Abstract
Recruitment of blood monocytes into tissues is a central event in the inflammatory response and in atherogenesis. The mechanisms leading to monocyte adhesion and migration through endothelium are not completely defined. We recently reported that MAb L11, against the leukocyte sialomucin CD43, blocks T-lymphocyte binding to lymph node and Peyer's patch high endothelial venules (HEV) and inhibits T-cell extravasation from the blood into organized secondary lymphoid tissues. We have now assessed the ability of L11 to inhibit monocyte-endothelial (EC) interactions and trafficking. L11 blocks binding of WEHI78/24 cells, a murine monocytoid cell line, to inflamed lymph node HEV and inhibits recruitment of monocytes and neutrophils to thioglycollate-inflamed peritoneum. Because monocyte adhesion to the endothelium and diapedesis in lesion-prone regions of the vasculature is among the earliest events in atherogenesis, leading to formation of lipid-laden foam cells, the ability of L11 to block monocyte recognition of aortic endothelial cells was assessed in a novel ex vivo assay of monocyte binding to intact rabbit aortic endothelium. Cholesterol feeding of rabbits induces enhanced aortic adhesiveness for monocytes and WEHI78/24 monocytoid cells, and this adhesion is inhibited by L11. The inhibitory effect of L11 is additive with that of a cocktail of anti–L-selectin and anti-α4 and β2 integrin monoclonal antibodies. Thus, CD43 represents a novel target for manipulation of monocyte recruitment in inflammation and atherogenesis.
A PROMINENT FEATURE of inflammation and atherogenesis is the adherence of monocytes to local vascular endothelium, with subsequent diapedesis and migration of these cells into the inflamed tissue. Although several monocyte-endothelial (EC) adhesion pathways have been described (including L–selectin-peripheral node addressin [PNAd], β2 integrin–intracellular adhesion molecule-1 [ICAM-1], α4 integrin–vascular cell adhesion molecule-1 [VCAM-1], sialyl Lewisx–E-selectin, and P–selectin-P–selectin glycoprotein-1), studies in multiple models indicate that important monocyte adhesion receptors and/or regulatory pathways remain to be identified.1-3 We have initiated efforts to identify novel molecular mechanisms of monocyte-EC interactions that are involved in or can modulate monocyte recruitment from the blood in inflammation and atherogenesis.
One accessible model for studying monocyte interactions with EC, and the mechanisms of their alteration during inflammation, is the Stamper-Woodruff assay of leukocyte adhesion to lymph node-high endothelial venules (HEV) in frozen sections. HEV in frozen sections of mouse lymph node normally support lymphocyte, but not monocyte binding.4 This lack of monocyte adhesion is intriguing, as monocytes constitutively express L-selectin and β2 integrin receptors for PNAd and ICAM-1 and -2, ligands that are constitutively expressed by HEV. Thus, although L-selectin and β2 integrins are available to support monocyte-HEV interactions, these molecules alone are not sufficient for monocyte binding. Inflammatory stimuli such as complete Freund's adjuvant (CFA), however, induce monocyte recruitment via lymph node-HEV in vivo, the kinetics of which are paralleled by a remarkable monocyte-selective increase in adhesive capacity of lymph node venules as assessed in the ex vivo Stamper-Woodruff frozen section assay.5 Thus this ex vivo system provides a model for characterization of additional components regulating monocyte adhesion to inflamed endothelium, and we have taken advantage of this model to select monoclonal antibodies (MoAbs) capable of blocking monocyte recognition of inflamed endothelial cells. We recently reported one MoAb selected using this screen, L11, that blocks T-lymphocyte binding to lymph node HEV and T-cell homing in vivo.6 We now describe L11 inhibition of monocyte-EC interactions as well: L11 blocks monocyte binding to inflamed lymph node HEV by greater than 70% and also inhibits monocyte and neutrophil recruitment to inflamed peritoneum in vivo.
Atherogenesis shares many features with chronic inflammation, including recruitment of monocytes and T lymphocytes, expression of major histocompatability complex (MHC) class II molecules by T cells and endothelial cells, as well as local expression of immune and inflammatory mediators.7-9 Focal adherence of peripheral blood monocytes to vascular endothelium in lesion-prone areas is one of the earliest observable events following initiation of an atherogenic diet. Adherent monocytes migrate into the intima, accumulate lipid, and become monocyte-derived foam cells, a major component of early lesions in humans and other species. The mechanisms governing monocyte-specific adhesion in preatherosclerotic vessels, although the subject of intense investigation, remain incompletely defined.2,10 We have shown that the aortic luminal EC of cholesterol fed rabbits displays markedly enhanced adhesiveness for monocytes in an ex vivo assay.10 11 Anti-CD43 MoAb L11 inhibited this interaction by greater than 70%.
Taken together, our findings suggest that CD43 can modulate monocyte-EC interactions and monocyte recruitment in inflammation and atherogenesis.
MATERIALS AND METHODS
Monoclonal Antibody Generation and Characterization
The derivation of L11 has been described previously described in the context of its effects on T-cell trafficking.6 Briefly, using WEHI78/24 cells12 (gift of R. Coffman, DNAX, Palo Alto, CA) as immunogen, rat antimouse MoAbs were generated using traditional polyethylene glycol (PEG) fusion methods. Hybridoma supernatants were screened for their ability to block binding of lymphocyte and WEHI78/24 cells to HEV in normal and inflamed peripheral lymph nodes (PLN) in the Stamper-Woodruff frozen section assay.13
Stamper-Woodruff Assay on Inflamed PLN
Normal and inflamed PLN were harvested from Balb/c mice (6 to 8 weeks of age) frozen in OCT (Miles Scientific, Elkhart, IN) and used within 3 months. To prepare inflamed nodes, mice were anesthetized with Metofane (Pittman-Moore, Mundelein, IL) and their hind foot-pads injected with 50 μL complete Freund's adjuvant (GIBCO, Gaithersburg, MD). Three days later mice were killed by cervical dislocation and popliteal lymph nodes were harvested and frozen in OCT. Fresh 10 μm sections were cut and used within 30 minutes.
The Stamper-Woodruff assay was performed essentially as described.6 13-15 Briefly, WEHI78/24 cells were grown in Dulbecco's modified Eagle's medium (DMEM) (BioWhittaker, Walkersville, MD) supplemented with 5% fetal calf serum (Gemini Scientific, Calabasas, CA; endotoxin <10 pg/mL) and 5% fetal clone (HyClone, UT; endotoxin <10 pg/mL). Cells were harvested in plateau phase (2.0 to 2.5 × 106 per mL), chilled, and preincubated with L11 or binding control MoAb 30G12 (ATCC, Rockville, MD), against the common leukocyte antigen T200, at 0.5 μg/106 cells. In other experiments, WEHI78/24 cells were incubated with blocking MoAbs against: L-selectin (Mel-14), α4 integrin (PS/2; ATCC), αL (CD11a; FD344; ATCC), αM (CD11b; M1/70; ATCC), β2 (CD18; M18.2.a.8; ATCC), or control binding MoAbs 30G12 (T200; ATCC) and RB5-8C5 (ATCC), all at 5× saturating concentrations (generally 1 to 10 μg/106 cells). In some experiments, WEHI78/24 cells (2 × 106 per mL) were mixed with rat lymph node lymphocytes (1 × 106 per mL) in binding buffer and 100 μL added to freshly cut sections within a 22.2 mmol/L diameter ring (drawn with a technical pen). (Rat lymphocytes are not recognized by any of the rat MoAbs used and are included as an internal standard.) Cells were allowed to adhere for 30 minutes under constant rotation (75 rpm) at 5 to 6°C. Nonadherent cells were drained off and slides were placed in 2% glutaraldehyde in phosphate-buffered saline (PBS) containing 2 mmol/L Ca2+, 2 mmol/L Mg2+, and allowed to fix for at least 15 minutes. Sections were examined under dark field illumination. WEHI78/24 cells were easily distinguished from the rat lymphocytes by their substantially larger size. The number of WEHI78/24 and lymphocytes bound to at least 25 HEV was determined and the data are expressed as the number of WEHI78/24 cells bound per rat lymphocyte. In other experiments, the binding of WEHI78/24 cells to inflamed HEV was measured without the addition of rat lymphocytes. Binding was quantified as the number of cells/HEV from four separate slides in each of three experiments. To determine if any cell aggregation occurred during the binding assay, additional slides (not used for analysis) were included and examined under dark field illumination before draining off the nonadherent cells.
Recruitment of leukocytes to the inflamed peritoneal cavity. Mice were preinjected intravenously (IV) with 250 μg of control or L11 MoAb followed by an intraperitoneal (IP) injection of thioglycollate (Becton Dickinson, Microbiology Systems, Cockeysville, MD). Peritoneal cells were isolated by injecting and withdrawing 10 mL of calcium and magnesium-free Hanks' balanced salt solution (HBSS) spiked with 106 fluorescent beads (6 μ; Polysciences, Warrington, PA) per mL. Cells were stained either with fluorescein isothiocyanate (FITC)-labeled RB5-8C5 and biotinylated-CD44 followed by phycoerythrin (PE)-conjugated streptavidin (Tago, Burlingame, CA) or with PE-conjugated RB6-8C5 (Pharmingen, San Diego, CA) and FITC-labeled M1/70 (anti-αM). The numbers of neutrophils (8C5high, αMmed, CD44med) and monocyte/macrophages (8C5lo-med, αMmed, CD44high), and beads were determined by flow cytometry and the total number of each cell type calculated based on the known number of fluorescent beads added to each sample.
To determine blood levels of monocytes and neutrophils, 100 μL of peripheral blood, collected by heart puncture, was spiked with 106 fluorescent beads and stained as described above. Before fluorescence-activated cell sorting (FACS) analysis, erythrocytes were lysed using a mouse erythrocyte lysis kit (R&D Systems, Minneapolis, MN) according to the manufacture's instructions.
Preparation of Aortic Endothelium and Binding Assay
Male New Zealand White rabbits were fed either normal rabbit chow or rabbit chow enriched with 1% cholesterol (ICN Biomedical, Cleveland, OH). Total cholesterol and HDL levels (mg/dL) of the control animals were 53.8 ± 11.1 and 28.7 ± 3.7, respectively, while cholesterol feeding for 2 weeks resulted in 803 ± 113 and 25.8 ± 3.8 mg/dL of total cholesterol and high-density lipoprotein (HDL), respectively.
Rabbits were killed and intact aortic segments were prepared as previously described.11 Briefly, thoracic aortas were removed and placed in cold, oxygenated HBSS and a 15-mm segment of thoracic aorta was excised from a point immediately distal to the left subclavian artery. The segments were opened longitudinally and placed into 35-mm culture dishes previously coated with a thick layer of 3% agarose equilibrated with binding buffer (HBSS supplemented with 2 mmol/L Ca2+, 2 mmol/L Mg2+, and 20 mmol/L HEPES, pH 7.0). Aortic segments were pinned to the dish exposing the endothelium to the medium.
WEHI78/24 cells were labeled with tetramethyl rhodamine B isothiocyanate (TRITC) as previously described6,15 16 and were preincubated with binding control MoAb 30G12 or with blocking MoAbs to L-selectin (Mel-14), alpha 4 (PS/2 ), αM (M1/70), αL (FD344), each at 3× saturating concentrations, L11 (0.5 μg/106 cell) or a combination of all blocking MoAbs for 20 minutes at room temperature (RT). A total of 2 × 106 cells in 2 mL of binding buffer was added to each dish and coincubated with continuous rocking for 30 minutes at RT. At least one dish of each condition was examined to determine if any cell aggregation occurred during the assay. The medium was gently aspirated and replaced by 2 mL fresh binding medium to remove nonadherent cells. After a second wash, the dishes were placed on the rocking platform for an additional 5 minutes to remove nonadherent cells. The aortic segments were then examined under epifluorescence microscopy and the number of adherent cells in >30 fields was determined on at least five independent segments from at least two control and three cholesterol-fed rabbits in each of three experiments.
WEHI78/24 adhesion to vascular selectins and ICAM-1. Mock transfected, human P-selectin and E-selectin transfected CHO cells17 (kindly provided by E. Berg, Protein Design Labs, Mountain View, CA) were plated in 35 mm dishes, grown to confluence, and were either left untreated or were treated with blocking anti–E-selectin MoAb CL-218 or anti–P-selectin MoAb WAPS12.219 for 15 minutes at RT. Adhesion assays were performed as described.15 Briefly, 1 × 106 WEHI78/24 cells, preincubated with L11 or control IgG2a MoAb 30G12, were added to dishes and coincubated for 30 minutes with constant agitation at RT. Nonadherent cells were removed and adherent cells were counted by microscopy.
RESULTS
Anti-CD43 MoAb L11 Blocks WEHI 78/24 Binding to HEV in Inflamed PLN
WEHI78/24 cells were preincubated with hybridoma supernatants and their binding to HEV in normal and inflamed PLN was assessed. As previously reported5 and reproduced here, WEHI78/24 cells failed to bind normal lymph node HEV, but WEHI78/24 cells bound well to HEV following CFA-induced inflammation (Figs 1 and 2). MoAb L11, but not class-matched, binding negative control MoAbs, blocked binding of WEHI78/24 cells to inflamed HEV by greater than 70% (Fig 2). For comparison, the effects of anti–L-selectin, αL, αM, β2, and α4 MoAbs, as well as two cell binding isotype matched control MoAbs (RB6-8C5 and 30G12), are presented in Table 1. None of the MoAbs used in these assays caused aggregation of WEHI78/24 cells.
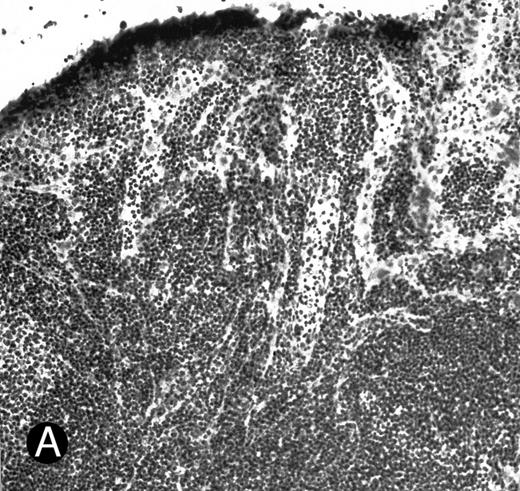
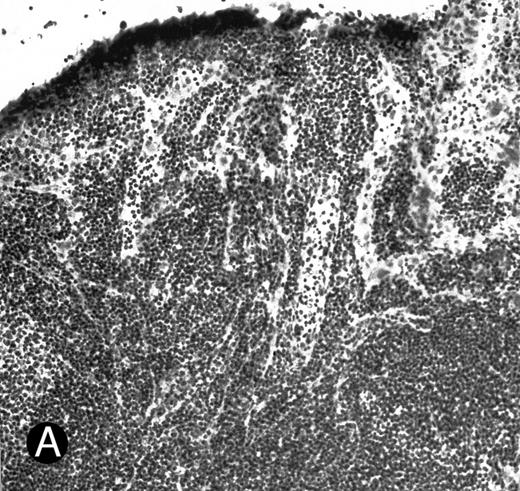
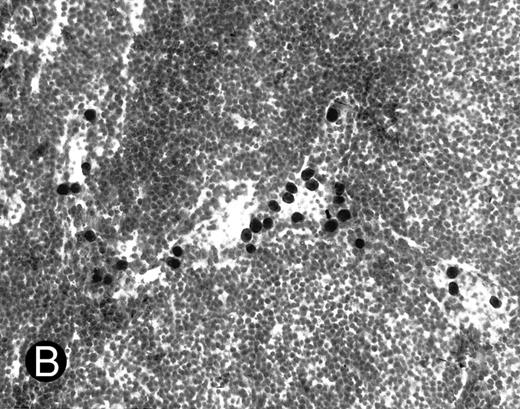
WEHI78/24 cells bind to inflamed, but not normal PLN HEV. Binding of WEHI78/24 cells to normal (A) and CFA-inflamed (B) PLN HEV was assessed in modified Stamper-Woodruff frozen section assays. WEHI78/24 cells were added to freshly cut frozen sections and incubated for 30 minutes at 5°C under constant rotation. Nonadherent cells were washed away and the sections fixed and stained with thionine as described.14 A lower magnification of the normal lymph node is shown to illustrate lack of binding on multiple HEV.
WEHI78/24 cells bind to inflamed, but not normal PLN HEV. Binding of WEHI78/24 cells to normal (A) and CFA-inflamed (B) PLN HEV was assessed in modified Stamper-Woodruff frozen section assays. WEHI78/24 cells were added to freshly cut frozen sections and incubated for 30 minutes at 5°C under constant rotation. Nonadherent cells were washed away and the sections fixed and stained with thionine as described.14 A lower magnification of the normal lymph node is shown to illustrate lack of binding on multiple HEV.
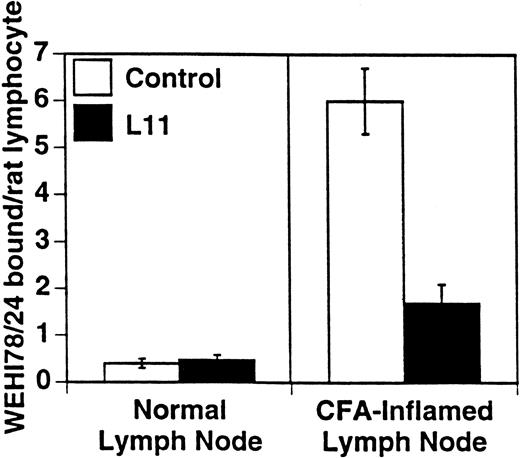
L11 blocks monocytoid cell binding to inflamed PLN HEV. Binding of WEHI78/24 cells to normal and CFA-inflamed lymph nodes was assessed in modified Stamper-Woodruff frozen section assays: WEHI78/24 cells were mixed with internal control cells (rat mesenteric lymph node lymphocytes), preincubated with isotype matched control MoAb or with L11 (0.5 μg/106 cells), added to freshly cut frozen sections and incubated for 30 minutes at 5°C under constant rotation. The number of WEHI78/24 bound per rat internal standard cell in greater than 30 HEV on each of quadruplicate sections was determined by microscopy. The mean and standard error of three independent experiments are pooled and presented.
L11 blocks monocytoid cell binding to inflamed PLN HEV. Binding of WEHI78/24 cells to normal and CFA-inflamed lymph nodes was assessed in modified Stamper-Woodruff frozen section assays: WEHI78/24 cells were mixed with internal control cells (rat mesenteric lymph node lymphocytes), preincubated with isotype matched control MoAb or with L11 (0.5 μg/106 cells), added to freshly cut frozen sections and incubated for 30 minutes at 5°C under constant rotation. The number of WEHI78/24 bound per rat internal standard cell in greater than 30 HEV on each of quadruplicate sections was determined by microscopy. The mean and standard error of three independent experiments are pooled and presented.
Anti-CD43 MoAbL11 Blocks Recruitment of Monocytes to the Inflamed Peritoneal Cavity
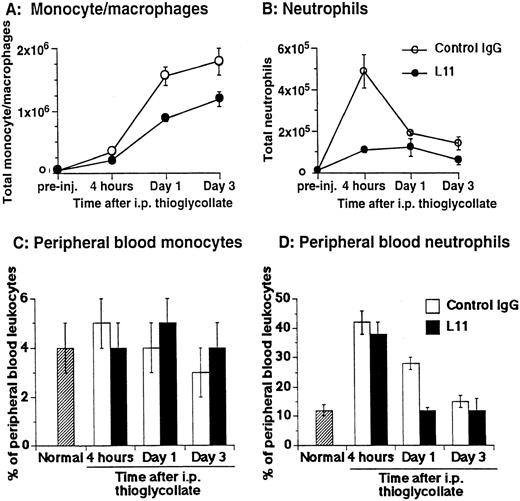
The ability of anti-CD43 MoAb L11 to modulate monocyte recruitment in inflammation was assessed by testing the ability of L11 to inhibit monocyte and neutrophil accumulation in the thioglycollate-inflamed peritoneum.22 Mice were injected with 250 μg of L11 or control antibody and 1 hour later received 2 mL of thioglycollate broth in the peritoneum. The number of monocytes, characterized by their 8C5low-med, αMhi, CD44med staining pattern23,24 and neutrophils, distinguished by their 8C5hi, αMmed, CD44med phenotype23 24 and light scatter properties and were evaluated at 4, 24, and 72 hours. Monocyte recruitment was dramatically increased at 24 and 72 hours following induction of inflammation (Fig 3A); preinjection of L11 resulted in a statistically significant (P < .001) reduction in monocyte recruitment at both time points (Fig 3A). Neutrophil recruitment begins rapidly, peaking at 4 hours (Fig 3B). In comparison with controls, L11 treatment resulted in a substantial reduction in the accumulation of neutrophils during the initial phase of recruitment, with less dramatic, but still significant inhibition of neutrophil numbers at later time points, when neutrophils were diminishing (Fig 3B). To rule out the possibility that L11-mediated blocking of recruitment was due to reduced availability of leukocytes in the blood, total blood leukocyte counts, as well as the percentage of peripheral blood monocytes and neutrophils in control MoAb and L11-treated animals, were compared at each time point. There was no significant difference in the number of circulating peripheral blood leukocytes in control IgG versus L11-treated mice at any time point (although all were elevated compared with untreated/uninflamed mice). In addition, there was no significant difference in the percentage of monocytes in the blood of control IgG versus L11-treated mice at any time point (Fig 3C). Thus, the blocking of monocytes was not due to reduced monocyte availability in the blood. Similarly, L11 treatment had no effect on the percentage (or number) of circulating neutrophils at 4 hours (which were significantly elevated over control levels by thioglycollate treatment) suggesting that the dramatic inhibition of neutrophil accumulation during the phase of active neutrophil recruitment is also due to decreased efficiency of extravasation, and not to decreased availability in the blood pool (Fig 3D). The reduction in peripheral neutrophil numbers in L11-treated mice at 24 and 72 hours, when the number of neutrophils in the periphery is declining anyway, may largely reflect the blockade of initial recruitment, although inhibition of residual neutrophil recruitment or effects on neutrophil retention might also play a role. Interestingly, the statistically significant (P < .005) reduction in circulating neutrophils in L11 recipients at 24 hours was seen in two of three experiments; although unexplained, it may reflect a more rapid reversion to normal (preinflammation) blood neutrophil levels.
L11 blocks recruitment of monocytes and neutrophils to the thioglycollate-inflamed peritoneum. Balb/c mice were injected (IV) with 250 μg of control IgG or with L11 and then injected with thioglycollate to induce peritonitis. Recruited cells were harvested, stained with monocyte and neutrophil markers, and analyzed by flow cytometry. The total number of monocytes (A) and neutrophils (B) recruited to the peritoneal cavity at 4, 24, and 72 hours was determined. Peripheral blood was collected at each time point, stained as in (A) and (B) and the total number of leukocytes, as well as the relative number of monocytes (C) and neutrophils (D) was determined. Data shown are the mean and standard error of the total number of cells per animal found in three animals per condition in each of three independent experiments (N = 3).
L11 blocks recruitment of monocytes and neutrophils to the thioglycollate-inflamed peritoneum. Balb/c mice were injected (IV) with 250 μg of control IgG or with L11 and then injected with thioglycollate to induce peritonitis. Recruited cells were harvested, stained with monocyte and neutrophil markers, and analyzed by flow cytometry. The total number of monocytes (A) and neutrophils (B) recruited to the peritoneal cavity at 4, 24, and 72 hours was determined. Peripheral blood was collected at each time point, stained as in (A) and (B) and the total number of leukocytes, as well as the relative number of monocytes (C) and neutrophils (D) was determined. Data shown are the mean and standard error of the total number of cells per animal found in three animals per condition in each of three independent experiments (N = 3).
L11 Blocks WEHI78/24 Binding to Intact Aortic Endothelium of Cholesterol-fed Rabbits
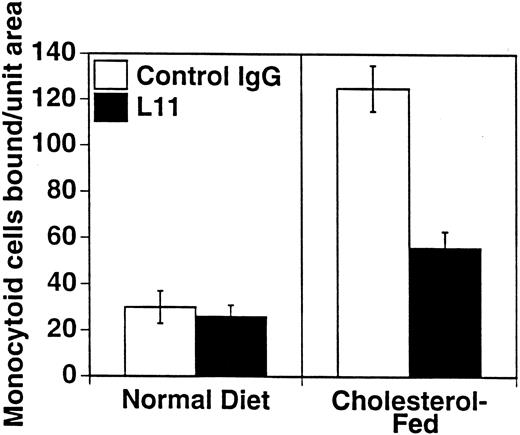
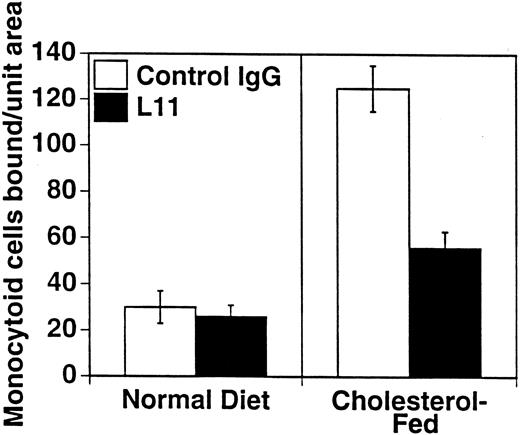
To assess the ability of anti-CD43 MoAb to modulate monocyte binding to the “dysfunctional” endothelium in atherogenesis, we used our ex vivo model of monocytoid cell interaction with the endothelium in lesion prone areas of intact aortic endothelium of cholesterol-fed rabbits. WEHI78/24 binding to aorta from hypercholesterolemic animals at RT is enhanced threefold compared with control animals, as previously described10 11 and reproduced here (Fig 4). Preincubation of the WEHI78/24 cells with L11 MoAb reduces binding to aortic endothelium by slightly more than 50% (Fig 4), suggesting that CD43 can modulate monocyte-endothelial interactions in this setting. No aggregation of WEHI78/24 cells was observed at any point in the binding assay.
L11 blocks binding of monocytoid cells to intact aortic endothelium from fat-fed rabbits. Fluorescent-labeled WEHI78/24 cells were incubated with control IgG or L11 (1 μg/106 cells) for 20 minutes, added to intact aortic segments from control and cholesterol-fed New Zealand White rabbits, and allowed to bind for 30 minutes at RT under constant rotation. Following extensive washing to remove nonadherent cells, the number of adherent cells in 15 fields (totaling 1.4 cm2 ) was determined on at least five independent segments from at least two control rabbits and three cholesterol-fed rabbits in each of three experiments. The mean and standard error of the pooled data are shown.
L11 blocks binding of monocytoid cells to intact aortic endothelium from fat-fed rabbits. Fluorescent-labeled WEHI78/24 cells were incubated with control IgG or L11 (1 μg/106 cells) for 20 minutes, added to intact aortic segments from control and cholesterol-fed New Zealand White rabbits, and allowed to bind for 30 minutes at RT under constant rotation. Following extensive washing to remove nonadherent cells, the number of adherent cells in 15 fields (totaling 1.4 cm2 ) was determined on at least five independent segments from at least two control rabbits and three cholesterol-fed rabbits in each of three experiments. The mean and standard error of the pooled data are shown.
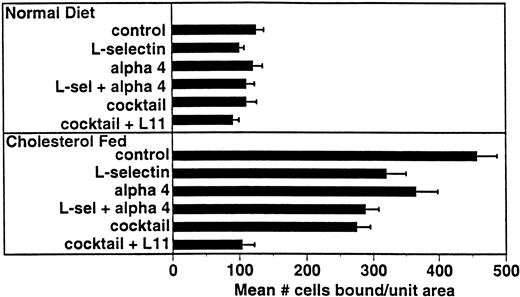
Several adhesion receptor/ligand pairs have been implicated in monocyte adhesion in models of atherogenesis including α4β1/VCAM-12 and β2 integrins/ICAM-1.25-27 For comparison with L11, we examined the involvement of these pathways in WEHI78/24 binding to intact aortic endothelium from cholesterol-fed rabbits (Fig 5). Preincubation of WEHI78/24 cells with anti–L-selectin MoAb MEL-14 blocked binding by approximately 30%, while anti-α4 MoAb PS/2 blocked binding by 20%. Together these MoAbs blocked WEHI78/24 binding 37%. Although binding was not inhibited by incubation of WEHI78/24 cells with anti-β2 integrin MoAbs M1/70 (anti-αM) or FD388 (anti-αL), alone or mixed, binding was reduced to 50% of control if anti-β2 MoAbs were added to the anti–L-selectin, anti-α4 integrin (MEL-14 and PS/2) mixture, blocking all three pathways. Addition of L11 to this cocktail blocked this residual adhesion by approximately 60% (essentially to control binding; Fig 5). These data suggest that CD43 inhibits one or more adhesive mechanisms that can act independently of or in parallel with L-selectin, α4 integrin and β2 integrins to mediate monocyte binding to preatherosclerotic endothelium in the rabbit.
L11-mediated blocking of WEHI78/24 binding to intact aortic endothelium is additive with partial blocking by anti–L-selectin and anti-α4. Adhesion assays were performed as described in Fig 3 using anti–L-selectin MoAb MEL-14, anti-α4 MoAb PS/2, a combination of these two antibodies, a “cocktail” of MEL-14, PS/2 and anti-β2 integrin MoAbs M1/70 and FD344.1 or the “cocktail” plus L11. Bound cells were counted as described for Fig 3. Shown are the mean and standard error of multiple (four to six) independent segments from two control or three cholesterol-fed rabbits from a single experiment in which all conditions were examined in parallel. Each condition (compared with control) was repeated in two to four independent experiments with similar results.
L11-mediated blocking of WEHI78/24 binding to intact aortic endothelium is additive with partial blocking by anti–L-selectin and anti-α4. Adhesion assays were performed as described in Fig 3 using anti–L-selectin MoAb MEL-14, anti-α4 MoAb PS/2, a combination of these two antibodies, a “cocktail” of MEL-14, PS/2 and anti-β2 integrin MoAbs M1/70 and FD344.1 or the “cocktail” plus L11. Bound cells were counted as described for Fig 3. Shown are the mean and standard error of multiple (four to six) independent segments from two control or three cholesterol-fed rabbits from a single experiment in which all conditions were examined in parallel. Each condition (compared with control) was repeated in two to four independent experiments with similar results.
L11 Does Not Influence WEHI78/24 Binding to Vascular Selectins or ICAM-1
The additive inhibition by L11 and anti–L-selectin, α4 and β2 MoAbs suggests that L11 acts independent of these adhesion pathways. This observation is consistent with our previous studies6 in which L11 failed to inhibit lymphocyte binding to purified vascular ligands for L-selectin (PNAd), α4β1 (VCAM-1) and α4β7 (MAdCAM-1) or αL (ICAM-1). To ask if L11 inhibits monocyte recognition of vascular selectins, we assayed WEHI78/24 binding to E and P-selectin transfected Chinese hamster ovary (CHO) cells.17 Anti–E-selectin MoAb17 blocked WEHI78/24 binding to E-selectin (but not P-selectin) transfectants by 72% ± 6% and anti–P-selectin MoAb17 block binding to P-selectin (but not to E-selectin) transfectants by 82% ± 9%, MoAb L11 had no effect on adhesion to either E or P-selectin transfectants (94% ± 4% and 108% ± 4% of control MoAb binding, respectively; P > .1). Although controversial, it has been suggested that CD43 is a ligand for ICAM-1.28 Accordingly, we assessed the binding of WEHI78/24 cells to purified ICAM-1 and found that blocking MoAbs against αL and αM reduce binding by 97% suggesting that these β2 integrins are the dominant ligands for ICAM-1 and that CD43 is not sufficient to mediate adhesion of WEHI78/24 cells to ICAM-1. Furthermore, the minimal residual binding observed in the presence of anti-αL and αM (<4% of control binding) is not blocked by addition of L11 MoAb. Together these data suggest that L11 blocks via a novel mechanism.
DISCUSSION
Monocytes are often described as the orchestrators of the inflammatory response and clearly play a key role in multiple settings of acute and chronic inflammation. Monocyte recruitment in inflammation and atherogenesis is thought to be mediated by local changes in endothelial adhesiveness for monocytes, as well as by local generation of monocyte chemoattractants such as IP-1029 and monocyte chemoattractant protein-1 (MCP-1)30 and members of the GRO family of chemoattractants.31 The multifactorial nature of events leading to leukocyte emigration, both in the normal recirculation of lymphocytes in immune surveillance and the extravasation of granulocytes, monocytes, and lymphocyte effector cells in inflammation, led to the multistep model of leukocyte recruitment.31-35 In vitro studies suggest that this multistep model of leukocyte-EC adhesion is relevant for monocyte interactions as well; the interaction of human monocytes under flow with interleukin-4 (IL-4)–stimulated human umbilical vein endothelial cells requires sequential contributions of L-selectin, β1 and β2 integrin adhesion events.32 Thus, the adhesion molecules involved in monocyte adhesion have been described in some models; however, studies in many other models indicate that important monocyte adhesion receptors and/or regulatory pathways remain to be identified.1-3
We have defined an additional molecule, CD43, with the potential to modulate monocyte-endothelial recognition in inflammation and atherogenesis. CD43 is an abundant leukocyte surface sialomucin expressed on B and T lymphocytes, monocytes, neutrophils, and platelets.33 Early observations of defective CD43 expression by T lymphocytes from many patients with Wiskott-Aldrich syndrome,34 an X chromosome-linked recessive immunodeficiency disorder, focused much attention on this molecule and multiple, diverse functions have been suggested. CD43 is a member of a growing family of cell-associated mucins,35 characteristically bearing extensive O-linked glycan substitutions that exert a chain-stiffening effect because of steric interactions of the highly charged O-glycans.35 In fact, electron microscopy analysis has shown that the extracellular region of CD43 has an extended filamentous shape with an average length of 45 nm,36 surpassing the typical glycocalyx in the plasma membrane. This extended structure and negative charge has led to the hypothesis that these molecules may provide a repulsive barrier around a cell that limits nonspecific adhesive interactions.37,38 Evidence for such an antiadhesive function was provided by transfecting CD43 into HeLa cells, which diminished their adhesion to T-lymphocyte adhesion mediated by αLβ2 (leukocyte function antigen-1 [LFA-1]) and ICAM-1. In addition, the targeted disruption of CD43 genes in a T-lymphocyte line37,38 resulted in enhancement of T-lymphocyte adhesiveness. Furthermore, phorbol myristate acetate (PMA)-treated thymocytes from CD43 knock-out mice showed substantially increased homotypic adhesion compared with thymocytes from wild-type littermates, and anti–CD3-stimulated binding of CD43-deficient splenic T cells to fibronectin or ICAM-1 was enhanced, as well.39 These investigators favor the hypothesis that the gross negative charge and extended structure of CD43 normally serves to retard T-cell interactions, which might, in turn, impede cell activation, and that lack of CD43 allows interaction to occur.
In contrast, other investigators have shown that CD43 has proadhesive properties. Numerous studies have shown that certain anti-CD43 MoAbs induce homotypic cell adhesion of different hematopoietic cells.40-44 In addition, others have shown that certain anti-CD43 MoAbs exert a modest costimulatory effect on T cells and several are capable of directly stimulating T-lymphocyte proliferation.40,41,45 Sanchez-Mateos et al46 have suggested that CD43 has a regulatory role on both integrin-mediated T-cell adhesion and cellular morphology. They have found that CD43 may function as a positive signaling molecule for some specific adhesion receptors such as β1 and β2 integrins, but not for E-selectin. Thus, it is possible that a physiologic ligand, mimicked by anti-CD43 MoAbs, may transduce activation signals to specific adhesion receptors. In fact, CD43 has a highly conserved cytoplasmic tail,47 which appears to be required for CD43-mediated enhancement of antigen-specific T-cell activation,42 and cross-linking of CD43 by specific MoAbs can result in generation of certain second messengers, calcium mobilization, activation of second protein kinase C, and induction of homotypic adhesion40,41,43,48 49 further supporting an active, potentially signaling role for CD43 in T-cell activation.
Here we have assessed the effects of anti-CD43 antibody on monocyte-EC recognition, and we report that anti-CD43 MoAb L11 can modulate monocyte binding to activated endothelium ex vivo, as well as interfering with recruitment of monocytes and of neutrophils during inflammation in vivo. In contrast to the reported proadhesive effects described for other anti-CD43 MoAbs, however, L11 substantially inhibits monocyte-EC interactions. We have recently shown that L11 also inhibits T cell/endothelial cell interactions in a number of normal and inflammatory settings6 and that inhibition requires cross-linking.50 Together these data suggest that CD43 activity reflects a common pathway capable of modulating leukocyte adhesion and trafficking.
As mentioned above, unlike lymphocytes and neutrophils, monocytes do not constitutively bind in appreciable numbers to lymph node HEV. They do, however, bind to HEV in complete Freund's adjuvant-inflamed lymph nodes in vivo and in the Stamper-Woodruff frozen section assay ex vivo.4,5 The alteration in HEV adhesiveness, as assessed in the frozen section assay in lymph nodes taken 3 days after CFA injection into the footpad, is selective for monocytes — no change in constitutive lymphocyte binding or in low levels of neutrophil binding is observed. The adhesion involves several molecules: binding of 2-day thioglycollate-elicited peritoneal exudate mononuclear cells (PEMC) to HEV in inflamed PLN is blocked by more than 80% by anti–L-selectin MoAb MEL-14 and by anti- αL, αM, and β2 integrin MoAbs by approximately 50%.4 As shown here, L-selectin and the β2-integrins also participate in binding of the WEHI78/24 monocytoid cells to HEV in inflamed lymph nodes. L-selectin and αLβ2 integrin support lymphocyte binding to HEV as well, and yet monocyte, but not lymphocyte, binding is dependent on inflammatory changes in the endothelium. The monocyte-selective increase in adhesion to HEV in inflamed lymph nodes could be due to induction of a monocyte-selective activator or chemoattractant, a monocyte-selective adhesion molecule, or both. Anti-CD43 MoAb L11 blocks WEHI78/24 cell binding to HEV in inflamed lymph nodes by greater than 70%, suggesting that L11 could block CD43 interaction with an upregulated (thus far unidentified) CD43 ligand in inflamed PLN or could potentially interfere with activating steps involved in HEV recognition. It is unlikely that L11 blocks L-selectin–mediated adhesion because we have previously shown that L11 also blocks lymphocyte-HEV recognition, but does not inhibit L-selectin–mediated rolling on purified PNAd.6
Monocytes and the monocytoid cell line WEHI78/24 also bind better to endothelium of aortic segments isolated from cholesterol-fed as opposed to normal rabbits.11 A combination of MoAbs capable of blocking L-selectin, α4 integrin and β2-integrin–dependent adhesion inhibited this enhanced binding by only ≈40%, suggesting that additional molecules also contribute to this interaction. Pretreatment of the WEHI78/24 cells with L11 alone blocked WEHI78/24 cell binding by more than 50%. Addition of L11 to the cocktail described above resulted in blocking of more than 75% of binding indicating that CD43 can participate in or modulate one or more adhesion mechanism that acts independently of or in parallel with L-selectin, α4 integrin and β2 integrins.
We conclude that anti-CD43 MoAb L11 blocks monocyte adhesion to activated endothelium in several ex vivo and in vivo models. A number of potential mechanisms for this function may be considered. L11 failed to block WEHI78/24 binding to P- and E-selectin indicating that the CD43-dependent adhesive activity is not mediated through interaction with these vascular selectins. In addition, L11 had no effect on WEHI78/24 binding to ICAM-1 transfectants, which appeared almost entirely β2-integrin–dependent. Together these results render it unlikely that L11 is inducing a passive barrier activity of CD43. Furthermore, inhibition of T cell50 and WEHI78/24 binding to lymph node HEV requires divalent antibody. In view of published studies showing that cross-linking by other anti-CD43 MoAbs elicits adhesion and proadhesive intracellular signals, our data suggests that CD43 may serve as a molecular switch, dynamically modulating leukocyte activation and adhesion; however, our results do not exclude involvement of a previously unidentified vascular ligand for CD43, with CD43-endothelial interaction representing an additional step in the multistep process of monocyte interactions. In summary, the present findings suggest the potential for CD43 to participate in the regulation of monocyte adhesion in atherogenesis, as well as in other settings of inflammation, and define CD43 as a novel target for experimental (and potentially for therapeutic) manipulation of monocyte traffic.
ACKNOWLEDGMENT
The authors thank June Twelves and Jean Jang for excellent technical assistance and Dr Ellen Berg for generously supplying E- and P-selectin transfectants and for comments on the manuscript.
Supported in part by Grants No. AI37319, HL48638, and GM37734 from the National Institutes of Health, Bethesda, MD. L.M.M. was a Senior Fellow of the American Heart Association, California Affiliate and the National Multiple Sclerosis Society during portions of this work. P.S.T. was the recipient of a National Service Research Award. J.P.C. was a recipient of the Vascular Academic Award from the National Heart, Lung, and Blood Institute and is an Established Investigator of the American Heart Association.
Address reprint requests to Leslie M. McEvoy, PhD, Department of Pathology, L235, Stanford University, Stanford, CA 94305.