/L factor VIIa⋅TF in the absence (open triangles) or presence of factor VIII (100%, open circles). Data points are the mean of two experiments.
Factor VIII titration at 5 pmol/L factor VIIa⋅TF. Thrombin formation was initiated by 5 pmol/L factor VIIa⋅TF in the presence of 0% (solid stars), 3.125% (open squares), 6.25% (solid triangles), 12.5% (open diamonds), 25% (solid squares), 50% (open stars), and 100% (solid circles) of the normal plasma concentration of factor VIII. Thrombin generation is also shown for reactions initiated by 1 pmol/L factor VIIa⋅TF in the absence (open triangles) or presence of factor VIII (100%, open circles). Data points are the mean of two experiments.
In reactions initiated by 5 pmol/L factor VIIa⋅TF in the absence of factor VIII (solid stars) ∼12 nmol/L thrombin was generated after 30 seconds; however the reaction did not accelerate after 30 seconds as is the case for reactions containing plasma concentrations of factor VIII. Instead, the thrombin generation rate decreased after 1 minute resulting in 32 nmol/L thrombin at 2 minutes, which persisted over 20 minutes. Reactions at factor VIII concentrations of 3.1% (0.022 nmol/L, open squares) and 6.3% (0.044 nmol/L, solid triangles) displayed only marginal increases of ≤25% in thrombin generation over that observed in the total absence of factor VIII. In contrast, at 12.5% factor VIII (0.088 nmol/L, open diamonds) a substantial increase (threefold) is observed in the maximum level of thrombin generated (∼105 nmol/L). Reactions with factor VIII at 25% (0.175 nmol/L, solid squares) and 50% (0.35 nmol/L, open stars) displayed further increased thrombin generation. However, in these latter reactions the increases in thrombin generation are relatively small compared with that observed at 12.5% factor VIII. At 50% factor VIII the peak thrombin value (180 nmol/L) is 50% that observed in the reaction with a normal plasma factor VIII concentration (0.7 nmol/L).
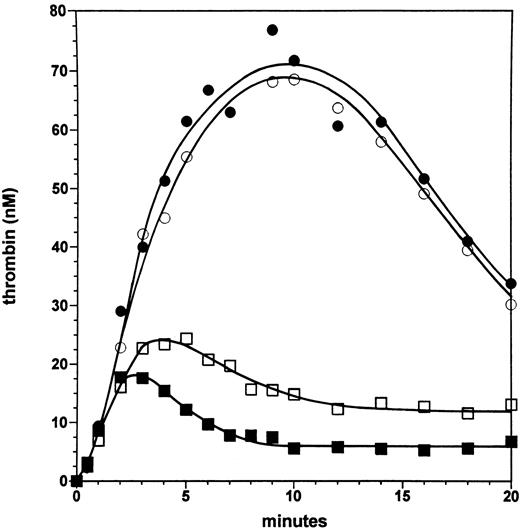
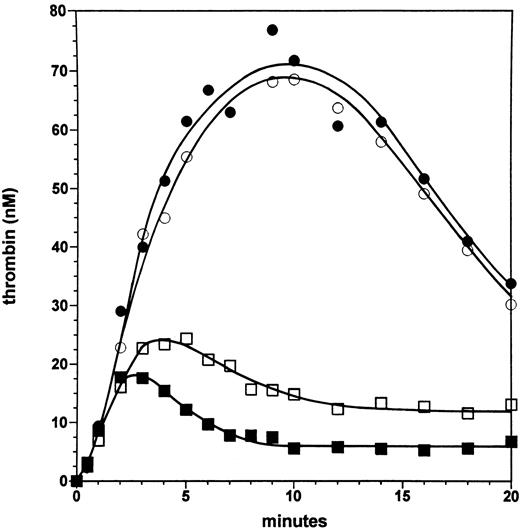
Effect of the factor VLEIDEN mutation in the absence and presence of the protein C pathway. Figure 2 displays experiments in which a moderate-severe hemophilia A is mimicked at a factor VIII level of 6% with normal factor V and with factor VLEIDEN. In the absence of protein C and thrombomodulin, thrombin generation is equivalent in the presence of normal factor V (closed circles) and factor VLEIDEN (open circles), and the maximal level of thrombin (∼70 nmol/L) is generated at 7 to 10 minutes in the reaction, subsequently the thrombin level declines to ∼35 nmol/L at 20 minutes. The lack of an effect of the factor VLEIDEN mutation in the absence of the protein C pathway rules out a direct positive effect of factor VLEIDEN on the reaction.
Effect of the factor VLEIDEN mutation in the absence and presence of the protein C pathway. Thrombin formation is shown for reactions initiated by 5 pmol/L factor VIIa⋅TF at 6% of the normal plasma concentration of factor VIII in the presence of normal factor V (solid symbols) and factor VLEIDEN (open symbols) in the absence (circles) or presence of protein C and 1 nmol/L thrombomodulin (squares).
Effect of the factor VLEIDEN mutation in the absence and presence of the protein C pathway. Thrombin formation is shown for reactions initiated by 5 pmol/L factor VIIa⋅TF at 6% of the normal plasma concentration of factor VIII in the presence of normal factor V (solid symbols) and factor VLEIDEN (open symbols) in the absence (circles) or presence of protein C and 1 nmol/L thrombomodulin (squares).
In the presence of protein C (65 nmol/L) and 1 nmol/L soluble thrombomodulin, thrombin generation is initially not influenced with either normal factor V (solid squares) or factor VLEIDEN (open squares). However, after 2 minutes with normal factor V, thrombin generation is downregulated by the protein C pathway with 17 nmol/L thrombin formed. Subsequently, the thrombin level declines to ∼6.5 nmol/L at 10 minutes. In contrast, in the presence of factor VLEIDEN, while 18 nmol/L thrombin is formed at 2 minutes, the reaction persists such that a maximal level of 24 nmol/L thrombin is observed at 5 minutes. This thrombin concentration is ∼23% higher than for the reaction with normal factor V. Subsequently, the level of thrombin declines in the reaction with factor VLEIDEN to ∼13 nmol/L at 10 minutes. The formation-inhibition steady state processes maintain thrombin at this concentration up to 20 minutes. Hence, at 6% factor VIII the thrombin levels generated in the presence of the protein C pathway are twofold higher with factor VLEIDEN in the later phases of the reaction compared with the reaction with normal factor V.
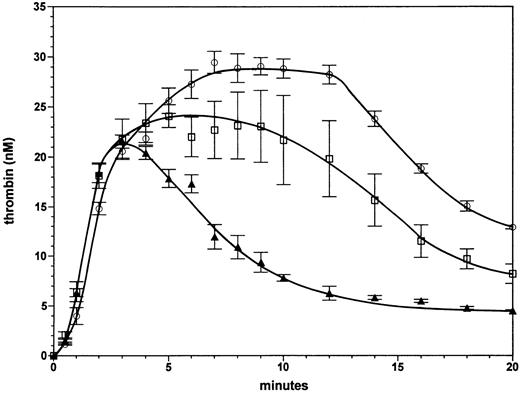
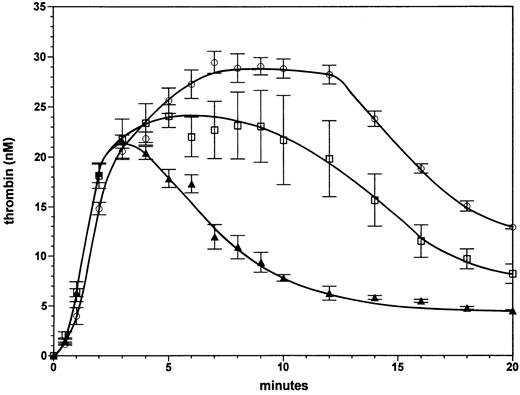
Homozygous and heterozygous factor VLEIDEN with severe hemophilia A. The effect of the factor VLEIDEN on thrombin generation in the complete absence of factor VIII is illustrated in Fig 3. Progress curves are shown of thrombin generation for reactions containing protein C and 1 nmol/L thrombomodulin with either 100% normal factor V (solid triangles), with 100% factor VLEIDEN (open circles), or a mixture of 50% normal factor V and 50% factor VLEIDEN (open squares). Thrombin formation is similar in all three reactions for the first 3 minutes, at which time approximately 21 nmol/L thrombin are formed. In the case of normal factor V (triangles) the thrombin level subsequently declines to 6 nmol/L at 12 minutes. In contrast, in the reaction with 100% factor VLEIDEN (open circles), thrombin increases from 21 nmol/L at 3 minutes to 29 nmol/L at 7 minutes. This level of ∼29 nmol/L thrombin persists for up to 12 minutes. Subsequently, the thrombin level declines to 13 nmol/L at 20 minutes. Thus, the homozygous phenotype of factor VLEIDEN has the potential to increase thrombin formation in a severe hemophilia A situation almost fivefold. The area under the thrombin generation curve (up to 20 minutes) was 120% larger (2.2-fold) with VLEIDEN compared with normal factor V. In the reaction with 50% normal factor V and 50% factor VLEIDEN (open squares), the thrombin level persists at a concentration of ∼20 nmol/L for up to 12 minutes. The thrombin level then declines to 8 nmol/L at 20 minutes. Hence, the heterozygous phenotype of factor VLEIDEN increases thrombin formation in the case of severe hemophilia up to threefold. The area under the thrombin generation curve was 1.9-fold greater than that for the reaction with normal factor V.
Effect of factor VLEIDEN in the case of severe hemophilia A. Thrombin formation in the absence of factor VIII in the presence of protein C and 1 nmol/L thrombomodulin with either normal factor V (triangles), a mixture composed of 50% normal factor V and 50% factor VLEIDEN (squares), or 100% factor VLEIDEN (circles). Thrombin generation was initiated by 5 pmol/L factor VIIa⋅TF. Error bars represent the standard error of mean (n = 3).
Effect of factor VLEIDEN in the case of severe hemophilia A. Thrombin formation in the absence of factor VIII in the presence of protein C and 1 nmol/L thrombomodulin with either normal factor V (triangles), a mixture composed of 50% normal factor V and 50% factor VLEIDEN (squares), or 100% factor VLEIDEN (circles). Thrombin generation was initiated by 5 pmol/L factor VIIa⋅TF. Error bars represent the standard error of mean (n = 3).
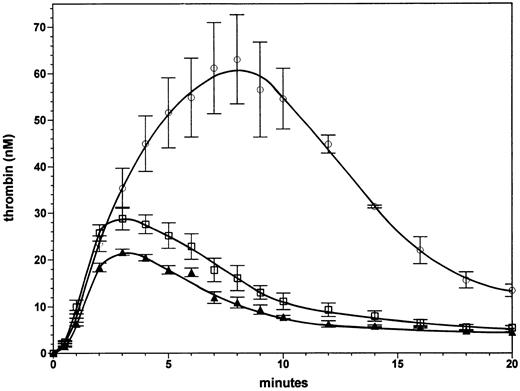
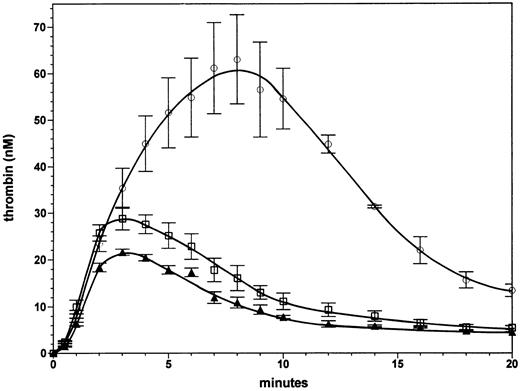
Effect of a 50% increase in factor V or factor VLEIDEN concentration with severe hemophilia A. Figure 4 shows the effect of factor V at 30 nmol/L (150% of the normal plasma concentration) in a reaction without factor VIII in the presence of protein C (65 nmol/L) and thrombomodulin (1 nmol/L). This experiment was performed to evaluate whether the beneficial effect of the factor VLEIDEN mutation in hemophilia could be mimicked by increases in normal factor V concentrations within the range observed in normal individuals. The set of experiments shown in Figs 3 and 4 can be compared quantitatively, because they were performed contemporaneously. Thrombin generation in the presence of 30 nmol/L normal factor V results in a small (25% to 40%) increase in thrombin levels formed when compared with the reaction at the normal plasma concentration (20 nmol/L, 100%) of factor V (triangles). At 20 and 30 nmol/L of factor V the thrombin concentration declines with a similar rate after 3 minutes. The area under the thrombin generation curve was 40% higher. Thus, an increase in normal factor V does not result in a very significant enhancement or prolongation of thrombin formation. In contrast, an increase in factor VLEIDEN to 150% of the plasma concentration (Fig 4, open circles) resulted in a threefold increase in the maximum level of formed thrombin compared with the reaction with 100% normal factor V. The area under the thrombin generation curve was 3.75-fold larger. Compared with the reaction with 100% factor VLEIDEN (Fig 3, open circles) the reactions with 150% factor VLEIDEN reached a twofold higher maximum thrombin level and led to a 1.7-fold greater area under curve. The increase in thrombin generation by a 50% increase in factor VLEIDEN is not only higher in an absolute sense compared with the reaction with 100% normal factor V. The relative increases of the reaction with 150% factor VLEIDEN compared with 100% factor VLEIDEN (open circles, Fig 3), which are a 100% increase in maximum thrombin and a 70% increase in area under the curve, are also higher compared with the 25% increase in maximum thrombin and 40% larger area under the curve with a 50% increase in normal factor V. These data show that increases in the concentration of the APC-resistant factor VLEIDEN to the upper range of the normal level will lead to significant amplification and extension of thrombin formation.
Effect of increases in normal factor V and factor VLEIDEN levels in severe hemophilia A. Thrombin formation was initiated under the same conditions as described in Fig 3 in the absence of factor VIII in the presence of either a normal plasma level (100%) of factor V (triangles), 150% factor V (squares), or 150% factor VLEIDEN circles). Error bars represent the standard error of mean (n = 3).
Effect of increases in normal factor V and factor VLEIDEN levels in severe hemophilia A. Thrombin formation was initiated under the same conditions as described in Fig 3 in the absence of factor VIII in the presence of either a normal plasma level (100%) of factor V (triangles), 150% factor V (squares), or 150% factor VLEIDEN circles). Error bars represent the standard error of mean (n = 3).
DISCUSSION
We have speculated that the factor VLEIDEN phenotype might be beneficial in hemophilia A by partially compensating for a defective factor VIIIa molecule.18 This speculation was apparently verified by the recent report of Nichols et al,19 who reported a varying phenotype (<1% versus 2% to 7% factor VIII activity) for hemophilia A patients with identical genotype. Screening for the factor VLEIDEN mutation showed that two of the patients who had the less severe phenotype also carried the G to A substitution in the factor V gene responsible for the Arg506 → Gln mutation, which renders the molecule partially resistant to inactivation by APC. Nichols at al19 hypothesized that the beneficial effect of the factor VLEIDEN mutation could be caused by the extended amplification of the limited signal produced by the defective factor VIII. In this study we recreate these combined hemophilic and thrombophilic phenotypes in a model that reconstitutes the TF pathway to thrombin,23 including the stoichiometric inhibitors TFPI and AT-III24 and the dynamic protein C pathway.25
At an initiating factor VIIa⋅TF concentration sufficient for explosive thrombin generation at normal concentrations of factor VIII, reduction of the factor VIII concentrations to ≤6.25% the normal plasma level led to only marginal increases in thrombin generation under these conditions. At 12.5% factor VIII thrombin generation is significantly upregulated compared with the reaction without factor VIII, resulting in a threefold increase in the maximal level of thrombin formed. The significant boundary in thrombin generation between 6.25% and 12.5% factor VIII is analogous to the borderline for the classification of hemophilia patients as mildly (5% to 25% factor VIII activity) or moderately (1% to 4% factor VIII activity) deficient.29 Thus, the model shows a similar factor VIII concentration dependence to that required for adequate hemostasis; the latter inferred from the correlation of factor VIII level with the severity of the hemorrhagic symptoms. When normal factor V was replaced with factor VLEIDEN no differences were observed in the absence of the protein C pathway in thrombin generation in our hemophilia model. This excludes the possibility that factor VLEIDEN has higher intrinsic cofactor activity under hemophilia conditions. Consistent with this, purified factor VLEIDEN possesses the same clotting activity when added to an activated partial thromboplastin time (APTT) assay in the presence of low levels of factor VIII.19
In the presence of the protein C pathway constituents, increased thrombin formation is observed with the factor VLEIDEN mutation. The extent of increase in thrombin generation ranges from threefold to sevenfold in the artificial hemophilia A situation when the concentration of factor VLEIDEN is varied from 50% to 150% of the mean normal plasma factor V concentration. The potential beneficial effect in the hemophilia A situation by factor VLEIDEN, when thrombin generation is surpressed by the protein C pathway, is ∼threefold with heterozygous factor VLEIDEN. The increase in thrombin level caused by the factor VLEIDEN mutation in the hemophilia A situation observed in this model provides biochemical data that may explain the reports of beneficial effect of the inheritance of the factor VLEIDEN mutation in severe hemophilia A.
The effects we observed are consistent with the report by Nichols et al.19 The hemophilia A patients studied by Nichols et al in whom factor VLEIDEN appeared to partially compensate for a factor VIII defect, were bearing a factor VIII R1689C or a R2209Q mutation. Patients identified with the same factor VIII mutations, which were homozygous for the normal factor V allele, displayed severe hemorrhagic disease. The factor VIII R1689C mutation occurs at a thrombin cleavage and prevents the formation of active cofactor. The factor VIII R2209Q mutation is located in the C2 domain, which is involved in the lipid binding of the molecule. However, Chan et al30 describe a group of severe hemophiliacs in whom the severity of hemorrhagic symptoms did not correlate with the factor VLEIDEN mutation. Also, Arbini et al31 have reported on the low prevalence of factor VLEIDEN in a cohort of severe hemophiliacs with a hemorrhagic disorder of lesser severity. However, these two studies used a random testing of hemophiliacs for the factor VLEIDEN mutation without correlating specific amino acid substitutions in the factor VIII gene with the presence of factor VLEIDEN, as is the case for the study of Nichols et al.19 However, caution should be employed when generalizing a beneficial effect of factor VLEIDEN in hemophiliacs. Our data show that in an in vitro system factor VLEIDEN will partially compensate for a defective factor VIII molecule. In this respect it should be mentioned that the cases of factor VLEIDEN in hemophilia have been identified by the presence of the mutation at the DNA level. However, this does not necessarily correlate with the plasma levels of the mutant factor V protein, as other factors are involved in the synthesis of factor V. For instance, the inherited combined deficiency of factor V and factor VIII32 has been hypothesized to be the result of a defect in the post-translational process involved in the synthesis of these cofactors. The dependence of the increase in thrombin levels on the concentration of factor VLEIDEN observed in our hemophilia A model suggests that the levels of factor VLEIDEN dictate the degree of a beneficial effect in hemophilia A. Quantitative analyses of factor VLEIDEN at the protein level in the case of hemophilia A may, therefore, shed more light on apparent discrepancies of a beneficial effect of factor VLEIDEN in hemophiliacs. Also, as mentioned by Nichols et al,19 the effect of the factor VLEIDEN mutation may be most pronounced in the context of specific factor VIII mutations.
Hemostatic plugs formed at the end of transected vessels are reported to be more fragile in the case of hemophilia.33,34 The central area of hemostatic plugs formed in hemophilia patients become eroded, and channels are formed, filled with red blood cells through the central area, which are consistent with delayed bleeding.34 Only a rim of fibrin deposition was observed at the periphery of the eroded plugs in hemophiliacs34 in contrast to thick fibrin fibers throughout the stable plugs formed in normal individuals. It should be mentioned that most thrombin is generated in blood after clot formation,35 which is the endpoint characterized in most plasma coagulation tests. Our data suggest that factor VLEIDEN increases and maintains thrombin generation in hemophilic blood by reducing thrombin downregulation by APC. APC-related factor Va fragments are formed during the clotting of whole blood35 without addition of exogenous thrombomodulin, indicating the existence or formation of APC in native blood. Also, hemostatic plugs extend into the lumen of the transected vessels and are in close proximity of thrombomodulin on the endothelium. Thus, APC formation may be expected during the formation of a hemostatic plug, either by endothelial-derived thrombomodulin or by the intrinsic APC generating potential of blood. If APC downregulates thrombin generation during the formation of a hemostatic plug, our data show how the factor VLEIDEN mutation may provide increased fibrin formation or increased stability against fibrinolytic attack36 37 in hemophilia patients.
Supported by HL-46703 to K.G.M. and by a TALENT-stipendium of the Netherlands Organization of Scientific Research to C.v.V.
Address reprint requests to Kenneth G. Mann, PhD, Department of Biochemistry, C401 Biven Bldg, The University of Vermont, College of Medicine, Burlington, VT 05405-0068.