Abstract
Hereditary von Willebrand factor (vWF ) deficiency in Dutch Kooiker dogs, which have undetectable levels of vWF, causes spontaneous hemorrhage of mucosal surfaces similar to the clinical picture of von Willebrand disease in humans. Therefore, we used this canine model to study the in vivo effects of a new recombinant von Willebrand factor (rvWF ) preparation containing all species of vWF multimers compared with a rvWF fraction containing only low molecular weight multimers (LMW-rvWF ) and with a plasma-derived factor VIII/vWF concentrate (pdvWF ). In the vWF-deficient dogs, the half-life of vWF:Ag was 21.6 and 22.1 hours for rvWF, 7.7 hours for pdvWF, and 9 hours for LMW-rvWF; in vivo recovery of vWF:Ag was 59%, 64%, and 70% for rvWF, 33% for pdvWF and 92% for LMW-rvWF; in vivo recovery of RCoF was 78%, 110%, and 120% for rvWF, and 25% for pdvWF. Both rvWF and pdvWF caused increases in factor VIII, which were sustained even when vWF:Ag had decreased to nearly undetectable levels and only monomeric or dimeric species were detectable on agarose gels. At the dosages used, no effect was seen on bleeding time, but the rate of blood flow from cuticle wounds was reduced after a single bolus administration of rvWF. The rvWF was able to control a severe nose bleed in one dog.
VON WILLEBRAND disease (vWD) is the most common bleeding disorder in humans, with more than 20 distinct subtypes.1,2 It is caused by genetic transmission of quantitative or qualitative defects in von Willebrand factor (vWF ), a multimeric adhesive glycoprotein required for platelet adhesion at sites of vascular damage and for stabilization of FVIII in the circulation.3,4 Thus, vWF plays a dual role in hemostasis: (1) It mediates the binding of platelets to the subendothelium, which is pivotal for primary hemostasis, and (2) it protects FVIII from proteolytic inactivation, which is essential for normal intrinsic coagulation.5-7
The manifestations of vWD show the pathophysiological significance of the different biological functions of this protein. The disease is characterized by prolonged bleeding time, defective platelet adhesion, and an accompanying deficiency in FVIII. The clinical symptoms include mucosal bleeding and epistaxis, which reflect the defect in primary hemostasis, as well as postoperative soft tissue bleeds and joint bleeds, which reflect defective intrinsic coagulation due to FVIII deficiency.8 The treatment of vWD involves raising the endogenous levels of vWF. Depending on the subtype and severity, this has heretofore been accomplished by administration of the synthetic vasopressin analogue DDAVP or by replacement therapy with plasma-derived FVIII concentrates that contain vWF protein.9 A human recombinant vWF preparation is under development and a candidate has been characterized in vitro.10 11
The biological and therapeutic effects of recombinant preparations intended for treatment of human bleeding disorders have often been studied in canine models. For example, before their use in humans, recombinant FVIII and recombinant factor IX preparations were evaluated in canine models of hemophilia A and B, respectively.12,13 Canine models of vWD exist in several breeds.14-17 The clinical spectrum of canine vWD closely parallels that of human vWD. Such dogs have therefore been previously used to study the half-life of infused plasma-derived FVIII with or without vWF and the effects of such preparations in controlling hemophilic bleeding15,18-21 as well as to assess the roles of vWF and FVIII in arterial thrombosis.22 Hereditary vWF deficiency in the Dutch “Kooiker” breed causes spontaneous hemorrhage of mucosal surfaces that manifests most commonly as epistaxis and oral or gingival hemorrhage as well as gastrointestinal bleeding.
MATERIALS AND METHODS
Assay methods. Von Willebrand factor antigen (vWF:Ag) was determined by enzyme-linked immunosorbent assay using a rabbit antihuman vWF antibody from Diagnostica Stago (Asserachrom vWF; Boehringer Mannheim, Mannheim, Germany) and was expressed in human plasma equivalent units per mL (U/mL) using the standard preparation from the test kit.
Ristocetin cofactor activity (RCoF ) was determined by measuring the ristocetin-induced aggregation of formaldehyde-fixed human platelets23 under conditions as described in the literature,24 using a 570-VS whole blood aggregometer (Chrono-Log, Havertown, PA) equipped with a chart recorder and was expressed in human plasma equivalent units per mL (U/mL) as described for the FVIII activity assays. Canine vWF cannot be measured using this method. Thus, the levels of RCoF given in this article refer to the infused human recombinant or plasma-derived vWF. Therefore, pharmacokinetics of human vWF can be assessed even in normal dogs in which endogenous vWF is present.
vWF multimer analysis was performed with horizontal sodium dodecyl sulfate (SDS)-agarose gel electrophoresis using 1% and 2% agarose gels according to Ruggeri et al,25 but with visualization of vWF multimers by immunoenzymatic staining, modified according to Aihara et al.26 A rabbit antihuman vWF antiserum, #A082 (Dakopatts, Glostrup, Denmark), which strongly cross-reacts with both human and canine vWF, was used as the primary antibody. Alkaline phosphatase-conjugated affinity-purified goat antirabbit IgG (Axell Accurate Chemical and Scientific Corp, Westbury, NY) was used as the secondary antibody. When selective visualization of human vWF in the presence of canine vWF was necessary, the mouse monoclonal antihuman vWF antibody ESvWF-8 (American Diagnostica, Inc, Greenwich, CT), which has low cross-reactivity with canine vWF, was used as the primary antibody. In such cases alkaline phosphatase-conjugated, affinity-purified sheep antimouse IgG (Axell Accurate Chemical and Scientific Corp, Westbury, NY) was used as the secondary antibody. Staining was performed with the nitroblue tetrazolium chloride/bromochloro indolyl phosphate substrate system.
Factor VIII (FVIII) activity was measured against a human FVIII standard using the two-stage clotting method and the chromogenic method. The two-stage clotting method was performed as described by Austen and Rhymes27 using the reagents from the two-stage FVIII assay kit from IMMUNO (Vienna, Austria) including a reference plasma FVIII standard containing 1 IU FVIII/mL calibrated against the 3rd International Standard 91/666 for FVIII and vWF. The chromogenic assay was the Immunochrom FVIII:C kit from IMMUNO as described recently.28 Human FVIII plasma-equivalent units were expressed in U/mL.
VWF-mediated adhesion of platelets to collagen was assessed in perfusion experiments under flow conditions at a high shear rate (2,500 sec−1) using published methods.29 RvWF or pdvWF was added to a perfusate and platelet adhesion to coverslips coated with collagen (Kollagen; Hormon Chemie, Munich, Germany) was evaluated using light microscopy interfaced with an image analyzer (Quantimet 600 Image Processing and Analysis System; Leica Cambridge Ltd, Cambridge, UK). Adhesion was expressed as the percentage of the surface of the coverslip covered with platelets. The perfusate consisted of washed erythrocytes and washed acetyl salicylic acid-treated platelets (200 × 106 mL) in Krebs-Ringer buffer, pH 7.4 (107 mmol/L NaCl, 20 mmol/L NaHCO3 , 4 mmol/L KCl, 2 mmol/L Na2SO4 , 19 mmol/L tri-Na-citrate, 2.5 mmol/L CaCl2 , 0.5% glucose) containing 4% human serum albumin with a final hematocrit of 40%. The monoclonal antibodies CLB-Rag 35,30 which is directed against the platelet glycoprotein Ib [GPIb] binding site of vWF, and CLB-Rag 50,29 a murine monoclonal antibody to vWF that does not inhibit vWF function, were used in concentrations of 10 μg per mL of perfusate to control for the specificity of the effect. A control experiment was also performed in which no vWF was added to the perfusate.
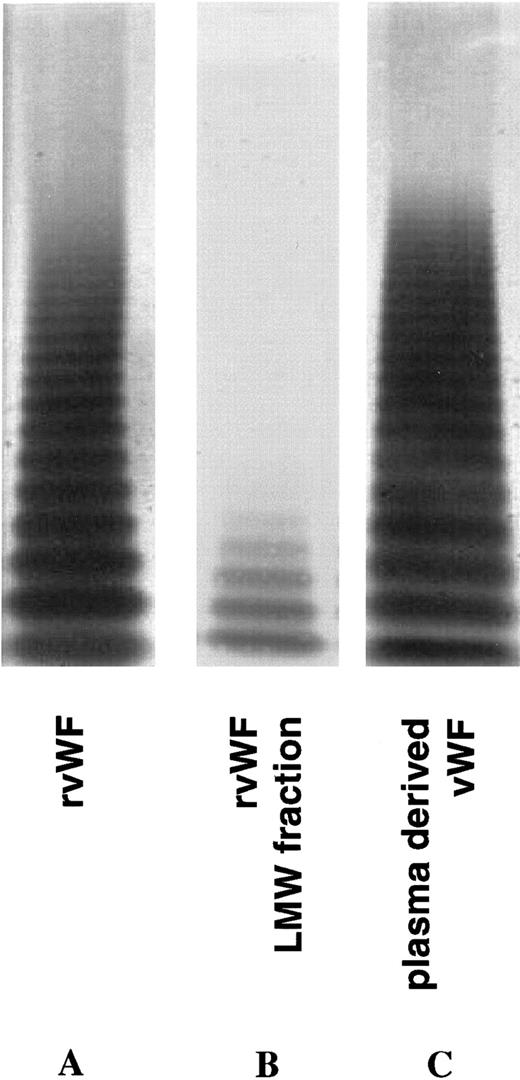
Test substances.The human rvWF (IMMUNO) has been previously described.10 11 The plasma-derived FVIII/vWF concentrate (pdvWF ) Haemate HS (Behringwerke, Marburg, Germany batches #2346641 and #2956641) was used for comparison. The rvWF contained more than 20 multimers. Since its purification process, which used affinity chromatography on immobilized heparin, allows the separation of a low molecular weight fraction of vWF multimers, a rvWF fraction containing only low molecular weight (LMW) multimers of vWF with relatively low platelet-aggregating (RCoF ) activity was also produced and used for one in vivo experiment. The biochemical characteristics of the vWF-containing preparations are summarized in Table 1; their multimer compositions are shown in Fig 1 (lanes A through C).
Multimer composition of the three different human vWF preparations used in this study.
Multimer composition of the three different human vWF preparations used in this study.
Animals. The 4 vWF-deficient Kooiker dogs were obtained from a Dutch breeder in Marum, The Netherlands. Beagle dogs purchased from Harlan (Zeist, The Netherlands) and a mongrel dog purchased from Harlan Sprague Dawley (Madison, WI) served as normal controls. The animals were housed in the animal care facilities of the medical faculty of the University of Vienna in Himberg, Austria, and of IMMUNO AG in Vienna, Austria. All animal studies were performed in accordance with Austrian federal law regulating animal experimentation (BG 501, 1988).
In vitro recovery of vWF in dog plasma. The in vitro recovery of human vWF in dog plasma was determined by adding 1.8 RCoF U/mL of the human rvWF preparation to plasma from a vWF-deficient dog. The mixtures were frozen and stored at −20°C for 24 hours. After thawing, vWF RCoF activity and rvWF:Ag were measured. The freezing and thawing cycle was performed to simulate the quality of ex vivo plasma samples that were also frozen after sampling and thawed before assay on the next day. The experiment was performed in triplicate.
Effect of human rvWF (▴) and pdvWF (•) on vWF-mediated platelet adhesion expressed as mean coverage (±SD) of surface of the perfusion chamber. Line graph: Response increases with increasing concentrations of vWF. Inset: At 5 μg/mL rvWF (▪) or pdvWF (), the effect is abolished by the anti-GPIb monoclonal antibody CLB-Rag 35 but not by the irrelevant antibody CLB-Rag 50 (Control [□] = no vWF ).
Effect of human rvWF (▴) and pdvWF (•) on vWF-mediated platelet adhesion expressed as mean coverage (±SD) of surface of the perfusion chamber. Line graph: Response increases with increasing concentrations of vWF. Inset: At 5 μg/mL rvWF (▪) or pdvWF (), the effect is abolished by the anti-GPIb monoclonal antibody CLB-Rag 35 but not by the irrelevant antibody CLB-Rag 50 (Control [□] = no vWF ).
Assessment of bleeding. In addition to the measurement of bleeding time,31,32 the intensity of bleeding was assessed using a method we developed to allow quantification of the rate of blood flow.32 An incision of the nail cuticle was made31 and blood from the cuticle wound was collected onto a filter in 1-minute time intervals. The filters were extracted with a hemolytic buffer and the hemoglobin was measured photometrically. The blood volume of each individual portion was calculated from a standard calibration curve that had been obtained according to the same procedure using defined volumes of dog blood. The rate of blood flow was calculated for an observation interval from 8 to 15 minutes post incision, when bleeding had already stopped in the normal dog. If bleeding did not stop after 15 minutes, the cuticle wound was electrically cauterized.
Test procedures. The animals were anesthetized with 10 mg/kg ketamine (Ketasol; Dr E. Graub AG, Bern, Switzerland) and 1 mg/kg xylazine (Xylasol; Dr E. Graub AG). The forearm veins were cannulated and the vWF preparations were administered via the venous cannula as single bolus injections, with the dosage of each preparation calculated on the basis of RCoF activity. Using a different cannula, blood samples were drawn at baseline (0 hour), 15 minutes, 30 minutes, and 1, 2, 3, 24, and 48 hours after administration for analysis of vWF:Ag, RCoF, and FVIII. Bleeding time and rate of blood flow were assessed for each animal as described above at baseline (0 hour), 30 minutes, 3 hours, and 24 hours after the administration of the test substance, using a different cuticle for each time point. Anesthesia was maintained for 3 hours and repeated for the 24- and 48-hour assessments. If a dog was treated twice with human vWF, the second application was performed under an antihistamine dose of 1 mg/kg diphenhydramine hydrochloride (Benadryl parenteral; Parke-Davis, Berlin, Germany) to prevent allergic reactions.
Biometrical methods. Half-life of vWF and in vivo recovery of vWF:Ag and RCoF were determined after injection of rvWF, LMW-rvWF, or human pdvWF. Half-life was calculated using the method of Lee et al.33 In vivo recovery was calculated according to the formula:
RESULTS
Perfusion experiments. The vWF-mediated platelet adhesion to collagen was assessed in perfusion experiments with the rvWF as well as with the pdvWF concentrate. Both preparations had the same effect on platelet adhesion under high shear rate conditions (Fig 2). The monoclonal antibody CLB-Rag 35 directed against GPIb abolished vWF-mediated adhesion, while the control monoclonal antibody CLB-Rag 50 had no effect.
In vitro recovery of vWF. The mean in vitro recovery of human vWF in vWF-deficient dog plasma was 102% (±5%) for vWF:Ag and 83% (±10%) for vWF RCoF activity (n = 3). Although the recovery is normal, the absolute amount of RCoF is only 8% of the amount of vWF:Ag (see Table 1).
In vivo experiments. The baseline characteristics of the animals involved in the study are shown in Table 2. No vWF is detectable in the vWF-deficient dogs, and this deficiency is accompanied by reduced plasma concentrations of FVIII (ranging from 1.4 to 2 U/mL in the 2-stage assay as compared to 3.3 U/mL in the normal dog) as well as hemorrhagic diathesis with bleeding times longer than 15 min. Dog no. 750 (Fig 3) was treated with 35 RCoF U rvWF per kg body weight. Dog no. 751 received 70 RCoF U rvWF per kg (Fig 4A) and 7 days later 63 RCoF U per kg (Fig 4B) of the pdvWF (batch no. #2346641). The treatment of dog no. 754 with 75 RCoF U rvWF per kg body weight was not a planned experiment but an emergency intervention to treat severe epistaxis. Dog no. 1140 (Fig 5) received LMW-rvWF at a dose of 45 mg per kg body weight equivalent to 6 RCoF U per kg. The normal dog no. H9510176 was first treated with 35 RCoF U per kg body weight of rvWF (Fig 6A) and then 3 days later with 32 RCoF U per kg body weight of the pdvWF (batch no. #2956641) (Fig 6B). There were no changes in platelet counts (not shown) and no adverse events were observed in any of the dogs.
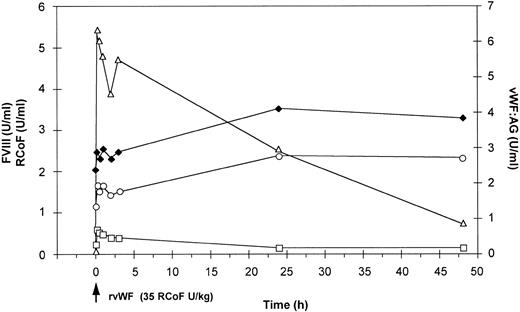
Effect of a single bolus injection of human rvWF (35 RCoF U/kg) on plasma levels of vWF:Ag (▵), RCoF (□) and FVIII-(♦ 2-stage, ○ chromogenic) in a vWF-deficient dog (no. 750).
Effect of a single bolus injection of human rvWF (35 RCoF U/kg) on plasma levels of vWF:Ag (▵), RCoF (□) and FVIII-(♦ 2-stage, ○ chromogenic) in a vWF-deficient dog (no. 750).
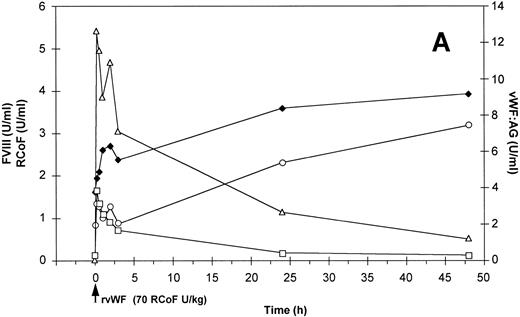
Effect of a single bolus injection of human (A) rvWF (70 RCoF U/kg) and 7 days later of (B) pdvWF (63 RCoF U/kg) on plasma levels of vWF:Ag-(▵), RCoF (□), and FVIII-(♦ 2-stage, ○ chromogenic) in a vWF-deficient dog (no. 751).
Effect of a single bolus injection of human (A) rvWF (70 RCoF U/kg) and 7 days later of (B) pdvWF (63 RCoF U/kg) on plasma levels of vWF:Ag-(▵), RCoF (□), and FVIII-(♦ 2-stage, ○ chromogenic) in a vWF-deficient dog (no. 751).
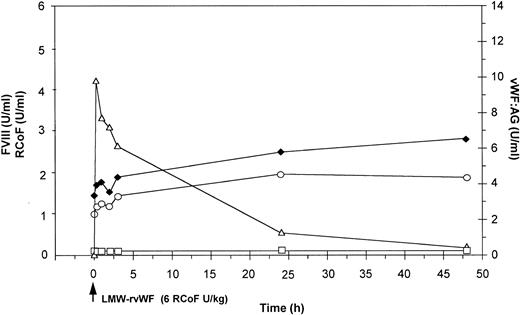
Effect of a single bolus injection of human LMW-rvWF (6 RCoF U/kg) on plasma levels of vWF:Ag (▵), RCoF (□), and FVIII-(♦ 2-stage, ○ chromogenic) in a vWF-deficient dog (no. 1140).
Effect of a single bolus injection of human LMW-rvWF (6 RCoF U/kg) on plasma levels of vWF:Ag (▵), RCoF (□), and FVIII-(♦ 2-stage, ○ chromogenic) in a vWF-deficient dog (no. 1140).
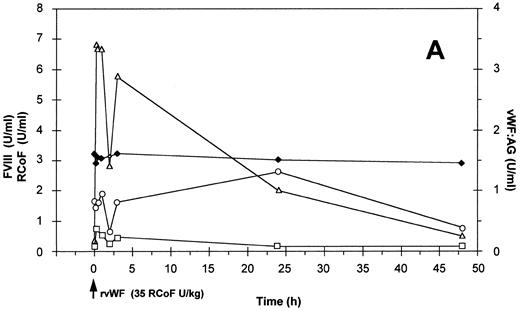
Effect of a single bolus injection of human (A) rvWF (35 RCoF U/kg) and 3 days later of (B) pdvWF (32 RCoF U/kg) on plasma levels of vWF:Ag-(▵), RCoF (□), and FVIII-(♦ 2-stage, ○ chromogenic) in a normal dog (no. H9510176).
Effect of a single bolus injection of human (A) rvWF (35 RCoF U/kg) and 3 days later of (B) pdvWF (32 RCoF U/kg) on plasma levels of vWF:Ag-(▵), RCoF (□), and FVIII-(♦ 2-stage, ○ chromogenic) in a normal dog (no. H9510176).
VWF activity. As shown in Table 3, the half-life of vWF:Ag was substantially longer after administration of rvWF than after administration of pdvWF in both vWF-deficient dogs and in the normal dog; for LMW-rvWF the half life of vWF:Ag was shorter than that seen after rvWF and was close to that seen after pdvWF. In vWF-deficient dogs, the in vivo recovery of vWF:Ag was substantially higher after administration of either rvWF or LMW-rvWF than after administration of pdvWF; in the normal dog the in vivo recovery of vWF:Ag after rvWF was comparable to that after pdvWF.
In vWF-deficient dogs the recombinant material could not be compared with pdvWF in regard to half-life of RCoF activity; however, the in vivo recovery of RCoF was higher for both recombinant preparations than for the pdvWF (Table 3). In the normal dog both half-life and recovery of RCoF activity were greater for rvWF than for pdvWF.
Distribution of vWF multimers. The multimer distribution of vWF analyzed in low and high resolution agarose gels before and after infusion is shown for the vWF-deficient dogs in Figs 7-9. Multimers of the plasma-derived preparations as well as of the recombinant vWF were removed from the circulation gradually. The high molecular weight species disappeared first. The infused rvWF had a polydisperse multimeric structure without the satellite bands that would have resulted if proteolytic degradation had taken place. The mobility of these multimers did not change after infusion.
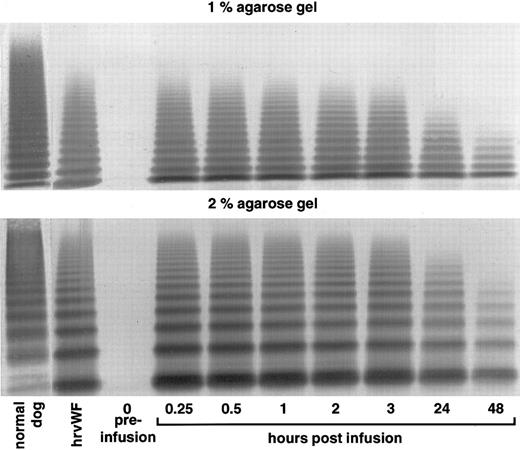
vWF multimer pattern over time in vWF-deficient dog no. 750 after a single bolus injection of human rvWF (35 RCoF U/kg). Plasma samples were analyzed on 1% and 2% SDS-agarose gel electrophoresis. Multimers visualized using a cross-reacting antidog/antihuman vWF antibody.
vWF multimer pattern over time in vWF-deficient dog no. 750 after a single bolus injection of human rvWF (35 RCoF U/kg). Plasma samples were analyzed on 1% and 2% SDS-agarose gel electrophoresis. Multimers visualized using a cross-reacting antidog/antihuman vWF antibody.
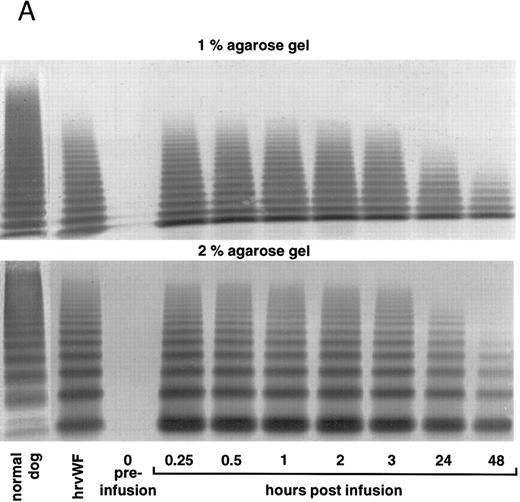
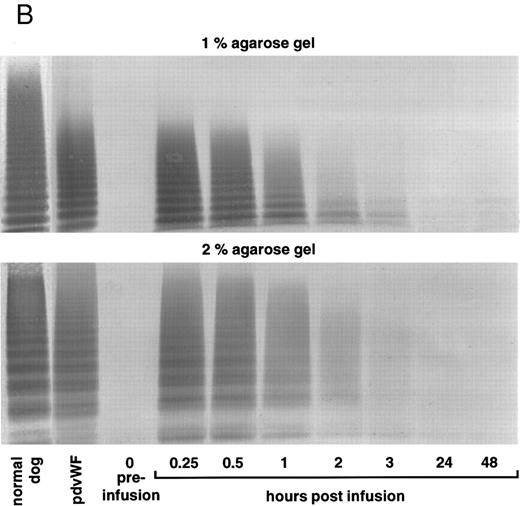
vWF multimer pattern over time in vWF-deficient dog no. 751 after a single bolus injection of human (A) rvWF (70 RCoF U/kg), and 7 days later of (B) pdvWF (63 RCoF U/kg). Plasma samples were analyzed on 1% and 2% SDS-agarose gel electrophoresis. Multimers visualized using a cross-reacting antidog/antihuman vWF antibody.
vWF multimer pattern over time in vWF-deficient dog no. 751 after a single bolus injection of human (A) rvWF (70 RCoF U/kg), and 7 days later of (B) pdvWF (63 RCoF U/kg). Plasma samples were analyzed on 1% and 2% SDS-agarose gel electrophoresis. Multimers visualized using a cross-reacting antidog/antihuman vWF antibody.
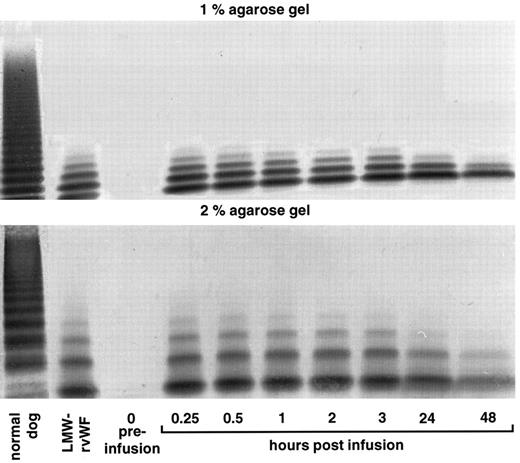
vWF multimer pattern over time in vWF-deficient dog no. 1140 after a single bolus injection of LMW-rvWF (6 RCoF U/kg). Plasma samples were analyzed on 1% and 2% SDS-agarose gel electrophoresis. Multimers visualized using a cross-reacting antidog/antihuman vWF antibody.
vWF multimer pattern over time in vWF-deficient dog no. 1140 after a single bolus injection of LMW-rvWF (6 RCoF U/kg). Plasma samples were analyzed on 1% and 2% SDS-agarose gel electrophoresis. Multimers visualized using a cross-reacting antidog/antihuman vWF antibody.
For monitoring the multimer pattern over time in the normal dog, a detection antibody that did not cross-react with canine vWF was used for visualization of the vWF oligomers. Thus, the elimination of human rvWF and pdvWF could be monitored even in the presence of normal levels of canine vWF and showed a gradual clearance of the vWF multimers as seen in the vWF-deficient animals (Fig 10A and B).
vWF multimer pattern over time in a normal dog after a single bolus injection of human (A) rvWF (35 RCoF U/kg) and 3 days later of (B) pdvWF (32 RCoF U/kg). Plasma samples were analyzed on 1% and 2% SDS-agarose gel electrophoresis. Multimers visualized using a cross-reacting antidog/antihuman vWF antibody and a noncross-reacting antihuman vWF antibody for the post infusion samples.
vWF multimer pattern over time in a normal dog after a single bolus injection of human (A) rvWF (35 RCoF U/kg) and 3 days later of (B) pdvWF (32 RCoF U/kg). Plasma samples were analyzed on 1% and 2% SDS-agarose gel electrophoresis. Multimers visualized using a cross-reacting antidog/antihuman vWF antibody and a noncross-reacting antihuman vWF antibody for the post infusion samples.
FVIII activity. In the vWF-deficient dogs, infusion of both rvWF and pdvWF caused sustained increases in endogenous FVIII levels (Figs 3 and 4) but the maximum FVIII activity (Table 3) obtained with the 2-stage assay was higher after treatment with rvWF (an increase of more than 240% in one dog) than with pdvWF. Infusion of LMW-rvWF in a vWF-deficient dog (Fig 5) caused an increase in endogenous FVIII plasma levels similar to that seen after the infusion of rvWF containing the full range of multimers. The assays used to determine FVIII and vWF were not able to differentiate between endogenous and infused material in the normal dog. Although the administration of rvWF resulted in an increase in the plasma concentration of vWF:Ag in the normal dog, there was virtually no change in FVIII measured in the 2-stage assay (Fig 6A). Whereas in vWF-deficient dogs the 2-stage and chromogenic assays yielded almost parallel curves, this was not the case for the normal dog. After administration of pdvWF FVIII levels increased in the normal dog (Fig 6B).
Cuticle bleeding. Neither recombinant nor plasma-derived vWF was able to reduce the abnormally prolonged bleeding time to less than 15 minutes in any of the vWF-deficient dogs (Table 4). In dog no. 750 the rate of blood flow from the cuticle wound (Table 4) was remarkably reduced at 30 minutes and 3 hours and slightly reduced 24 hours after injection of rvWF. In dog no. 751 the rate of blood flow also decreased after injection of rvWF, increased slightly at 3 hours but remained below baseline, and then decreased again at 24 hours. When pdvWF was administered in the same dog 7 days later, the baseline level was relatively low and the rate of blood flow decreased only at 3 and 24 hours. The baseline rate of blood flow was also relatively low in dog no. 1140, which received LMW-rvWF. The rate of blood flow was low at 30 minutes, but increased again at 3 and 24 hours. Emergency treatment of dog no. 754 with rvWF resulted in a reduction of the nose bleeding within 1 hour post infusion of the rvWF preparation. Bleeding further decreased after 2 hours and ceased completely within 2 to 3 hours. In the normal dog, bleeding had completely ceased before the start of each observation interval for determining the rate of blood flow (8 to 15 minutes after the injury to the cuticle).
DISCUSSION
Congenital vWF deficiency is the most common disorder of coagulation in humans as well as in dogs.18 The vWF-deficient Kooiker dogs used in this infusion study have a bleeding disorder similar to severe type 3 vWD in humans,34 characterized by undetectable vWF antigen, undetectable vWF multimers on agarose gels, and — since FVIII is stabilized by vWF in dogs as well as in humans — reduced FVIII levels in the circulation. The FVIII levels in these dogs, which were about 50% of normal canine values, were higher than those observed in type 3 vWD in humans, possibly indicating that canine FVIII is less dependent than human FVIII on vWF for stabilization. Similar FVIII levels have also been reported to occur in other dog breeds with vWD.14 As in hemophilic dogs, injury to the nail cuticles of vWF-deficient dogs results in prolonged bleeding.
The availability of this natural animal model of the human deficiency state allowed us to evaluate the biological activity of the human recombinant vWF preparation in vivo. When this material was added to plasma from the vWF-deficient dogs the in vitro recovery of vWF:Ag was approximately 100% and that of RCoF approximately 85%, demonstrating that the plasma values for vWF measured post infusion are valid, although the assays were carried out in test systems that were designed and based on reagents for human proteins. Infusion of rvWF into these dogs, which are virtually completely deficient in vWF, resulted both in an immediate rise of ristocetin cofactor activity and vWF antigen, with peaks at 15 minutes post injection, as well as in the appearance of vWF multimers with a distribution indistinguishable from that of the infused material (Figs 7-8, compare Fig 1). The in vivo recovery of RCoF activity ranged from approximately 80% to 120%, and the in vivo recovery of vWF:Ag was approximately 60% to 70%, although the absolute amount of vWF RCoF was approximately 10% of the level of vWF:Ag (see Table 1). The half-life of RCoF was between 4 and 10 hours. A half-life of RCoF of 1.3 hours after infusion of an intermediate purity human FVIII concentrate was previously reported in a dog with vWD.16
The half-life of rvWF protein in our vWF-deficient dogs was approximately 22 hours, which was longer than that of the pdvWF we used for comparison in one vWF-deficient dog. Since the pdvWF was administered 7 days after the rvWF the possibility of an accelerated clearance of the pdvWF mediated by an immune response cannot be ruled out. The half-life we found for rvWF was also slightly longer than that previously reported after the infusion of a human pdFVIII/vWF complex in vWF-deficient dogs.15 In another report, canine cryoprecipitate infused into Dobermans with vWD resulted in half-life values of vWF antigen ranging from 16.3 to 24.4 hours with a mean of 21.3 ± 1.8.21 The half-life of our recombinant vWF preparation was also seen to be longer than that of human or porcine pdvWF in a porcine model of vWD.35 In any case, the clearance of rvWF is not faster than that of pdvWF, either in dogs or in pigs, which — since carbohydrate moieties could play a role in vWF clearance — would be the case if defects in carbohydrate processing were present.36,37 Our in vivo data therefore agree with the previous in vitro finding that regular carbohydrate processing occurs with the rvWF used here.10 38 Subtle differences in glycosylation of this recombinant protein may still be present, which may have a potential for inducing an immune response in humans. To determine the immunogenicity of rvWF, careful clinical studies in individuals with vWD will need to be performed according to standard guidelines for other blood products used in replacement therapy.
Neither in the vWF-deficient dogs nor in the normal dog was there any sign of in vivo proteolytic degradation of the infused rvWF, especially no appearance of satellite bands that would be indicative of cleavage at Tyr841Met842, which is known to be caused by a protease specific for vWF.39-41 An absence of satellite band formation after infusion of a CHO-derived rvWF has also been shown in normal rats.37 However, our rvWF is susceptible to specific cleavage by the purified human vWF-cleaving protease in vitro.42 The reasons for this apparent discrepancy need to be investigated, especially whether this protease exists in canine plasma and if so, whether it can degrade human rvWF in vitro. Since in contrast to rvWF, the pdvWF does show satellite bands in agarose gel electrophoresis, the prolonged half-life of rvWF could be due to the integrity of the rvWF multimers.
We also monitored the metabolic clearance of multimers over time. The high molecular weight forms were cleared more rapidly from the circulation than low molecular weight forms. A more rapid elimination of the high molecular weight multimers than of the corresponding dimers of HUVEC- or CHO-derived vWF has also recently been observed in rat infusion studies.37 Whether this represents selective removal due to enhanced binding to platelet receptors, differential compartmentalization, degradation of high molecular weight multimers into smaller species, or is simply an artifact due to the relatively greater mass of low molecular weight forms in the infused preparation, is unclear at present. The changes in the multimeric pattern after transfusion are in accord with the substantially longer half-life of rvWF as compared to pdvWF.
In the vWF-deficient dogs the appearance of vWF antigen in the circulation was associated with a rapid rise in endogenous FVIII from around 50% to normal canine levels, peaking at 24 to 48 hours and remaining at this level for at least a further 24 hours. This resembles the “secondary rise” in FVIII seen in previous human infusion studies with vWF-containing FVIII preparations and corresponds to the prolongation of the half-life of endogenously secreted FVIII observed after infusion of a vWF concentrate containing very little FVIII.43 These data show that human rvWF can bind to and stabilize canine FVIII in vivo.
The FVIII levels remained elevated even when vWF antigen had decreased to nearly undetectable levels and only monomeric or dimeric species were detectable on agarose gels. Thus, the half-life of FVIII in these dogs was independent of the survival of all of the multimeric vWF species in the circulation. This is also corroborated by the infusion experiment using LMW-rvWF, in which endogenous FVIII levels were similar to the post infusion levels of FVIII obtained with the fully multimerized rvWF. Since the elevated FVIII levels were sustained for so long, the half-life could not be calculated. In contrast, when purified human FVIII is infused into dogs with vWD, due to the lack of vWF for stabilization it is rapidly removed from the circulation, with a half-life of less than one hour.16 Our in vivo studies confirm in vitro observations that multimers of different sizes have equivalent affinities for FVIII.6 Multimerization does not affect the binding of FVIII, nor is the protective effect of vWF related to the size of multimers. Our data would suggest that only a small amount of vWF protein with restricted multimerization is required to protect canine FVIII. Although this effect appears due to protection from proteolytic degradation, an effect of rvWF on factor VIII synthesis and/or secretion cannot be excluded.
Despite its imprecision, bleeding time is generally taken as a surrogate for determining efficacy of treatment in correcting the defect of primary hemostasis in patients with vWD. FVIII concentrates have been shown to have a variable effect on bleeding time in dogs with vWD; some are unable to correct bleeding time,16 whereas others partially or completely correct it.15 Moreover, shortening of bleeding time does not seem to correlate with vWF antigen values obtained in individual dogs.15 In the present study, bleeding time remained prolonged after the single infusion of both rvWF and pdvWF. However, bleeding intensity expressed as the rate of blood flow from the sites of injury decreased after administration of rvWF in all experiments, suggesting that the sustained increase in FVIII produced by a single infusion of rvWF generated sufficient thrombin to lessen the rate of blood flow. However, apparently not enough thrombin was generated to amplify FVIII-dependent intrinsic coagulation, nor was the local concentration of vWF obtained adequate to achieve sufficient platelet adhesion to form a normal hemostatic plug. Although few quantitative data are available that relate dose to shortening of bleeding time in humans or in animals,44 recent observations in humans would suggest that no shortening of bleeding time can be expected until the plasma levels of ristocetin cofactor exceed 100 U/dL.45 In our study the RCoF activity was far below these levels, which could explain the bleeding time results. Further studies will be required to determine whether different treatment modalities with rvWF such as repeated administration or continuous infusion with or without factor VIII would shorten the prolonged bleeding time.
The successful treatment of a severe nose bleed in one of the vWF-deficient dogs suggests its clinical efficacy. Bleeding was well controlled after a single infusion of rvWF without any rebleeding, nor were any other local interventions or concomitant treatments necessary. To our best knowledge this is the first observation of the efficacy of a recombinant vWF protein in a canine bleeding model. The rvWF was well tolerated, and in this canine model of vWD it exhibited biological properties which make it a promising therapeutic agent. Considering the natural limitations of animal models, it remains to be seen whether the effects observed here can be translated into clinical benefits for human patients.
ACKNOWLEDGMENT
We are grateful to Dr Derk de Korte and Dr Jürgen Siekmann for analysis of platelet adhesion and aggregation, to Ingrid Neunteufl for performing the multimer electrophoresis, to Günter Richter and Dr Sandor Fritsch for biometrical evaluation, to Kathryn Nelson for editing, and to Sylvia C. Maurer for preparation of the manuscript.
Presented in part at the 37th Annual Meeting of the American Society of Hematology, Seattle, WA, December 1-5, 1995.
Address reprint requests to Hans Peter Schwarz, MD, Immuno AG, Industriestr 67, A-1220 Vienna, Austria.

![Fig. 2. Effect of human rvWF (▴) and pdvWF (•) on vWF-mediated platelet adhesion expressed as mean coverage (±SD) of surface of the perfusion chamber. Line graph: Response increases with increasing concentrations of vWF. Inset: At 5 μg/mL rvWF (▪) or pdvWF (), the effect is abolished by the anti-GPIb monoclonal antibody CLB-Rag 35 but not by the irrelevant antibody CLB-Rag 50 (Control [□] = no vWF ).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3555/4/m_bl_0038f2.jpeg?Expires=1767779432&Signature=WSUuAuaBdC1RXtEdSPaQAL7fuz4y9DhlCnLP7qN4AnbvG-DRbNsCGGSzMayKvZBM3njPQUuJ0-0Hx6yIlw-g1xp~PE9nNsxbF9y1tlU51IwnLGwufLsVp7EROUarQjB41BwSfNBeQ6Ib1KRGBQNDj3XAJt2W9cZQc2nzp1NSSN1cG-sxXN8r6suFxSbIOmLuU2Y~YSLIGfgRCuQZNO5srYyFoRBuMqqQMd8nLncclEPaE-Vs8xtoi3cmgvDVDpnApJDqHvd5zCk0HczkxOY0AIi9jK~L6tCBXZOKnKY~LShfvpoY4wxs0b5PmCLw1pxUmW1F2Oga8HDe48-9ceptkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)