Abstract
von Willebrand factor (vWF) is stored and released from endothelial secretory granules called Weibel-Palade (WP) bodies. Acute release can be induced by thrombin, histamine, and other mediators of thrombosis or inflammation. Their effect is thought to be mediated by an increase in intracellular free calcium ([Ca2+]i). Purine nucleotides such as adenosine triphosphate (ATP) and adenosine diphosphate (ADP) are released from platelet dense granules and from ischemic tissues and are important regulators of platelet function and vascular tone. In the present study, we investigated whether they could also induce exocytosis from cultured endothelial cells. ATP (1 to 100 μmol/L) induced a dose-related increase in vWF release, with a 2.3-fold maximal increase after 30 minutes. Similar responses were observed with ADP. ATP induced calcium mobilization from intracellular stores, an effect mimicked by 2-methylthio-ATP, a selective agonist for P2y receptors. However, 2-methylthio-ATP–induced vWF release was only 43% of the ATP response. ATP-induced vWF release was also associated with a twofold increase in cellular cyclic adenosine monophosphate (cAMP) content, and was potentiated by 3-isobutyl-1-methylxanthine ([IBMX] added to increase cAMP levels by blocking cellular phosphodiesterases) and 8-bromo-cAMP and inhibited by more than 50% by Rp-8-CPT-cAMPS, a competitive protein kinase A inhibitor. Adenosine but not 2-methylthio-ATP mimicked the ATP-induced increase in cAMP. ATP-induced vWF release was partly inhibited by adenosine deaminase, which degrades adenosine generated from ATP in the incubation medium. Adenosine (1 to 100 μmol/L) failed to induce vWF release, but potentiated the secretory response to 2-methylthio-ATP and thrombin without modifying the calcium response to these agents. Our results suggest that ATP/ADP can induce vWF release from endothelial cells via dual activation of P2y and adenosine A2 receptors. ATP/ADP-induced exocytosis could be involved in the regulation of thrombus formation and ischemia-reperfusion injuries. Further, we provide evidence that a receptor-mediated increase in cellular cAMP can potentiate the secretory response to calcium-mobilizing agents.
THE VASCULAR endothelium plays a central role in blood coagulation and the inflammatory response. Upon activation, endothelial cells acquire a procoagulant state and also become adhesive for circulating leukocytes, which allows their migration to the extravascular space. One important activation mechanism is agonist-induced exocytosis.1 By this process, preformed secretory granules fuse with the plasma membrane. These granules, called Weibel-Palade bodies (WP bodies), store and release von Willebrand factor (vWF), an adhesive glycoprotein involved in primary hemostasis.1,2 Indeed, vWF is essential for platelet adhesion to the vascular subendothelium, at least under conditions of high shear stress.3 Exocytosis of WP bodies also induces P-selectin translocation to the cell membrane. This granule membrane protein is an adhesion molecule that mediates rolling and subsequent extravasation of circulating leukocytes.4Expression of P-selectin at the cell surface is thought to play a major role in the inflammatory response4 and in neutrophil-mediated damage in ischemia-reperfusion injuries.5 6
In cultured endothelial cells, WP bodies can release their contents after stimulation with thrombin,7 histamine,8fibrin,9 complement components C5a and C5b-9,10,11 leukotrienes,12 and superoxide anions.13 The principal recognized intracellular signaling event involved in the response to these activators is a rapid increase in cytosolic free calcium ([Ca2+]i) and the consequent activation of calmodulin-dependent processes.7,14 In addition, we have recently observed that in vitro, epinephrine can induce vWF release and potentiate the secretory response to thrombin, a well-characterized calcium-mobilizing agonist. The effect of epinephrine was associated with an increase in cyclic adenosine 3′,5′ monophosphate (cAMP), providing evidence that cAMP-dependent signaling can modulate exocytosis of WP bodies.15
Extracellular purine nucleotides may play an important physiologic role in the regulation of vascular tone and platelet function.16Extracellular adenosine triphosphate (ATP) and adenosine diphosphate (ADP) are released at high concentrations from platelet dense granules.17 The main additional source is ischemic tissue. Indeed, ATP is released from perfused myocardium after only short periods of coronary occlusion.18 ATP or ADP can modulate vascular tone via regulation of the production of prostacyclin and nitric oxide from endothelial or smooth muscle cells.16 ADP is an important activator of platelet aggregation.16 Its key role in hemostasis is suggested by the bleeding tendency observed in patients with several forms of storage pool disorders, associated with impaired ADP release from platelet dense granules.19In endothelial cells, ATP or ADP binds to specific membrane receptors, which induces an increase in [Ca2+]i. Several receptor subtypes have been described and are classified according to the relative potency of ATP-related agonists.16 The P2y subtype, activated by 2-methylthio-ATP > ATP = ADP > uridine triphosphate, seems to predominate in human endothelial cells, although expression of additional subtypes has not been excluded.20Both in vivo and in vitro, ATP is rapidly converted to ADP, AMP, and adenosine through sequential reactions catalyzed by ecto-ATPases and 5′ nucleotidase.21 The biologic functions of ATP can therefore be modulated by the actions of its metabolites, particularly adenosine. Adenosine A2 receptors are expressed in endothelial and other vascular cells, and mediate the pleiotropic biologic functions of adenosine via activation of cAMP-dependent signaling pathways. They are involved in the regulation of vascular tone, endothelial permeability, and neutrophil activation.22
Since ATP is a calcium-mobilizing agent, via activation of P2y receptors, it could be predicted to induce exocytosis from endothelial cells. However, a previous report found that ATP is only a weak stimulator of vWF release from cultured human umbilical vein endothelial cells (HUVECs), in apparent contradiction to the central role of calcium in the regulation of endothelial exocytosis.23 In the present study, we investigated the role of purine nucleotides in exocytosis assessed by acute vWF release and characterized the associated intracellular signaling events.
MATERIALS AND METHODS
Materials.
RPMI 1640 medium was obtained from GIBCO-BRL (Gaithersburg, MD), and fetal calf serum (FCS) and collagenase were from Seromed (Berlin, Germany). Endothelial Cell Growth Supplement (ECGS) was from Upstate Biotechnology Inc (Lake Placid, NY). Anti-vWF antibodies were from Dako (Glostrup, Denmark). ATP, adenosine, ADP-βS, and adenosine deaminase (ADA) were from Fluka (Buchs, Switzerland). 2-Methylthio-ATP and 2-chloro-ATP were from RBI (Natick, MA). UTP, human thrombin, 3-isobutyl-1-methylxanthine (IBMX), and forskolin were from Sigma (St Louis, MO). Rp-8-CPT-cAMPS was from Biolog (Bremen, Germany). Fura-2 acetoxymethylester (fura 2-AM) was from Teflabs (Austin, TX), and ionomycin was from Calbiochem (La Jolla, CA).
Cell culture.
Primary cultures of endothelial cells (HUVECs) were obtained from individual human umbilical veins by collagenase digestion as described previously.13 They were grown in RPMI 1640 medium supplemented with 10% FCS, 90 μg/mL heparin, and 15 μg/mL ECGS. Cells were used during passages 1 to 3. The tissue culture dishes, 24-well plates (Costar, Cambridge, MA), and glass coverslips were coated with 0.1% gelatin.
Secretion studies.
Confluent monolayers of HUVECs grown in 24-well dishes were washed three times and preincubated in 1 mL Krebs-Ringer bicarbonate buffer (120 mmol/L NaCl, 4.75 mmol/L KCl, 1.2 mmol/L KH2PO4, 0.6 mmol/L MgSO4, 1.2 mmol/L CaCl2, 25 mmol/L NaHCO3, and 25 mmol/L HEPES, pH 7.4 [KRBH], supplemented with 0.1% BSA) for 10 minutes at 37°C. After a fourth wash, cells were incubated in 0.3 to 0.5 mL KRBH with the different agents. All pharmacologic agents were directly dissolved in incubation medium; only forskolin and IBMX were dissolved in dimethylsulfoxide (DMSO). The final concentration of DMSO in the incubation medium did not exceed 0.2%, a level that has no effect on vWF release.
To control for cell lysis, the activity of the cytosolic enzyme lactate dehydrogenase was measured fluorometrically as previously described.13
vWF measurements.
vWF levels were measured by enzyme-linked immunosorbent assay (ELISA) as described previously.13 A standard curve was constructed from serial dilutions of normal pooled plasma assuming a plasma concentration of 10 μg/mL. Results are usually expressed in nanograms per well per time unit. We observed considerable variation in the cellular vWF content and rate of secretion between cell batches. When necessary, the results are therefore expressed in relative values, ie, as a percentage of release from unstimulated control cells from the same cell preparation. Unless indicated otherwise, results are shown as the mean ± SEM. Statistical analysis was performed using the two-tailed, paired Student's t-test.
vWF immunoprecipitation and sodium dodecyl sulfate–agarose gel electrophoresis.
To examine the multimeric composition of released vWF, HUVECs were metabolically labeled with 50 μCi/mL [35S]-cysteine and [35S]-methionine (PromixTM; Amersham, Little Chalfont, UK) for 24 hours in methionine and cysteine–deficient RPMI 1640. The cells were then stimulated with various agonists as already described. The supernatants and labeling medium were supplemented with Tris (50 mmol/L, pH 8.0), EDTA (2 mmol/L), phenylmethylsulfonyl fluoride (1 mmol/L), iodoacetic acid (1 mmol/L), and N-methyl-maleimide (1 mmol/L). vWF was immunoprecipitated from the samples with an anti-vWF antiserum preadsorbed to protein A–Sepharose, and was resolved on a nonreducing 2% sodium dodecyl sulfate (SDS)-agarose gel as previously described.24 The gel was processed using a phosphorImager (Molecular Dynamics, Sunnyvale, CA).
Measurement of [Ca2+]i.
HUVECs grown on glass coverslips were loaded with 1 μmol/L fura 2-AM in culture medium (containing 10% FCS) for 30 to 60 minutes at 37°C. The coverslips were rinsed, incubated for 5 to 10 minutes in KRBH at room temperature, and immersed in a glass cuvette containing 1.5 mL KRBH under constant stirring. The cuvette was held at constant temperature (37°C). Fluorometric readings were performed with a Jasco (Hachiogi City, Japan) CAF-110 fluorimeter. The fluorescent excitation was 340 and 380 nm, and the emission wavelength was 490 nm. The [Ca2+]i was calculated according to the equation of Grynkiewicz et al25: [Ca2+]i = KD × B × (R − Rmin)/(Rmax − R), where R is the ratio of fura-2 fluorescence at 340 nm excitation divided by the fluorescence at 380 nm excitation, Rmax is the ratio when all fura-2 is bound to calcium, Rmin is the ratio when all fura-2 is in the free form, B is the ratio of the fluorescence of free fura-2 divided by the fluorescence of calcium-bound fura-2 with 380 nm excitation, and KD is the dissociation constant of calcium binding to fura-2 (224 nmol/L). Rmax was measured at the end of the recording after addition of ionomycin 10 μmol/L and CaCl2 to a final concentration of 3.2 mmol/L. EGTA 8 mmol/L and Tris 40 mmol/L, pH 9.0, were then added for measurement of Rmin.
cAMP measurements.
Confluent HUVECs grown in culture dishes (diameter, 35 mm) were handled for secretion studies as already described. At the end of the incubations, the cell monolayer was extracted with 0.6 mL ice-cold ethanol (70% vol/vol). After separation from proteins by centrifugation, the extract was dried in a Speedvac and reconstituted in assay buffer. cAMP levels were measured by radioimmunoassay using a commercial kit (Amersham).
RESULTS
ATP and ADP induce vWF release via the regulated secretory pathway.
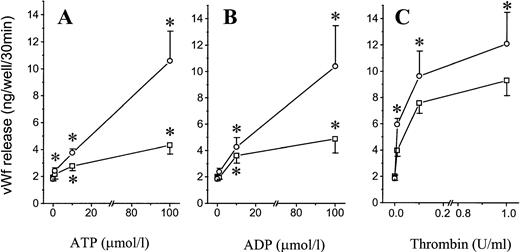
To investigate the effects of extracellular adenosine nucleotides on exocytosis from endothelial cells, we incubated confluent HUVECs with ATP, ADP, and thrombin for 30 minutes. vWF release was measured by ELISA in the supernatant (Fig 1). Weobserved a dose-related increase in vWF release, with a 2.3-fold increase (from 1.9 + 0.3 to 4.3 + 0.7 ng/well/30 min,P < .02, n = 8) with ATP and a 2.4-fold increase (from 2.0 ± 0.3 to 4.9 ± 1.1 ng/well/30 min, P = .04, n = 5) with ADP at concentrations of 100 μmol/L (Fig 1A and B). The response to ATP or ADP was smaller than the response to thrombin, which induced a 4.6-fold increase (from 2.0 ± 0.3 to 9.3 ± 1.2 ng/well/30 min, P < .02, n = 5) at a concentration of 1 U/mL (Fig 1C). Incubation with IBMX, a phosphodiesterase inhibitor added to prevent cAMP degradation, failed to induce vWF release, as previously reported.15 However, in the presence of IBMX, the response to ATP and ADP was much stronger, with a 5.9- and 5.7-fold increase, respectively (P < .05), and was detectable at nucleotide concentrations as low as 1 μmol/L. In contrast, IBMX had only a minor potentiating effect on the response to thrombin. Thus, in the presence of IBMX, the responses to ATP, ADP, and thrombin were similar (Fig 1A v C).
Effect of ATP and ADP on vWF release. Confluent HUVECs grown in 24-well plates were incubated for 30 minutes at 37°C at the indicated concentrations of ATP (A), ADP (B), and thrombin (C) in either the absence (□) or the presence (○) of IBMX (100 μmol/L). vWF release was measured in the supernatant by ELISA. Results are the mean ± SEM of 8 (A) and 5 (B and C) experiments. *P < .05v unstimulated control cells.
Effect of ATP and ADP on vWF release. Confluent HUVECs grown in 24-well plates were incubated for 30 minutes at 37°C at the indicated concentrations of ATP (A), ADP (B), and thrombin (C) in either the absence (□) or the presence (○) of IBMX (100 μmol/L). vWF release was measured in the supernatant by ELISA. Results are the mean ± SEM of 8 (A) and 5 (B and C) experiments. *P < .05v unstimulated control cells.
vWF released from WP bodies consists of high–molecular weight multimers, whereas constitutively released vWF consists predominantly of dimers and small multimers.24 We therefore determined the multimer pattern of vWF released in response to ATP (Fig 2). Confluent HUVECs were incubated for 24 hours with [35S]-labeled methionine and cysteine. After stimulation with ATP, ATP/IBMX, or thrombin for 30 minutes, released vWF was immunoprecipitated from the supernatant and resolved on a nonreducing SDS-agarose gel. vWF released from cells stimulated with ATP (± IBMX) consisted of high–molecular weight forms in a pattern similar to the one observed after stimulation with thrombin. In contrast, vWF from unstimulated cells consisted of a small amount of dimers. vWF constitutively released over 24 hours into the culture medium during the labeling period also consisted predominantly of dimers and low–molecular weight multimers. These results suggest that ATP induces vWF release from WP bodies, ie, via regulated secretion. This conclusion was confirmed by immunofluorescence experiments. ATP stimulation caused a decrease in the number of WP bodies and the appearance of extracellular deposits of vWF associated with the subendothelium, typical of regulated secretion.13 24 Again, the pattern was similar to that observed after stimulation with thrombin (data not shown).
Multimer pattern of vWF released in response to ATP. Confluent HUVECs were metabolically labeled with [35S]-cysteine and [35S]-methionine for 24 hours. vWF was immunoprecipitated from the labeling medium (medium) and from the supernatant of cells incubated for 30 minutes with ATP (100 μmol/L), ATP/IBMX (100 μmol/L), thrombin (1 U/mL), or KRBH-BSA 0.1% alone (control). vWF multimers were resolved on a horizontal nonreducing 2% SDS-agarose gel. Top arrowhead, the origin of the gel; bottom arrowhead, position of vWF dimers. vWF released constitutively into the labeling medium consists predominantly of dimers and small multimers, whereas vWF released in response to ATP and thrombin consists of high–molecular weight multimers.
Multimer pattern of vWF released in response to ATP. Confluent HUVECs were metabolically labeled with [35S]-cysteine and [35S]-methionine for 24 hours. vWF was immunoprecipitated from the labeling medium (medium) and from the supernatant of cells incubated for 30 minutes with ATP (100 μmol/L), ATP/IBMX (100 μmol/L), thrombin (1 U/mL), or KRBH-BSA 0.1% alone (control). vWF multimers were resolved on a horizontal nonreducing 2% SDS-agarose gel. Top arrowhead, the origin of the gel; bottom arrowhead, position of vWF dimers. vWF released constitutively into the labeling medium consists predominantly of dimers and small multimers, whereas vWF released in response to ATP and thrombin consists of high–molecular weight multimers.
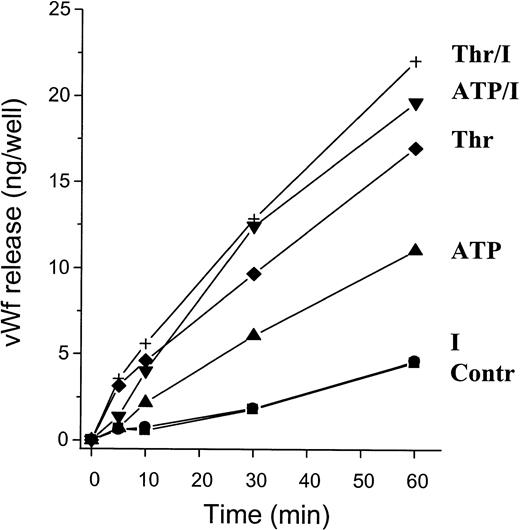
We next performed a time-course study of ATP-induced vWF release (Fig3). Release from cells stimulated with 100 μmol/L ATP was unchanged as compared with control cells after 5 minutes. However, there was a 2.9-fold increase after 10 minutes (P < .02), which continued during the 60 minutes of the study. Although the increase in vWF release was larger, addition of IBMX had no significant effect on the lag time of the ATP response. In contrast, the response to thrombin was much faster, with a 3.9-fold increase over control cells already after 5 minutes (P < .05). IBMX had no significant effect on thrombin-induced vWF release after 5 and 10 minutes, with a minor potentiating effect becoming obvious only after 30 minutes. Thus, ATP-induced vWF release (although delayed when compared with thrombin) occurs in less than 30 minutes, againsuggesting release from preformed stores.
Time course of ATP-induced vWF release. Confluent HUVECs grown in 24-well plates were incubated for the indicated times with 100 μmol/L ATP, ATP/IBMX (both 100 μmol/L, ATP/I), 1 U/mL thrombin (Thr), or 1 U/mL thrombin and 100 μmol/L IBMX (Thr/I). Control cells were incubated with KRBH-BSA 0.1% alone (Contr) or supplemented with 100 μmol/L IBMX (I). Results are the mean ± SEM of 5 experiments; error bars were omitted for the sake of clarity. The SEMs were less than 25% of the mean of all values.
Time course of ATP-induced vWF release. Confluent HUVECs grown in 24-well plates were incubated for the indicated times with 100 μmol/L ATP, ATP/IBMX (both 100 μmol/L, ATP/I), 1 U/mL thrombin (Thr), or 1 U/mL thrombin and 100 μmol/L IBMX (Thr/I). Control cells were incubated with KRBH-BSA 0.1% alone (Contr) or supplemented with 100 μmol/L IBMX (I). Results are the mean ± SEM of 5 experiments; error bars were omitted for the sake of clarity. The SEMs were less than 25% of the mean of all values.
Involvement of P2y receptor activation in vWF release.
The slower time course and the modulation by IBMX suggest that ATP induces vWF release through signaling mechanisms at least in part distinct from those elicited by thrombin. To characterize the receptor subtype(s) involved, we studied vWF release in response to receptor-specific pharmacologic agonists. Defining the secretion produced by ATP (100 μmol/L for 30 minutes) as 100%, the response to UTP was negligible (17 ± 6%, NS, n = 4), arguing against the involvement of a P2u receptor in vWF release. The response to the P2y agonists 2-chloro-ATP, MeS-ATP, and ADP-βS (all tested at 100 μmol/L) was 126% ± 16%, 43% ± 5% and 48% ± 9%, respectively (n ≥ 4, P < .05 v unstimulated controls for all three agonists). These results suggest that P2y activation elicits vWF release. However, the relative potency of the agonists, in particular the weaker responses to 2-methylthio-ATP and ADP-βS, suggest that P2y activation does not fully account for ATP-induced vWF release.
Implication of adenosine in the ATP response.
Both in vivo and in vitro, ATP is rapidly converted to AMP and adenosine through sequential reactions catalyzed by ectonucleotidases.16 21 We therefore wondered whether the effect of ATP could be mediated by adenosine (Fig 4A). Adenosine added alone had no effect, but when added together with IBMX it induced a 2.2-fold increase (P < .05) in vWF release. However, 2-methylthio-ATP and adenosine addedtogether induced a secretory response comparable to that of ATP. The synergistic effect of adenosine and 2-methylthio-ATP was observed in both the absence and presence of IBMX. This observation suggests that the response to ATP may result from activation of both P2y and adenosine receptors. This interpretation implies that adenosine is generated from ATP in the incubation buffer in concentrations sufficient to activate its receptor. We performed a dose-response study of the effect of adenosine on 2-methylthio-ATP–induced vWF release (Fig 4B). In the absence of IBMX, we observed a maximal response to adenosine at 10 μmol/L, with a significant effect already seen at 1 μmol/L. The dose-response profile was similar in the presence of IBMX, except for a larger potentiating effect at high adenosine concentrations (100 μmol/L). Thus, adenosine produced by conversion of less than 10% of the added ATP is sufficient to potentiate the secretory response to activation of P2y receptors.
Synergistic effect of adenosine and 2-methylthio-ATP on vWF release. (A) Confluent HUVECs were incubated for 30 minutes at 37°C in the presence of ATP adenosine (Ado), and 2-methylthio-ATP (MeS) (all agonists tested at 100 μmol/L) in the presence or absence of 100 μmol/L IBMX. Results are the mean ± SEM of 5 experiments. Because of large variations in vWF release in this series, the results are shown as relative values, with release at 30 minutes from unstimulated cells defined as 100%. The effects of MeS and Ado alone were small, but the response to the combination of Mes + Ado was similar to or greater than the response to ATP. *P < .02v control (C) cells; **P < .02 v control cells and cells treated with Ado or MeS alone. (B) Adenosine-induced vWF release: dose-response in the presence of IBMX (100 μmol/L), MeS (100 μmol/L), and IBMX + MeS. Each combination was added to confluent HUVECs for 30 minutes at 37°C. Results are the mean ± SEM of 5 experiments. *P < .002 v control cells not stimulated with adenosine.
Synergistic effect of adenosine and 2-methylthio-ATP on vWF release. (A) Confluent HUVECs were incubated for 30 minutes at 37°C in the presence of ATP adenosine (Ado), and 2-methylthio-ATP (MeS) (all agonists tested at 100 μmol/L) in the presence or absence of 100 μmol/L IBMX. Results are the mean ± SEM of 5 experiments. Because of large variations in vWF release in this series, the results are shown as relative values, with release at 30 minutes from unstimulated cells defined as 100%. The effects of MeS and Ado alone were small, but the response to the combination of Mes + Ado was similar to or greater than the response to ATP. *P < .02v control (C) cells; **P < .02 v control cells and cells treated with Ado or MeS alone. (B) Adenosine-induced vWF release: dose-response in the presence of IBMX (100 μmol/L), MeS (100 μmol/L), and IBMX + MeS. Each combination was added to confluent HUVECs for 30 minutes at 37°C. Results are the mean ± SEM of 5 experiments. *P < .002 v control cells not stimulated with adenosine.
To confirm this contention, we added ADA to the cells to degrade any adenosine produced during the incubation with ATP (Fig 5). ADA (1 U/mL) inhibited the ATP response by 23% ± 3% and 52% ± 3% in the presence and absence of IBMX, respectively (P < .001, n = 8). Higher concentrations of ADA failed to achieve greater inhibition. The secretory response could be reconstituted by addition of 2-chloro-adenosine, an adenosine analog resistant to ADA. These results confirm that ATP-induced vWF release is mediated in part by adenosine, most likely generated by partial degradation of the added ATP. The inhibition by ADA and rescue by 2-chloro-adenosine suggest that adenosine itself, rather than inosine or other adenosine degradation products, is involved. Indeed, when adenosine and 2-chloro-adenosine were tested in parallel, together with IBMX and/or 2-methylthio-ATP, the effect of 2-chloro-adenosine was always equal to or stronger than the effect of adenosine itself (not shown).
ATP-induced vWF release: Effect of adenosine removal by ADA. Confluent HUVECs were incubated with ADA (1 U/mL) ± 2-chloro-adenosine (2Cl, 10 μmol/L) as indicated; 3 minutes later, 100 μmol/L ATP was added where indicated, and the incubation continued for 30 minutes at 37°C. The experiment was performed in the absence or presence of 100 μmol/L IBMX. Data are the mean ± SEM of 5 to 8 experiments. *P < .05.
ATP-induced vWF release: Effect of adenosine removal by ADA. Confluent HUVECs were incubated with ADA (1 U/mL) ± 2-chloro-adenosine (2Cl, 10 μmol/L) as indicated; 3 minutes later, 100 μmol/L ATP was added where indicated, and the incubation continued for 30 minutes at 37°C. The experiment was performed in the absence or presence of 100 μmol/L IBMX. Data are the mean ± SEM of 5 to 8 experiments. *P < .05.
Involvement of cAMP in ATP-induced vWF release.
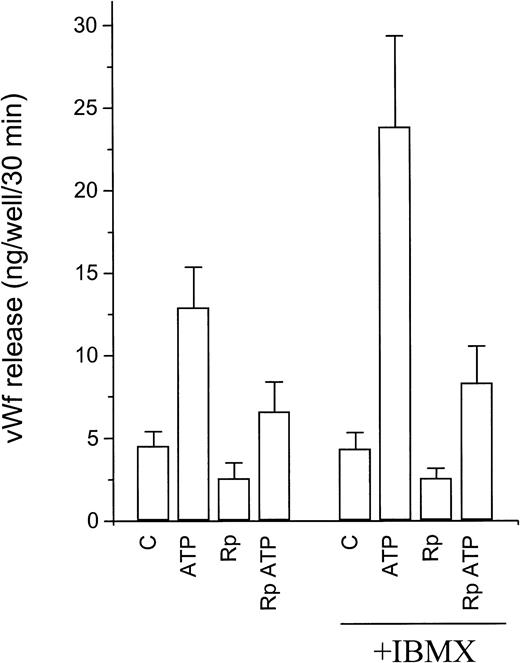
Activation of P2y receptors by ATP or ADP is known to be associated with an increase in [Ca2+]i due to calcium mobilization from intracellular stores.20 Numerous reports have convincingly demonstrated the key role of cytosolic calcium in the regulation of vWF release from WP bodies. However, the relative efficacy of ATP and 2-methylthio-ATP, ADP-βS, and 2-chloro-ATP indicates that the activation of P2y receptors is a poor stimulus for vWF release and suggests the participation of additional messenger systems. We tested the involvement of the cAMP signaling pathway in ATP-induced vWF release using Rp-8-CPT-cAMPS, a competitive inhibitor of protein kinase A (Fig 6). In the presence of Rp-8-CPT-cAMPS (500 μmol/L), basal vWF release was inhibited by 47% ± 11% (P < .05, n = 4). Even when the lower basal release was taken into account, ATP-induced vWF release was inhibited by 49% ± 7% and the response to ATP/IBMX was inhibited by 71% ± 7% (P < .05, n = 4). vWF release in response to forskolin, used as a control activator of the cAMP pathway, was reduced by greater than 50% (not shown). Lower concentrations of the inhibitor were ineffective. The related inhibitors Rp-cAMPS and Rp-MB-cAMPS had only weak inhibitory effects on the response to either ATP or forskolin, in keeping with their lower lipophilicity.
Effect of protein kinase A inhibition on ATP-induced vWf release. Confluent HUVECs grown on 24-well plates were incubated for 30 minutes in the presence or absence of Rp-8-CPT-cAMPS (Rp). ATP and/or IBMX were then added, and the incubation continued for 30 additional minutes. Results are expressed as vWf released per well during the last 30 minutes. Basal release was calculated as half the vWF released during the 1-hour incubation (assuming continuous constitutive release over 1 hour, see Fig 2). See text for statistical analysis.
Effect of protein kinase A inhibition on ATP-induced vWf release. Confluent HUVECs grown on 24-well plates were incubated for 30 minutes in the presence or absence of Rp-8-CPT-cAMPS (Rp). ATP and/or IBMX were then added, and the incubation continued for 30 additional minutes. Results are expressed as vWf released per well during the last 30 minutes. Basal release was calculated as half the vWF released during the 1-hour incubation (assuming continuous constitutive release over 1 hour, see Fig 2). See text for statistical analysis.
To confirm the involvement of cAMP in vWF release, we tested the effect of 8-bromo-cAMP, a cell-permeant cAMP analog. Cultured HUVECS were incubated for 40 minutes with 8-bromo-cAMP (1 mmol/L), and additional agonists were added after the first 20 minutes. In the presence of 8-bromo-cAMP (1 mmol/L) added alone, vWF release increased from 1.5 ± 0.2 to 1.9 ± 0.3 ng/well/20 min (nonsignificant,P = .14, n = 5). However, vWF release in response to ATP increased from 4.4 ± 0.8 to 10.0 ± 2.4 ng/well/20 min (+308%), to 2-methylthio-ATP from 3.1 ± 0.5 to 6.4 ± 1.5 ng/well/20 min (+344%), and to thrombin from 7.3 ± 1.2 to 12.8 ± 2.3 ng/well/20 min (+196%) (P < .01 for all three comparisons). Thus, 8-bromo-cAMP can potentiate the secretory response to several calcium-mobilizing agonists.
To better define the involvement of cAMP in ATP-induced vWF release, in particular to identify the receptor involved, we measured cellular cAMP content by radioimmunoassay (Table 1). ATP (100 μmol/L) added for 30 minutes caused a 2.0-fold increase in cAMP content (from 1.10 ± 0.19 to 2.23 ± 0.55 pmol/plate,P < .05, n = 5). Incubation with IBMX caused a 1.7-fold increase in cAMP content (P < .05, n = 5), while ATP added together with IBMX caused a 3.1-fold increase. This observation confirms that the potentiation of IBMX on ATP-induced vWF release is due to its effect on cAMP levels. The effect of ATP on cAMP content was mimicked by stimulation with adenosine (P < .05) but not with 2-methylthio-ATP (P = .19) (both agents tested at 100 μmol/L). Similar results were observed in the presence of IBMX (P = .01 and .07, respectively). These results indicate that the cAMP-mediated potentiation of ATP-induced vWF release involves activation of adenosine receptors. The effect of adenosine or ATP on cellular cAMP content is equal to if not greater than that of epinephrine, a known receptor-mediated activator of adenylate cyclase. cAMP levels are minimally increased by 2-methylthio-ATP and thrombin, and were only marginally increased in response to thrombin. It is therefore highly unlikely that the effect of these calcium-mobilizing agents directly implicates activation of a calcium-sensitive adenylate cyclase.
Effect of ATP and adenosine on [Ca2+]i.
P2y receptors are seven-transmembrane domain receptors that induce IP3-mediated calcium mobilization from intracellular stores.16,20 To confirm the involvement of P2y receptors in ATP-induced vWF release, we performed measurements of [Ca2+]i using the fluorescent calcium-sensitive probe fura-2. HUVECs were grown on glass coverslips, loaded with fura-2, immersed in a cuvette, and stimulated with ATP, adenosine and MeS-ATP, and thrombin (Fig 7). Thrombin induced a rapid but transient increase in [Ca2+]i (with a peak elevation from 79 ± 20 to 399 ± 43 nmol/L, n = 3), followed by a second phase of more stable elevation, with a mean value of 130 ± 25 nmol/L after 3 minutes. ATP caused a similar but smaller initial elevation (from 58 ± 16 to 279 ± 34 nmol/L), but with a maximal amplitude that was only 70% of that observed after thrombin. [Ca2+]i values returned rapidly to near baseline (79 ± 20 nmol/L after 3 minutes; Fig 7A). IBMX failed to alter the [Ca2+]i profile in response to ATP (not shown). An almost identical profile was observed in response to MeS-ATP (100 μmol/L), with a peak elevation from 83 ± 21 to 263 ± 3 nmol/L (n = 3; Fig 7B). These results are compatible with P2y receptor–mediated mobilization of calcium from an intracellular store, as reported in detail previously.20 Adenosine had no effect on [Ca2+]i even in the presence of IBMX (Fig7C). The [Ca2+]i response to 2-methylthio-ATP was not modified by preincubation with adenosine and IBMX as compared with 2-methylthio-ATP alone (maximal elevation from 68 ± 2 to 268 ± 60 nmol/L, n = 3; Fig 7C). This last observation was confirmed with longer recordings of up to 10 minutes (not shown). These results strongly suggest that an increase in cAMP due to exposure to IBMX and/or adenosine does not potentiate the calcium response to P2y receptor activation, ie, cAMP modulates ATP-induced vWF release at a more distal step than calcium mobilization.
Effect of ATP and adenosine on [Ca2+]i. HUVECs were grown on gelatin-coated glass coverslips, loaded with fura-2, and immersed in a quartz cuvette for radiometric fluorescent emission measurements. Each tracing shown is representative of at least 3 similar experiments. (A) Stimulation with 100 μmol/L ATP was followed by 0.5 U/mL thrombin (Thr). The [Ca2+]i response to thrombin looks virtually identical when tested alone, and is not modified by prior stimulation with ATP (not shown). (B and C) The response to 2-MeS-ATP was similar to the ATP response (B), and was not modified by preincubation with IBMX and adenosine (Ado), both tested at 100 μmol/L (C).
Effect of ATP and adenosine on [Ca2+]i. HUVECs were grown on gelatin-coated glass coverslips, loaded with fura-2, and immersed in a quartz cuvette for radiometric fluorescent emission measurements. Each tracing shown is representative of at least 3 similar experiments. (A) Stimulation with 100 μmol/L ATP was followed by 0.5 U/mL thrombin (Thr). The [Ca2+]i response to thrombin looks virtually identical when tested alone, and is not modified by prior stimulation with ATP (not shown). (B and C) The response to 2-MeS-ATP was similar to the ATP response (B), and was not modified by preincubation with IBMX and adenosine (Ado), both tested at 100 μmol/L (C).
Adenosine potentiates the secretory response to thrombin.
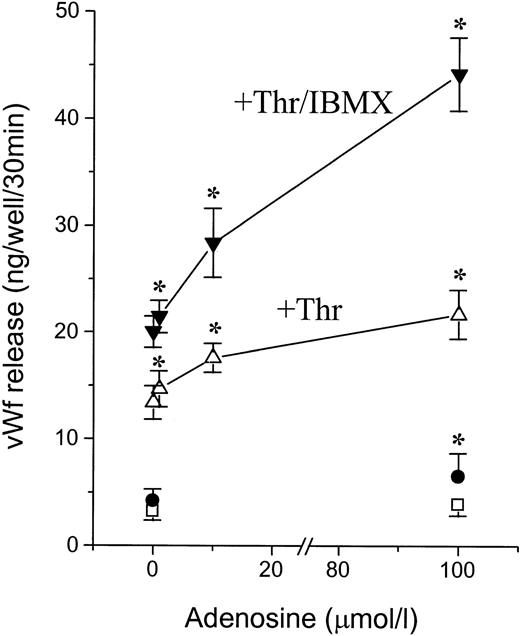
Adenosine may potentiate the secretory response not only to ATP but to other calcium-mobilizing agents. We therefore tested the effect of adenosine on thrombin-induced vWF release (Fig 8). Adenosine potentiated the response to thrombin whether added alone or together with IBMX. A significant potentiating effect occurred at adenosine concentrations of 1 to 100 μmol/L both alone and in the presence of IBMX, with a profile similar to that observed for MeS-ATP (Fig 4B).
Potentiating effect of adenosine on thrombin-induced vWF release. Confluent HUVECs were incubated for 30 minutes at 37°C with adenosine at the indicated concentrations alone (□) or together with 100 μmol/L IBMX (•), 1 U/mL thrombin (Thr, ▵), or Thr/IBMX (▾). Results are the mean ± SEM of four experiments. *P < .05v corresponding control without adenosine.
Potentiating effect of adenosine on thrombin-induced vWF release. Confluent HUVECs were incubated for 30 minutes at 37°C with adenosine at the indicated concentrations alone (□) or together with 100 μmol/L IBMX (•), 1 U/mL thrombin (Thr, ▵), or Thr/IBMX (▾). Results are the mean ± SEM of four experiments. *P < .05v corresponding control without adenosine.
DISCUSSION
Our results demonstrate that ATP and ADP induce vWF release from cultured HUVECs. Although the secretory response to ATP is slower than the response to thrombin, it occurs within minutes, suggesting that vWF secretion is due to release from preformed stores, ie, WP bodies, rather than to increased biosynthesis and constitutive release. This conclusion is borne out by the observation that ATP-induced vWF release consists of high–molecular weight multimers, typical of vWF released from WP bodies24 (Fig 2). Moreover, ATP causes a decrease in the number of WP bodies as observed by indirect immunofluorescence (not shown).
ATP activates P2y receptors, since it induces a rapid and transient increase in [Ca2+]i that is mimicked by the P2y-specific analog 2-methylthio-ATP. However, activation of P2y receptors appears to be a weak stimulus for vWF release, as indicated by the weaker secretory response to 2-methylthio-ATP and ADP-βS than to ATP. In addition, our results demonstrate that in vitro coactivation by adenosine is required for ATP-induced vWF release, a potentiating effect that is mediated by an increase in cellular cAMP content. The increase of cAMP content in response to adenosine in conjunction with the absence of an effect on [Ca2+]i indicate that endothelial cells express adenosine A2 receptors, in agreement with previous reports.16,21 ATP-induced vWF release is partially inhibited by ADA, indicating that its effect on cAMP depends on partial conversion to adenosine in the incubation medium. The adenosine-induced cAMP formation and protein kinase A activation are essential for the secretory response, as suggested by the inhibitory effect of the competitive PKA inhibitor Rp-8-CPT-cAMPS. Thus, adenosine, via a cAMP-dependent mechanism, is a weak agonist for vWF release but potentiates the response to ATP (via P2y activation). The potentiating effect of adenosine is not restricted to ATP, since it was also observed when added to thrombin, another well-characterized calcium-mobilizing agent. We have previously shown that in a similar way, epinephrine is only a weak secretagogue but a potentiator of thrombin-induced vWF release, an effect mediated by adenylate cyclase–coupled β-adrenergic receptors.15 Taken together, these observations suggest that a receptor-mediated increase in cAMP causes potentiation rather than initiation of exocytosis evoked by calcium-mobilizing agonists.
Exocytosis from cultured endothelial cells has been associated with an increase in [Ca2+]i, as observed in the secretory response to thrombin and histamine.7,8 Incubation with the calcium ionophore A23187 is sufficient to induce a near-maximal secretory response.26 Further, exocytosis stimulated by thrombin is suppressed by pretreatment with the intracellular calcium chelator MAPTA-AM (although only weakly by removal of extracellular calcium).7 These reports strongly suggest that exocytosis is dependent on calcium mobilization from intracellular stores.7,14 We were therefore surprised by the weak secretory effect of the P2y activators 2-methylthio-ATP and ADP-βS. The maximal secretory response to 2-chloro-ATP was stronger, but still only 55% of the thrombin response. (Even this effect of 2-chloro-ATP may be due to partial conversion to the potent adenosine analog 2-chloro-adenosine). The increase in [Ca2+]i in response to ATP and 2-methylthio-ATP was smaller and shorter than the response to thrombin (Fig 7). In particular, the second phase of elevation in [Ca2+]i, known to be secondary to calcium influx, was weak or absent in response to ATP or 2-methylthio-ATP. However, the plateau phase is thought to play a minor role in vWF release. Removal of extracellular calcium (by incubation with EGTA) abolishes the plateau phase and shortens the initial [Ca2+]i peak seen in response to thrombin and superoxide anions, without a significant inhibition of vWF release.13 14 The differences in the [Ca2+]i response therefore appear unlikely to explain the large discrepancy between ATP (and 2-methylthio-ATP) and thrombin in terms of vWF release. Thrombin induces only a weak cAMP response even in the presence of IBMX. Note also that the secretory response to either ATP or thrombin is hardly modified by IBMX at early time points; indeed, the full potentiating effect of IBMX is seen only by 30 minutes (Fig 3). It is therefore unlikely that the greater initial effect of thrombin is due to coactivation of cAMP formation. These observations suggest that in addition to calcium and cAMP, a third, unidentified signaling pathway may be involved in the secretory response to thrombin. A role for coactivators of vWF release may come as a surprise, given the well-established effect of [Ca2+]i on exocytosis, in particular in response to calcium ionophores. However, calcium ionophores induce massive and prolonged increases in [Ca2+]ithat are not achieved by receptor-mediated agonists.
cAMP is likely to be involved in the regulation of exocytosis, judging from the potentiating effect of the cell-permeant analog 8-bromo-cAMP on vWF release induced by ATP, 2-methylthio-ATP, and thrombin. The actions of adenosine and epinephrine on vWF release are associated with an increase in cellular cAMP, and the effect of adenosine is inhibited by Rp-8-CPT-cAMPS. The action of this competitive protein kinase A inhibitor provides strong evidence that a receptor-mediated increase in cAMP can stimulate exocytosis from cultured endothelial cells. In contrast, an increase in platelet cAMP in response to diverse agents such as adenosine, prostacyclin, and even nitric oxide (via inhibition of phosphodiesterases) results in inhibition of platelet secretion.17,27,28 From a physiologic point of view, it is surprising that a single second messenger, sometimes in response to the same hormone, activates endothelial cells and inhibits platelet aggregation. However, activation of exocytosis by cAMP alone and/or in synergism with intracellular calcium should not be surprising, since it has been observed in many secretory cell systems such as insulin-secreting β cells,29 the exocrine pancreas,30 and others. The mechanism of this cross-talk remains poorly understood at the molecular level, whether in endothelial cells or other secretory cells. An effect of cAMP on calcium mobilization is highly unlikely, since neither adenosine nor IBMX affected the calcium responses to ATP (Fig 7) or to thrombin (not shown). It is worth emphasizing that the potentiating effect of cAMP on calcium-mediated vWF release (in response to thrombin, ATP, and 2-methylthio-ATP) is not restricted to adenosine, but also occurs after stimulation with epinephrine15 and presumably with other cAMP-raising agonists, such as prostacyclin and other agents. This conclusion is compatible with rat hindlimb perfusion studies showing a potentiating effect of forskolin on vWF secretion induced by the calcium-mobilizing agents bradykinin and platelet-activating factor.31
vWF mediates platelet aggregation, in conjunction with fibrinogen, as well as platelet adhesion to the subendothelium. Platelet adhesion is mediated by the interaction of vWF with the platelet membrane protein complex GPIb-IX, which is enhanced at high shear stress.1-3vWF is therefore considered particularly important in the pathogenesis of arterial thrombosis. Although circulating vWF also plays a role, vWF released from endothelial cells and trapped in the subendothelium is particularly efficient in this process.3,32 During platelet activation, ATP and ADP are released from dense granules, where they are stored in high concentrations (<400 mmol/L).17Further, ATP and ADP are released from erythrocytes. This release may occur even in the absence of hemolysis and is enhanced under conditions of high shear stress.33 Our results suggest that ATP/ADP released from activated platelets or erythrocytes may induce vWF release from nearby endothelial cells. Thus, extracellular ATP/ADP could facilitate the process of thrombus formation, or at least mediate its extension from the initial site of vascular injury, via a dual-activating effect on platelet activation and endothelial exocytosis. Endothelial cells express an ATP/ADPase, recently identified as CD39, which is thought to play a major role in the regulation of thrombus formation.21,34 This enzyme may not only protect platelets from activation by ADP, but also control endothelial release of vWF and surface exposure of P-selectin by regulating the balance between ADP production and breakdown. Note that a role for P-selectin in thrombus formation has recently been described,35 although the relative importance of endothelial and platelet P-selectin has not yet been delineated.
Our results are at variance with an earlier study showing potent effects of ATP, ADP, and AMP on vWF release from cultured HUVECs at low, submicromolar concentrations.36 These low concentrations are hard to reconcile with the reported dose-response studies for endothelial purinergic (P2y) receptors.20 The response to ADP and AMP was observed after a 30-minute lag time. This suggests that part of the response observed may be a late effect of adenosine produced from ADP degradation rather than a result of activation of ADP/ATP receptors.
Exocytosis from WP bodies causes translocation of P-selectin from the granule membrane to the cell surface.4 This surface expression initiates leukocyte rolling and subsequent extravasation. Ischemia-reperfusion injuries are associated with tissue infiltration by neutrophils, which is at least in part inhibited by neutralization of P-selectin with blocking monoclonal antibodies.5,6 One group of mediators of P-selectin activation (via exocytosis from WP bodies) are oxygen free radicals, in particular superoxide anions generated during the reperfusion phase.13,37 In addition, Pinsky et al38 have identified hypoxia per se as an additional stimulus for WP body exocytosis, which cannot be accounted for by oxygen free-radical formation. ATP is released from the ischemic myocardium after only short periods of coronary occlusion, ie, before actual cell necrosis.18 Adenosine is also generated during ischemia as a result of both ATP degradation and direct release from ischemic cells.16 Our observation that ATP, in conjunction with adenosine, induces exocytosis from endothelial cells raises the possibility that ATP released from ischemic tissues is a mediator of P-selectin activation and subsequent neutrophil infiltration. At first sight, it is unlikely that ATP is released in concentrations sufficient to overcome degradation by endothelial ADPase. However, tissue concentrations of ATP released from ischemic cells have not been determined. Tissue-derived ATP production may be significantly underestimated in the content of venous effluent, since the washout may be incomplete.18 There could also be differences in the effect of ADPase on tissue-derived (ie, basolateral) as opposed to luminal ATP/ADP.
In cultured endothelial cells, extracellular ATP is an agonist for endothelial exocytosis that induces vWF release and, by inference, increases surface expression of P-selectin. ATP-induced exocytosis may be involved in thrombus formation, and also in ischemia-induced neutrophil infiltration and tissue damage. The effect of ATP is mediated by dual activation of ATP (P2y) and adenosine (A2) receptors. At the intracellular level, an increase in both [Ca2+]i and cAMP is required for ATP-induced vWF release. cAMP potentiates the secretory response to calcium-mobilizing agents. Future studies will clarify whether additional agents/hormones known to activate adenylate cyclase are also involved in the regulation of endothelial exocytosis.
ACKNOWLEDGMENT
We thank Nicole Aebischer for technical assistance, and Werner Schlegel for insightful advice.
Supported by Grants No. 32-41941.94 (U.M.V.) and 32-32376.91 (C.B.W.) and a SCORE subsidy (U.M.V.) from the Swiss National Science Foundation.
Address reprint requests to Ulrich M. Vischer, MD, Division de Biochimie Clinique, CMU, 1 rue Michel Servet, 1211 Geneva 4, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 2. Multimer pattern of vWF released in response to ATP. Confluent HUVECs were metabolically labeled with [35S]-cysteine and [35S]-methionine for 24 hours. vWF was immunoprecipitated from the labeling medium (medium) and from the supernatant of cells incubated for 30 minutes with ATP (100 μmol/L), ATP/IBMX (100 μmol/L), thrombin (1 U/mL), or KRBH-BSA 0.1% alone (control). vWF multimers were resolved on a horizontal nonreducing 2% SDS-agarose gel. Top arrowhead, the origin of the gel; bottom arrowhead, position of vWF dimers. vWF released constitutively into the labeling medium consists predominantly of dimers and small multimers, whereas vWF released in response to ATP and thrombin consists of high–molecular weight multimers.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.118/3/m_blod4011502.jpeg?Expires=1767856190&Signature=Fdf7V4WrYY0r7DAb04gcwBucRMw-eKpQZXvrilhBUvX~XXpCbZVCdbfOfFmm-ltYTmaT3DpO62ngukcuau~fkGRi9Yu3TbuYCLEvBYVISMuFMvXfpRwDC9vE30Qf5xb2uhaHMNRg5r8MQmnkgazHfEmokM1No0sDxKIggL6b2~gx3eR0jSuiAUvWGu~LEvSkFC6xK4x0QaaiBwnmw1hWzV7jeMR~HNeP1KcP5-RWf0eei2yLQTdSRs3dBbKsZ-v~2tItzcc7-t-feKHN0YEuvwjGRpmT8Iq9gUUym3FGlfZJv51WA8urxNgugPHb23igTG1rTLFeq2nWjM~gMUE5Cw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 7. Effect of ATP and adenosine on [Ca2+]i. HUVECs were grown on gelatin-coated glass coverslips, loaded with fura-2, and immersed in a quartz cuvette for radiometric fluorescent emission measurements. Each tracing shown is representative of at least 3 similar experiments. (A) Stimulation with 100 μmol/L ATP was followed by 0.5 U/mL thrombin (Thr). The [Ca2+]i response to thrombin looks virtually identical when tested alone, and is not modified by prior stimulation with ATP (not shown). (B and C) The response to 2-MeS-ATP was similar to the ATP response (B), and was not modified by preincubation with IBMX and adenosine (Ado), both tested at 100 μmol/L (C).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.118/3/m_blod4011507.jpeg?Expires=1767856190&Signature=wV0B62QLArNnFknmxK0zuCVZmmNpFVd1beE6-I3xDYn~OlCGw1ZOk3zmUq1jHoH1Goyt0NpoZK82Vh3NJEE5kx3T00DTBGX4IC2HTrw0Iii0uH-9P1pTv3sBS1Br8k5sGAg2nzoT1-s6yGSmRusCezpQFB48owpdm8UgmbKlsII-n-KsuIG89XxLiMr8XeI1CGXl7-utBgYf7mjdyXRupCHl5yD5KvBIxdc55ljzQO2rDmjUj2zpzy09zwBLasCk1lBlAlunltKd7DGrhAhNvrbo8GbslF9w4WcYr3NE6M917hJXMBlANc~sm8p50e-XroaodFo5L3Z3PH78pf5oeQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 2. Multimer pattern of vWF released in response to ATP. Confluent HUVECs were metabolically labeled with [35S]-cysteine and [35S]-methionine for 24 hours. vWF was immunoprecipitated from the labeling medium (medium) and from the supernatant of cells incubated for 30 minutes with ATP (100 μmol/L), ATP/IBMX (100 μmol/L), thrombin (1 U/mL), or KRBH-BSA 0.1% alone (control). vWF multimers were resolved on a horizontal nonreducing 2% SDS-agarose gel. Top arrowhead, the origin of the gel; bottom arrowhead, position of vWF dimers. vWF released constitutively into the labeling medium consists predominantly of dimers and small multimers, whereas vWF released in response to ATP and thrombin consists of high–molecular weight multimers.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.118/3/m_blod4011502.jpeg?Expires=1767856191&Signature=EjfReJcuTrmP94VqUD34ykxyoBk-eORjQ5Fifsgw0erbAnr-N6CgnRArWjNUgo08Dn4plRL-rpThOJ0q17zsGQ34SsfGhKYr7SNjWWDsmrOSiTMOOP9J~jpFZ2bCFPvV9lJ8XdINSfblqmVE-mfAE4JwoEs8Q1~ZdjqbxGtILVwRXtig03-Jk51TlgIBwyeVn-G6AG9sIVSIXECvyz8hw0dvkwBkCc3DXJ-jgd5BK6plUDga7CATlQbntWVAhkI-~XaXRDOwo37M95h2G-jNZBb-p3SKfaUYkVB4BAGxtR4f5Blg1697Meuc-KD7kLaPljqCQGUryQ6leH~0EKdDUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 7. Effect of ATP and adenosine on [Ca2+]i. HUVECs were grown on gelatin-coated glass coverslips, loaded with fura-2, and immersed in a quartz cuvette for radiometric fluorescent emission measurements. Each tracing shown is representative of at least 3 similar experiments. (A) Stimulation with 100 μmol/L ATP was followed by 0.5 U/mL thrombin (Thr). The [Ca2+]i response to thrombin looks virtually identical when tested alone, and is not modified by prior stimulation with ATP (not shown). (B and C) The response to 2-MeS-ATP was similar to the ATP response (B), and was not modified by preincubation with IBMX and adenosine (Ado), both tested at 100 μmol/L (C).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/1/10.1182_blood.v91.1.118/3/m_blod4011507.jpeg?Expires=1767856191&Signature=ph-3adKZ4oDVbThCKgUegr71DXitZ6acykR1~2XVHodYvgrIhtrnfHI~8ZjmSQwP0wmpULtwBT-2UqpCU0zt6V2zJ25UUMjSryFDZ3N13AtzPlhTWHPeC7zTPBNJ9b-oCQ9KjHZdjSLsuNNCNPc4O0NaXs-bvTdaYaN-FjLGV1kKjM8LJBfdaaLllHq36~brK9khpLLrljPkcnTDt4DVIJD-TvpUFMV7JFpQkTZvIIuepRGzzFphB5m7~gGsWoObqkafST0eEC98RY5Ip8xtBfh0hDnnL8orrXWCAyt8Ry73Uj8FWojyY6BI~Q5RXMIKaKF6cULrWX0mLvDGsSdimg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)