Abstract
Vascular endothelial growth factor (VEGF ) is a secreted endothelial cell-specific mitogen, which is induced by hypoxia and is angiogenic in vivo. Recently, elevated serum concentrations of VEGF (S-VEGF ) have been reported in patients with cancers of various histologies. However, the prognostic significance of S-VEGF in human cancer is unknown and the origin of S-VEGF remains unsettled. We measured S-VEGF by enzyme-linked immunosorbent assay from sera taken from 82 patients with non-Hodgkin's lymphoma before treatment and stored for 9 to 15 years at −20°C. All but one of the patients had been followed-up for at least 5 years or until death. S-VEGF ranged from 15 to 964 pg/mL; median, 228 pg/mL; mean, 291 pg/mL. A higher than the median S-VEGF level was associated with a poor World Health Organization performance status, a high International Prognostic Index, a high serum lactate dehydrogenase level, and a large cell histology. Patients with lower than the median S-VEGF at diagnosis had a 71% 5-year survival rate in comparison with only 49% among those with a higher than the median S-VEGF. We conclude that a high pretreatment S-VEGF level is associated with poor outcome in non-Hodgkin's lymphoma.
ANGIOGENESIS, the sprouting of new capillaries from preexisting ones, is an important component in many physiological and pathological processes. In healthy adults extensive angiogenesis occurs only in the female reproductive system. Angiogenesis may also take place in some pathological conditions, such as wound healing, rheumatoid arthritis, diabetic retinopathy, and tumor growth (reviewed by Folkman1 ).
During tumorigenesis, the vasculature can become activated to grow new capillaries in response to an appropriate stimulus (reviewed by Hanahan and Folkman2 ). One such stimulus is vascular endothelial growth factor (VEGF ), also called vascular permeability factor (VPF ), which is a soluble, dimeric 46-kD protein that is active as an endothelial cell-specific mitogen and as a vascular permeability factor (reviewed by Ferrara3 and Dvorak et al4 ). As a result of alternative splicing of VEGF mRNA, there exist at least three isoforms of VEGF encompassing 121, 165, and 189 amino acid residues.5 The two shorter forms are efficiently secreted from cells, whereas the longer one remains mostly cell associated. VEGF is induced by hypoxia in vitro and in vivo.6-10 Tumor cells have been shown to express both VEGF mRNA and protein in a variety of human cancers,11-14 and it has been concluded that VEGF has an important role in tumor biology and in the process of tumor angiogenesis (reviewed by Dvorak et al4 ). Expression of VEGF has been detected also in lymphoma.15 Furthermore, VEGF protein purified from a human histiocytic lymphoma cell line promoted dermal blood vessel leakage in guinea pigs at a dose of 20 ng and promoted in vitro endothelial cell growth at concentrations as low as 50 pmol/L.16 Expression of VEGF has been shown to increase tumor growth and angiogenesis in vivo in a nude mouse model.17-19 Similarly, anti-VEGF antibodies have the ability to inhibit the growth of several tumor cell lines in nude mice.20-22
Recently, elevated serum VEGF concentrations (S-VEGF ) have been observed in patients with various histological types of cancer.23,24 High S-VEGF has also been associated with a large size of the primary breast tumor and a short tumor volume doubling time in advanced colorectal cancer.23 25 However, little is known about the clinical significance of S-VEGF, and there are no studies available on the prognostic value of S-VEGF in any type of human cancer. In the present study we measured S-VEGF from lymphoma patients' sera stored for years at −20°C and found that S-VEGF values were associated with survival and several important clinical variables.
MATERIALS AND METHODS
Patients
S-VEGF was measured in 82 randomly selected adult patients with non-Hodgkin's lymphoma diagnosed and treated in the Department of Oncology, Helsinki University Central Hospital in 1981 to 1987, of whom frozen serum taken at the time of diagnosis but before possible lymphoma treatment was available. Histological classification was not reviewed in conjunction with the present study, but 18 lymphomas (22%) had been classified as low-grade, 40 (49%) intermediate-grade, and 22 (27%) as high-grade lymphoma according to the Working Formulation scheme26 by a pathologist with a special interest in lymphoma, and 2 (2%) cases were considered unclassifiable.
Thirty-eight (46%) of the patients were men, and the median age at diagnosis was 60 years. Clinical staging was done according to the Ann Arbor classification system. The clinical status, a chest radiograph, computed tomography (CT) scans of the mediastinum and abdomen, and a bone marrow biopsy were taken as staging examinations. Twenty-two (27%) of the patients had stage I, 24 (29%) stage II, 14 (17%) stage III, and 22 (27%) stage IV disease at diagnosis. Fifteen patients (18%) had B symptoms (weight loss, unexplained fever, or night sweats). The International Prognostic Index (IPI)27 could be determined in 79 (96%) cases.
A total of 68 patients were treated with combination chemotherapy. The patients with intermediate- or high-grade lymphoma and disseminated disease were treated usually with bleo-CHOP (bleomycin, cyclophosphamide, doxorubicin, vincristine, and prednisone), M-BACOD (methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone), or another anthracycline containing a combination chemotherapy regimen. Low-grade lymphomas were usually treated with a single agent chlorambucil if symptomatic. Twenty-eight patients received megavoltage radiotherapy.
All patients were regularly followed-up with a few months intervals in an outpatient department. The median follow-up time was 84 months (range, 33 to 136 months), and all but one patient were followed up over 60 months or until death. During the follow-up time 38 patients died.
Methods
Venous blood samples. Peripheral venous blood samples were collected in sterile test tubes a few hours or a few days before starting lymphoma therapy, centrifuged at 2,000g for 10 minutes, and then stored at −20°C.
S-VEGF immunoassay. S-VEGF concentrations were determined as S-VEGF immunoreactivity by using a quantitative sandwich enzyme immunoassay technique (Quantikine R; R & D Systems, Minneapolis, MN). The system uses a solid phase monoclonal antibody and an enzyme-linked polyclonal antibody raised against recombinant human VEGF. For each analysis 100 μL of serum was used. All analyses and calibrations were performed in duplicate. The calibrations on each microtiter plate included recombinant human VEGF standards. Optical densities were determined by using a microtiter palate reader (Multiscan RC Type 351; Labsystems, Helsinki, Finland) at 450 nm. The blank was subtracted from the duplicate readings for each standard and sample. A standard curve was created using StatView 4.02 (Abacus Concepts Inc, Berkeley, CA) by plotting the logarithm of the mean absorbance of each standard versus the logarithm of the VEGF concentration. Concentrations are reported as pg/mL. The coefficient of variation of interassay determinations reported by the manufacturer vary from 6.2% to 8.8% when the S-VEGF concentrations range between 50 and 1,000 pg/mL. When we measured 12 serum samples twice in two separate assays, the interassay variation ranged from 2% to 9% within the same concentration range. The possible effect of freezing on S-VEGF was investigated by measuring S-VEGF before and after a freeze-thawing cycle in serum samples taken from six cancer patients and from six healthy volunteers. S-VEGF levels were determined without any knowledge of the survival or other clinical data except age and sex of the patients, which could be found on the test tube labels.
Statistical analysis. Statistical analysis was done by using the BMDP software (BMDP Statistical Software Inc, Los Angeles, CA). Cumulative survival was estimated with the product-limit method from the date of diagnosis. The Mantel-Cox log-rank test and the generalized Wilcoxon test were used to compare survival between different groups. The relative importance of different variables was analyzed using Cox's proportional hazard model (BMDP 2L). Frequency tables were analyzed with the χ2 test or Fisher's exact test. The effect of freezing on S-VEGF was analyzed with the Wilcoxon signed rank test. All P values are two tailed.
RESULTS
The Effect of Freezing on VEGF Concentrations of Serum Samples
The effect of freeze thawing was studied in 12 serum samples. Before freezing, S-VEGF of the 12 samples tested ranged from 23 to 2,703 pg/mL; median, 261 pg/mL; mean, 670 pg/mL, and after freezing and thawing, the range was from 22 to 3,114 pg/mL; median, 263 pg/mL; mean, 719 pg/mL (P > .1; Table 1). Hence, freezing and thawing of the serum samples did not have any marked effect on S-VEGF values.
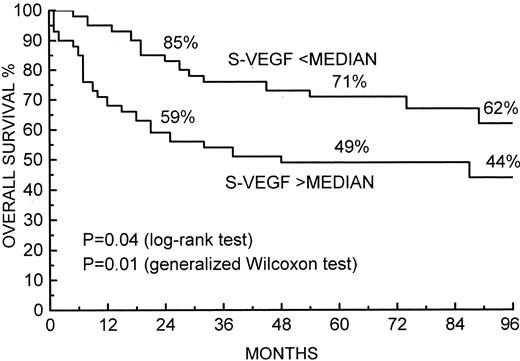
Survival of 82 patients with non-Hodgkin's lymphoma by the pretreatment S-VEGF level. The median (228 pg/mL) was used as the cut-off value. Survival rates at 24, 60, and 96 months are given.
Survival of 82 patients with non-Hodgkin's lymphoma by the pretreatment S-VEGF level. The median (228 pg/mL) was used as the cut-off value. Survival rates at 24, 60, and 96 months are given.
Serum VEGF in Non-Hodgkin's Lymphoma
Serum VEGF concentrations ranged from 15 to 964 pg/mL (median, 228 pg/mL; mean 291 pg/mL) among the 82 patients with non-Hodgkin's lymphoma. One third of the patients had S-VEGF lower than 122 pg/mL and one third higher than 314 pg/mL.
If the median S-VEGF concentration was used as the cut-off value, a high S-VEGF was strongly associated with a poor World Health Organization (WHO) performance status (>1, P = .002), a higher than the normal serum lactate dehydrogenase (S-LDH) level measured at the time of the diagnosis (P = .005), a high IPI (>2, P = .003), and a possible association was found with the female gender (P = .08; Table 2). No association between a high pretreatment S-VEGF and age at diagnosis, Ann Arbor stage, or the presence of B-symptoms was found (P > .10). Similarly, no association between S-VEGF and the Working Formulation grading was found (P = .82), but patients with large cell lymphoma (either large cell diffuse, G, n = 27; immunoblastic, H, n = 14; lymphoblastic, I, n = 2; or histiocytic lymphoma, n = 1) had more often a higher than the median pretreatment S-VEGF level in comparison with the rest of the patients (P = .03; Table 3).
S-VEGF and Survival
Patients with higher than the median S-VEGF had inferior survival in comparison with those with lower than the median pretreatment value (P = .04 by the log-rank test and .01 by the generalized Wilcoxon test; Fig 1). The 2- and 5-year survival rates of the patients with a lower than the median S-VEGF level were 85% and 71%, respectively, and those of the patients with a higher than the median level, 59% and 49%, respectively. A high S-VEGF level was associated with inferior survival also when tertiles and quartiles were used as cut-off values (Table 4). A higher than the median S-VEGF was associated with poor overall survival among patients with low-grade lymphoma (5-year survival 56% v 100%; P = .04) and those with high-grade lymphoma (5-year survival 25% v 50%; P = .03), but in intermediate-grade lymphomas the difference was not statistically significant (62% v 67%; P > .1). When survival in the subgoup of large cell diffuse and immunoblastic lymphomas (n = 41) was analyzed separately, patients with a higher than the median S-VEGF (269 pg/mL) had inferior survival in comparison with patients with lower than the median S-VEGF, but the difference was not statistically significant (2-year survival 55% v 71%; 5-year survival 44% v 52%; P > .1).
Several other factors correlated strongly with overall survival in univariate survival analyses in the present series (Table 4). Therefore, S-VEGF using the median as the cut-off value was tested in a multivariate analysis together with the IPI score, gender, and the presence of B symptoms using Cox's proportionate hazard model. In this analysis, only the IPI score had independent influence on survival (P < .001, the relative risk of death 2.3, 95% confidence interval from 1.7 to 3.2).
DISCUSSION
In the present study we found many patients with non-Hodgkin's lymphoma to have an elevated pretreatment S-VEGF concentration. The pretreatment S-VEGF of non-Hodgkin's lymphoma patients ranged from 15 to 964 pg/mL (median, 228 pg/mL; mean, 291 pg/mL). In comparison, the S-VEGF in 184 healthy individuals reported by Yamamoto et al23 varied from values that were under the detection limit to 228 pg/mL. The results of Yamato et al are in line with our experience, because we have only rarely measured VEGF concentrations higher than 150 pg/mL in sera of presumably healthy individuals (range from 1 to 177 pg/mL; n = 113).24
We found a high pretreatment S-VEGF to be associated with poor overall survival and a few established adverse prognostic factors in non-Hodgkin's lymphoma. S-VEGF did not have independent prognostic value when the IPI was included in a multivariate analysis, but it should be noted that in a univariate analysis S-VEGF was about as strong a prognostic factor as age at diagnosis, which is currently included as a component together with stage, S-LDH level, the number of extranodal sites, and the WHO performance status in the IPI.27 Although first designed for aggressive non-Hodgkin's lymphomas, the IPI has been shown to be useful in all grades of lymphoma.28 However, the present findings should be interpreted with some caution because the series is heterogeneous and includes several types of non-Hodgkin's lymphoma.
In lymphoma and several other types of human cancer the cancer cells produce VEGF.4,15 Hypoxia has been considered to be an important stimulus for VEGF production in tumor cells, but also various cytokines such as interleukin-6, epidermal growth factor, transforming growth factor-β1, keratinocyte growth factor, and tumor necrosis factor-α have been shown to induce the expression of VEGF in cultured cells.29,30 Lymphocytes infiltrating human cancers, macrophages, and peripheral blood T lymphocytes and platelets also contain VEGF.31-34 Therefore, circulating VEGF found in patients with cancer may originate from cancer cells but also from tumor infiltrating benign cells and circulating peripheral blood cells. However, it should be noted that not all of the proteins stored in blood cells are endogenously synthesized, but instead may originate primarily from plasma.35 36 Consequently, it is possible that VEGF secreted by cancer cells or by tumor infiltrating inflammatory cells escapes to the circulation and accumulates in the blood cells, such as platelets and lymphocytes.
In the present study we measured higher than the median S-VEGF values more frequently from large cell lymphomas than from small cell or mixed cell lymphomas, and high S-VEGF levels were also associated with a poor WHO performance status, a high serum LDH level, and the IPI. These findings are not in contradiction with the hypothesis that high serum VEGF levels are associated with growth of lymphoma and active angiogenesis. The biological role of circulating VEGF in cancer progression and dissemination may be of importance. VEGF in the tumor microenvironment not only stimulates proliferation of tumor blood vessels, but also increases vascular permeability (reviewed by Dvorak et al4 ), thus, possibly contributing to tumor cell extravasation and metastasis formation. Moreover, VEGF has recently been found to inhibit maturation of dendritic cells, which are important antigen-presenting cells.37 Hence, exposure of the immune system to high levels of local and possibly also circulating VEGF may play a role in allowing tumors to avoid induction of an immune response.
In conclusion, these data indicate that a high S-VEGF level is associated with poor outcome in non-Hodgkin's lymphoma. A high pretreatment S-VEGF may be associated with active tumor angiogenesis and tumor growth, and it is possible that similar associations with unfavorable survival can be found in other types of human cancer as well. Further studies are now needed in non-Hodgkin's lymphoma to find out whether S-VEGF is a prognostic factor in single histological types of the disease and whether S-VEGF values are associated with the proliferation rate of lymphoma. It will be of particular interest to find out whether S-VEGF levels predict response to antiangiogenic drugs in lymphoma and in other types of human malignancy. In addition, further studies are required to investigate the origin of circulating VEGF as well as its levels in conditions such as infectious and inflammatory states.
ACKNOWLEDGMENT
We thank Elina Roimaa for technical assistance.
Supported by grants from the Finnish Academy, the Finnish Cancer Foundation, and the Helsinki University Central Hospital Research Funds.
Address reprint requests to Heikki Joensuu, MD, PhD, Department of Oncology, Helsinki University Central Hospital, Haartmaninkatu 4, FIN-00290 Helsinki, Finland.