Abstract
Primary autoimmune neutropenia (AIN) is caused by granulocyte-specific autoantibodies and occurs predominantly in infancy. Clinical presentation and diagnosis have not been well established, resulting in burdening diagnostic investigations and unnecessary treatment with granulocyte colony-stimulating factor (G-CSF). In the present study, clinical, laboratory, and immunologic data of 240 infants with primary AIN were evaluated. Suspected association with parvovirus B19 infection was investigated using serologic and DNA-based methods. Primary AIN was mainly diagnosed at the age of 5 to 15 months but was observed as early as day 33 of life. In 90% of the cases, AIN was associated with benign infections despite severe neutropenia. Spontaneous remission, shown by 95% of the patients, usually occurred within 7 to 24 months. Autoantibodies in the patient's sera were not always present, and screening had to be repeated several times until antibody detection succeeded. About 35% of the autoantibodies showed preferential binding to granulocytes from NA1 and NA2 homozygous donors. Bone marrow was typically normocellular or hypercellular, with a variably diminished number of segmented granulocytes. A significant association with parvovirus B19 infection was not found. Symptomatic treatment with antibiotics was sufficient in most patients. Eighty-nine percent of the patients received antibiotics (cotrimoxazole) for prophylaxis of infections. For severe infections or for surgical preparation, G-CSF, corticosteroids, and intravenous IgG were administered, resulting in increased neutrophil counts in 100%, 75%, and 50% of the patients treated, respectively. In combination with the detection of granulocyte-specific antibodies, the typical clinical picture allowed diagnosis of AIN without burdening investigations. Treatment with G-CSF was found to be a reliable alternative to temporarily increase the neutrophil count.
AUTOIMMUNE neutropenia (AIN) is a disorder caused by increased peripheral destruction of neutrophils as a result of antibodies in the patient's blood that are directed against their own neutrophils. It could not be convincingly shown until 1975 that neutropenia can be caused by granulocyte-specific autoantibodies.1,2 Primary AIN, which is not associated with other diseases such as systemic lupus erythematosus, is often observed in infants.3,4 Although the etiology of the disease is unknown, a recent report about a possible association of AIN with Parvovirus B19 infection5 resulted in frequent testing for this virus in patients with suspected AIN. Because of the difficulties with the detection of granulocyte autoantibodies, many questions concerning clinical course, diagnostic approach, immunology, and therapy have remained open. In contrast to severe chronic neutropenias (SCN) caused by disorders of myelopoiesis,6AIN in infancy is less often considered in standard textbooks of pediatric hematology. As a result, AIN is less known among physicians and many cases are only diagnosed after expensive and burdening investigations excluding other forms of neutropenia. This and the availability of recombinant human granulocyte colony-stimulating factor (G-CSF) for treatment of chronic neutropenia6 make an accurate diagnosis of AIN necessary. Therefore, we evaluated the data of 240 infants with primary AIN.
MATERIALS AND METHODS
Patients and Sera
Infants with chronic neutropenia and detectable granulocyte-specific antibodies in their blood were included in the study. None of the patients received blood transfusions before antibody screening or suffered from additional underlying diseases that could be associated with neutropenia. In each patient, antibodies were detected in at least two different blood samples using different freshly isolated panel cells. All tests were performed for diagnostic reasons with the full consent of the patient's parents. Blood collections for antibody screening and for other laboratory tests were combined.
Detection of Granulocyte-Specific Autoantibodies
Granulocyte-specific antibodies were detected by the granulocyte immunofluorescence (GIFT) and agglutination (GAT) tests as described.7 The four panel cells typed for the NA1, NA2, NB1, and 5b antigens were freshly isolated from the donor's blood each day of the investigation. To exclude nongranulocyte-specific antibodies such as HLA antibodies, each serum was additionally tested by lymphocyte immunofluorescence or lymphocytotoxicity using the lymphocytes of the donors for the granulocyte panel. Lymphocyte immunofluorescence was performed using the same method as GIFT, but lymphocytes were used instead of granulocytes. Lymphocytotoxicity was performed according to the standard procedure of the National Institutes of Health (Bethesda, MD). Granulocytotoxicity was performed using the method for lymphocytotoxicity, but granulocytes were used instead of lymphocytes.
Location of Autoantigens
For location of autoantigens, the recently described antigen-specific luminescence immunoassay monoclonal antibody-specific immobilization of granulocyte antigens (MAIGA) was used.8 Briefly, 1 × 106 neutrophils from one individual fixed with 1% paraformaldehyde were incubated (30 minutes at 37°C) with human serum and a monoclonal antibody (MoAb). In different reaction mixtures we used the MoAbs 3G8 (Immunotech, Hamburg, Germany) and Bw 209/2 (Behringwerke, Marburg, Germany), which are specific for the Fcγ receptor IIIb, and the MoAbs 2E1, Bear 1, 7E4, and J3D3 (Immunotech), which are specific for the Fcγ receptor II, the subunits of the leukocyte adhesion molecule CD11b/CD18, and the C3b complement receptor I. The cells were washed and solubilized by adding 100 μL of lysis buffer (1% Triton-X 100, 5 mmol/L EDTA, 2 mmol/L phenylmethyl sulfonyl fluoride, 0.5 μg/mL leupeptin, and 500,000 U/mL aprotinin in 20 mmol/L Tris-buffered saline, pH 7.4) for 30 minutes at room temperature. After sonication (2 minutes) and centrifugation at 15,000g for 30 minutes, the supernatant of each reaction mixture was transferred to a separate tube coated with goat antimouse antibodies. Unattached antibodies were removed by washing, and goat antihuman IgG (heavy + light chain) antibodies conjugated with peroxidase were added. After washing and subsequent addition of a substrate containing luminol, hydrogen peroxide, and 4-iodophenol, the emitted light (chemiluminescence) was measured over a period of 15 minutes in a luminometer (Lumat LB 9501; Berthold, Wildbad, Germany).
Serologic and Molecular Biologic Detection of Parvovirus B19
RESULTS
Clinical Presentation
Patients.
In 240 children tested, positive antineutrophil antibodies could be detected. Female to male distribution was 54%:46%. All patients were white and less than 3 years of age. Diagnostic testing of these children with positive antibody results did not show any other conditions than neutropenia (primary AIN). Five patients appeared to have AIN, but granulocyte antibodies could not be detected even though they were tested three times. After the granulocyte count began to increase, no further testing was performed. Although the clinical picture did not differ from the other 240 patients, as a result of the failure to detect antibody, these patients were not included in the study. It appears likely that they had AIN, but, at the time of diagnosis, spontaneous remission of neutropenia had already begun. At that time granulocyte autoantibodies are no longer detectable in the patient's blood.
The average age at diagnosis of AIN was 8 months. In 1 patient, granulocyte-specific antibodies could be detected as early as 33 days after birth. In this patient, normal neutrophil counts were detected in the first week of life. In two thirds of the cases, AIN was diagnosed at the age of 5 to 15 months. The distribution of infections present at the time of diagnosis is shown in Table 1. Eighty percent of the patients suffered from mild infections, particularly skin infections, otitis media, and infections of the upper respiratory tract. In 8% of the patients, AIN was detected by chance and 12% suffered from severe infections such as pneumonia, meningitis, or sepsis.
Laboratory Findings
Blood count.
At the time of diagnosis, the neutrophil counts in the peripheral blood were distributed as follows: 70% had greater than 500 cells/μL, 23% had between 500 and 1,000 cells/μL, and 7% had between 1,000 and 1,500 cells/μL. Neutrophil counts varied significantly between 0 and 1,500/μL during the neutropenic phase in 30 patients who were tested repeatedly during the neutropenic period. Monocyte counts of more than 1,000 cells/μL were found in 28% of all patients. Eosinophilia was only observed in 1 case.
In 12 patients there was a documented transient increase of the neutrophil count from less than 500/μL to more than 1,500/μL during an infectious event. After successful treatment with antibiotics, the neutrophils decreased to the same neutropenic level as before.
Bone marrow findings.
Bone marrow findings are summarized in Table2. Ninety-seven percent of the patients showed normal or increased cellularity with or without a reduced number of metamyelocytes, bands, and mature neutrophils. Although in some cases a maturation arrest seemed to occur, in all cases maturation reached at least the myelocyte/metamyelocyte stage. Phagocytosis of granulocytes by bone marrow macrophages was observed in 5 cases, indicating that removal of sensitized granulocytes already occurs in the bone marrow.
Immunohematologic findings.
Granulocyte-specific antibodies were detected in 74% of the cases in the first investigation. In 26%, repeated antibody testing with additional blood samples from the same patient up to three times at intervals of 2 to 4 weeks was necessary for detection of antibodies. The GIFT was more sensitive in detection of granulocyte autoantibodies than the GAT (Table 3). Cytotoxic or noncytotoxic HLA antibodies were detectable in none of the sera. Ninety-seven percent of the sera contained granulocyte autoantibodies of the IgG type, and 15% of the sera contained granulocyte autoantibodies of the IgM type. In 30 of 60 sera tested, granulocyte autoantibodies activated complement and 15 reacted positively in the granulocyte cytotoxicity test.
Thirty-one percent of the autoantibodies showed a preferential binding to granulocytes isolated from NA1 homozygous donors and 3% of the autoantibodies showed a preferential binding to granulocytes from NA2 homozygous donors. These sera often failed in binding to granulocytes from NA1/NA2 heterozygous donors and antibody detection was only possible by the use of NA1- or NA2-homozygous cells. NA specificity could vary and was not found in all samples taken from the same patient at different times. The presence of NA-specific antibodies was not associated with monocytosis. To support the diagnostic value of antibody screening, the sera of 10 patients with congenital severe neutropenia and a maturation arrest on the promyelocyte/myelocyte stage were investigated for granulocyte antibodies. No granulocyte-specific autoantibodies were detectable.
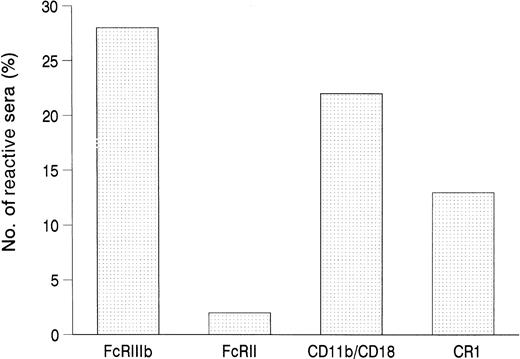
To determine the antigens that were recognized by the autoantibodies, we tested 125 randomly selected sera in the MAIGA assay for their binding to the granulocyte membrane glycoproteins Fcγ receptor IIIb (FcγRIIIb, CD16b) and Fcγ receptor II (FcγRII, CD32), the leukocyte adhesion molecule b (LeuCAMb, CD11b/CD18), and the C3b complement receptor (CR1, CD35). The results are summarized in Fig1. Autoantibodies to FcγRIIIb were found in 27% of the sera, to CD11b/CD18 in 21%, to CR1 in 14%, and to FcγRII in 2%. Of the FcγRIIIb-reactive antibodies, 25% showed binding only to one of the NA isoforms, mostly to the FcγRIIIb-NA1 form.
Location of autoantigens by MAIGA. Shown is the frequency of autoantibody binding (n = 125) to selected, isolated glycoproteins of the granulocyte membrane.
Location of autoantigens by MAIGA. Shown is the frequency of autoantibody binding (n = 125) to selected, isolated glycoproteins of the granulocyte membrane.
Parvovirus B19 diagnostics.
A total of 110 patients were tested for Parvovirus B19 infection. Thirty-six patients showed threshold values in ELISA (IgG and IgM; n = 9), in immunoblot (n = 12), and/or by virus-specific PCR (n = 28). Serum samples from 24 of the 36 patients were retested for Parvovirus B19 antibodies after spontaneous remission of neutropenia and all were found to be negative.
Treatment and Prognosis
Symptomatic treatment with antibiotics was sufficient in greater than 90% of the infectious events. Eighty-nine percent of the patients were treated prophylactically with cotrimoxazole; a few patients were treated with amoxicillin, erythromycin, or cephaclor; and 11% of the infants received no prophylaxis. Most patients prophylactically received antibiotics after recurrent infectious events, predominantly otitis media. Prophylaxis with antibiotics prolonged the infectious-free intervals in patients with previous recurrent infections.
To increase the neutrophil count before elective surgery or because of severe infections, 31 patients were treated either with corticosteroids (n = 7), high-dose intravenous IgG (IvIgG; n = 20), or recombinant human G-CSF (Filgastrim; n = 8). Corticosteroids and high-dose IvIgG were effective in 75% and 50% of the cases, respectively, whereas G-CSF increased the neutrophil count in all patients treated, as shown in Table 4. Corticosteroids and G-CSF treatment resulted in a sustained elevated neutrophil count during the treatment period, whereas IvIgG infusion resulted in a transient increase in neutrophils for 1 week.
In 30 of 34 infants who were observed for up to 6 years, neutropenia spontaneously disappeared after a median of 17 months (mode, 12 months; range, 1 to 38 months). In 80% of the patients observed, neutropenia lasted 7 to 24 months. Disappearance of autoantibodies preceded spontaneous normalization of the neutrophil count. To document the autoantibody nature, the initial sera of all patients drawn for the first antibody screening and stored frozen were tested with the patient's autologous neutrophils as soon as they appeared in sufficient number in the peripheral blood; all tests were positive. In the first months after the disappearance of the antibodies, the neutrophil counts strongly fluctuated in all patients. There was no evidence of other autoimmune disease during the follow-up period. One patient died of lung failure after repeated episodes of pneumonia.
DISCUSSION
In this study we present 240 children with primary AIN. The differential diagnoses of AIN of infancy are alloimmune neonatal neutropenia, cyclic neutropenia, and SCN. Alloimmune neonatal neutropenia, which can last up to 6 months, could be excluded by the detection of granulocyte alloantibodies in the maternal serum.7 Cyclic neutropenia is typically characterized by neutropenic periods of 3 to 6 days occurring approximately every 21 days. In AIN, neutrophil counts can also vary considerably from day to day. Based on inadequate serial data, a number of cases reported as cyclic neutropenia were probably AIN.11 In contrast to AIN, SCN is characterized by severe infections already in the first month of life, no spontaneous remission of neutropenia, and a maturation arrest of myelopoiesis at the promyelocyte stage. The promyelocytes are frequently morphologically atypical with vacuolization and atypical nuclei, but findings are variable.6 Overall, the number and severity of bacterial infections is much lower in AIN than in SCN. However, severe infections can occur in AIN patients and the antibody-mediated destruction of segmented neutrophils and band forms can mimic maturation arrest in the myelocyte/metamyelocyte stage. In addition, 3% of all AIN patients present a hypocellular marrow that is probably caused by antibodies binding not only to mature neutrophils but also to primitive hematopoietic cells.12 Therefore, differential diagnosis of SCN and AIN often depends on the detection of granulocyte-specific antibodies in the patient's sera. Testing the sera of 10 patients with SCN, we found no evidence of granulocyte antibodies. AIN is far more frequent than SCN, with 1:100,00013 versus 1:1,000,000,6 and very probably the incidence of AIN is much higher. Therefore, it is justified to postpone bone marrow examination until antibody screening shows a negative result. In case of a positive result, there is usually no need for bone marrow aspiration or biopsy.
For the detection of granulocyte autoantibodies and alloantibodies in the patient's serum, a combination of immunfluorescence and agglutination tests has proven to be the best antibody screening procedure, as shown by the Second International Granulocyte Serology Workshop.14 By studying the effect of leukocyte antibodies on the in vivo fate of indium 111-labeled granulocytes, McCullough et al15 could show the clinical significance of the antibodies detected by immunofluorescence and agglutination tests. Although immunfluorescence is more sensitive than the agglutination test, certain alloantibody specificities, such as anti-5b, anti-NB2, and anti-9a, are more detectable using the agglutination test. The same applies to a few autoantibody specificities and some autoantibodies may be missed if GAT is not performed. Because antibody titers are usually low, in about 30% of the cases, screening for granulocyte-specific antibodies in the patient's serum had to be repeated several times until antibody detection in serum samples succeeded. However, background problems due to unspecific IgG binding to Fcγ receptors in immunofluorescence and spontaneous aggregation in agglutination limit their sensitivity. The same applies to enzyme and radioimmunoassays.16 In patients who were diagnosed shortly before spontaneous remission of AIN, antibody detection may not be possible.
The direct testing of the patient's granulocytes for specifically bound antibodies is hampered by the need for a sufficient number of isolated granulocytes and by the unspecific binding of IgG immune complexes to the Fcγ receptors II and IIIb of the patient's activated neutrophils. Because cells from patients are often activated due to an inflammation process and their plasma contains large amounts of immune complexes, their neutrophils can show an extremely elevated amount of granulocyte-associated IgG without neutropenia. Therefore, a positive direct granulocyte immunofluorescence test or determination of granulocyte-associated IgG by an enzyme immunoassay cannot be considered as proof that autoantibodies are present.17,18Possibly, the high turnover of granulocytes contributes to the positive direct testing, because patients treated with G-CSF for nonimmune neutropenia also showed a false-positive direct testing due to additional expression of the high-affinity Fcγ receptor I.19
Most of the autoantibodies tested were directed against the FcγIIIb and, to a lesser extent, the leukocyte adhesion molecule CD11b/CD18. Antibodies to CD11b/CD18 have been reported to influence granulocyte function by inhibiting granulocyte adhesion.20 T and B lymphocytes normally do not express CD11b and the restriction of β2 subunit (CD18)-reactive antibodies to neutrophils may be due to the known influence of the α subunit in the αβ2 complex on the glycosylation pattern of CD18.21 The FcγRIIIb seems to be very immunogenic, because the clinically most important granulocyte-specific antigens NA1 and NA2, as well as the recently described SH antigen, are also located on this neutrophil-restricted glycoprotein.22,23 It is possible that the high expression of Fcγ IIIb in copy numbers of 100,000 to 300,000 and of CD11b/CD18 in about 600,000 molecules on maximally activated neutrophils contributes to their immunogenicity.24 25 Because 34% of the autoantibodies showed preferential binding to granulocytes from NA1 or NA2 homozygous donors, such cells should be included in the cell panel used for antibody screening. However, in the antigen-specific assay MAIGA, many of the NA1-specific antibodies showed strong binding to the FcγRIIIb-NA1 but also weak binding to the FcγRIIIb-NA2.
The etiology of AIN is not clear. Although an association between NA1-specific autoantibodies and HLA-DR2 has been found, the occurrence of AIN in only one of monozygotic twins is strong evidence against a strictly hereditary mechanism.26,27 Recently, an association between AIN and Parvovirus B19 infection was suggested by demonstration of anti-B19 IgM in 5 of 11 patients.5 We tested 110 serum samples from different patients for anti-B19 IgG/IgM and virus-specific DNA and found 36 sera showing only threshold values. These results indicate that Parvovirus B19 diagnosis is not helpful in cases of suspected AIN in infants.
AIN patients usually do not require specific treatment of neutropenia. Infections are treated symptomatically with antibiotics. A decision to apply prophylactic treatment with antibiotics such as cotrimoxazole depends on the circumstances in each isolated case. Prophylaxis with antibiotics is indicated in infants with recurrent infections, especially otitis media, to avoid tissue damage in the ear. Despite the concern of allergic reactions, of delayed marrow recovery, and of drug resistance, cotrimoxazole has continued to be a suitable prophylactic agent during profound neutropenia.28 However, the positive experience of long-term treatment with cotrimoxazole in patients with chronic granulomatous disease29 has resulted in a broader administration when uncertain compliance of the parents and/or additional physical handicaps were present or were to be expected. In cases with severe infections or surgical intervention, remission can be achieved by large doses of IvIgG30 or corticosteroids. However, steroids showed inconsistent results,3,4 and remission induced by IvIgG lasts only about 1 week. Intravenous administration of IgG is burdening for the child and IvIgG can also induce neutropenia.31 Corticosteroids alter host defense and long-term treatment can induce known side effects. Alternatively, recombinant human G-CSF has been administered to 8 patients and all responded within 4 days. G-CSF is administered subcutaneously and remission lasted until cessation of G-CSF administration. Successful treatment of AIN in adults with G-CSF has been also reported.32 The efficiency of G-CSF is not surprising, because during infectious events in some patients a transient increase of the neutrophil count is observed that is probably caused by an increased production of endogenous G-CSF. Thus, G-CSF seems to be very useful as a means to induce temporary remission of neutropenia. Although G-CSF has shown long-term safety in patients with SCN,33 there is no need in AIN patients for long-term treatment, because AIN shows a benign and self-limiting clinical course. Treatment with G-CSF should be restricted to patients with severe infections or before surgical intervention.
Supported in part by Grant No. Bu 770/3-2 from the Deutsche Forschungsgemeinschaft.
Address reprint requests to Juergen Bux, MD, Institute for Clinical Immunology and Transfusion Medicine, Justus-Liebig University, Langhansstrasse 7, D-35385 Giessen, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.