Abstract
The chromosomal translocation t(14;18) in lymphoma leads to an overproduction of the Bcl-2 protein on the basis of increased Bcl-2 mRNA levels. Whereas the juxtaposition of Bcl-2 with the Ig heavy chain locus causes a transcriptional activation, 70% of the lymphomas also produce Bcl-2–Ig fusion RNAs with Ig 3′ ends. Using S1 nuclease protection assays that can discriminate between nuclear RNA precursors and spliced mRNA, we found that the fusion RNAs in t(14;18) cell lines exhibit an additional posttranscriptional processing advantage. Transfection experiments with artificial genes containing various Bcl-2 or Ig 3′ ends show that this effect is (1) related to RNA splicing and/or nucleocytoplasmic transport; (2) independent of transcriptional activation by the heavy chain enhancer; (3) dependent on the presence of the JH-CH and C-γ1 Ig introns; and (4) tissue specific for B cells. This constitutes a novel mechanism of oncogene deregulation unrelated to transcriptional activation or half-life prolongation. The data further support the existence of a tissue-specific posttranscriptional pathway of Ig regulation in B cells.
THE CHROMOSOMAL translocation t(14;18)(q32;q21) constitutes the molecular hallmark of human follicular lymphoma. The breakpoint on the derivative 14 chromosome juxtaposes the Bcl-2 proto-oncogene from 18q21 with one of six Ig heavy chain joining (JH) regions of 14q32.1-3 The pathologic consequence is the overexpression of both Bcl-2 RNA and protein in cells bearing this translocation.4-6 Transgenic mice bearing a Bcl-2–Ig minigene established that this translocation was of primary pathogenic importance in lymphomagenesis. Bcl-2 mice demonstrate B-cell follicular hyperplasia that progresses to high-grade lymphoma.7 8
The breakpoints on chromosome segment 18q21 are not randomly distributed. Approximately 70% occur within the major breakpoint region (mbr), where most cluster within 150 nt.1-3 Up to 20% of breakpoints are found approximately 30 kb further telomeric within the minor breakpoint region (mcr).9,10 Although both breakpoints are associated with follicular lymphoma, important questions remained concerning the precise mechanisms that deregulate Bcl-2 production. We and others have shown that the rate of Bcl-2 transcription is increased in t(14;18)-bearing cell lines and constitutes one mechanism of deregulation.6,11 12 However, whether the magnitude of this transcriptional enhancement fully accounts for the log-fold increase in steady-state levels of Bcl-2–Ig fusion RNA within t(14;18) cells is uncertain.
Many interchromosomal translocations responsible for neoplasia generate fusion RNAs between genes on their chromosomal partners. Notable examples include BCR-ABL,13 PML-RARα,14DEK-CAN,15 LYT-10-Cα1,16 and MLL-AF4,17 to name a few. Similarly, breakpoints within the mbr but not the mcr of Bcl-2 result in a Bcl-2–Ig fusion RNA.4,6,9,10 However, one notable difference exists between the Bcl-2–Ig example and most of the aforementioned fusion RNAs. Most fusion RNAs found in neoplasias encode a chimeric protein product providing a clear rationale for the selection of clones bearing these events. In contrast, the mbr of Bcl-2 is located within the 3′ untranslated region of the gene and translocations into it result in Bcl-2–Ig hybrid RNA but not a chimeric protein. Yet, the majority of Bcl-2 translocations involve this region and generate Bcl-2–Ig fusion RNAs, suggesting that this event may also have a selective advantage. The most obvious potential mechanism would be an alteration in mRNA half-life. This was attractive, because the native Bcl-2 RNA had a relatively short half-life of 2.5 hours within B cells,6whereas Ig RNAs demonstrated a longer half-life approaching 20 hours.18,19 However, experiments showed that the Bcl-2–Ig fusion RNA exhibited an unaltered half-life of 2.5 hours.6
Posttranscriptional control by differential RNA splicing or transport has been documented for a variety of genes. A number of organisms show tissue specific splicing.20-22 Cellular transport mechanisms have been proven to be important in the expression of viruses such as human T-lymphotrophic virus (HTLV) I and II,23-25 influenza virus NS1,26 or hepatitis B.27,28 The existence of a posttranscriptional pathway related to intronic sequences has also been postulated for Ig RNA regulation.29 These observations prompted us to examine the processing of Bcl-2–Ig fusion versus native Bcl-2 RNAs. We developed a system that eliminated the potential influence of transcriptional initiation to examine the contribution of Ig versus Bcl-2 sequences on posttranscriptional control. This assay, which discriminates between precursor and spliced mRNA products, defined a processing advantage for Bcl-2–Ig fusion RNA, a novel mechanism of oncogene activation. Because the improvement is conferred by Ig sequences and is tissue specific for B cells, it may also contribute to the efficiency of normal Ig gene expression.
MATERIALS AND METHODS
Cell culture.
Cell lines were maintained in Iscove's modified Dulbecco's medium (GIBCO, Grand Island, NY) supplemented with 10% fetal calf serum, penicillin, streptomycin, and 50 mmol/L β-mercaptoethanol at a CO2 concentration of 7.5%. The following cell lines were used: Nall-1 (pre-B30); SU-DHL9 (mature B); SU-DHL-6 [t(14;18) bearing mature B31]; K562 (erythroleukemia); 70z/3 (mouse pre-B32); and S194 (mouse myeloma, ATCC TIB 10, 1985).
Construction of plasmids and transfection.
A 1.4-kb Sst I/BamHI human β-actin promoter fragment was excised from pHβAPr-I (kindly provided by L. Kedes33,34). This fragment contains 472 nt of 5′ flanking region, the β-actin promoter, exon I, IVS I including the β-actin enhancer, and an acceptor splice site and has been shown to retain 78% of promoter activity when transfected into Hela cells.35
To generate the backbone of the βApr-fusion vectors, this fragment was supplied with an Sst I linker on both ends and inserted into the Sst I site of Bluescript (Stratagene, La Jolla, CA) pKS (T7 = 5′; termed plasmid p229.6; see Fig 2). Thus, 95 nt of polylinker from the acceptor splice site to the uniqueEcoRI site become part of exon II of this vector. The different 3′ ends include (1) a 5.6-kb EcoRI genomic fragment from a normal Bcl-2 allele 3′ of the EcoRI site (at position 1856)3 in Bcl-2 exon III1,2,6(βApr-Bcl-2); (2) an 11.6-kb EcoRI genomic fragment from a Bcl-2–Ig fusion allele (SU-DHL-62;βApr-Ig); (3) a 2.4-kb EcoRI cDNA fragment from SU-DHL66 fused to the C-γ1 membrane genomic 3′ end including its polyadenylation signal (βApr-IgΔIntrons); and (4) a 2.5-kb EcoRI cDNA fragment as in (3) with the exception that a 134 nt non-Ig intron (IVS II of the human β-actin gene) was inserted into the 2.4-kb EcoRI cDNA as a substitute for the JH6 to C-γ1 intron (βApr-IgςIntron). These fragments were inserted into the EcoRI site of p229.6 to form the final βApr-fusion vectors. All constructs were partially sequenced to identify the correct orientation and checked for single-copy integration by restriction mapping.
Constructs for transfection. A 1.4-kb Sst I (S) fragment of the human β-actin promoter plus β-actin IVS-I was fused to various 3′ ends at the EcoRI (R) site:βAPr-Bcl-2 contains the normal genomic Bcl-2 3′ end, including the Bcl-2 poly(A) sites and 3′ flanking regions;βAPr-Ig contains a translocated allele cloned from SU-DHL-6, including the Bcl-2 mbr as well as the JH-CHand CH introns and the Cγ1 poly(A) sites and flanking regions; βAPr-Ig▵Introns contains a translocated cDNA from SU-DHL-6 and the genomic Cγ1 membrane (M) poly(A) signal and flanking regions; βApr-IgςIntron corresponds toβAPr-Ig▵Introns with the exception that a nonlymphoid intron (IVS-II of β-actin) is inserted as a substitute for the JH-CH intron. The transcriptional start site is indicated by an arrow. The EcoRI (R) and Xba I (X) sites were used for the S1 protections and primer extensions on the β-actin promoter.
Constructs for transfection. A 1.4-kb Sst I (S) fragment of the human β-actin promoter plus β-actin IVS-I was fused to various 3′ ends at the EcoRI (R) site:βAPr-Bcl-2 contains the normal genomic Bcl-2 3′ end, including the Bcl-2 poly(A) sites and 3′ flanking regions;βAPr-Ig contains a translocated allele cloned from SU-DHL-6, including the Bcl-2 mbr as well as the JH-CHand CH introns and the Cγ1 poly(A) sites and flanking regions; βAPr-Ig▵Introns contains a translocated cDNA from SU-DHL-6 and the genomic Cγ1 membrane (M) poly(A) signal and flanking regions; βApr-IgςIntron corresponds toβAPr-Ig▵Introns with the exception that a nonlymphoid intron (IVS-II of β-actin) is inserted as a substitute for the JH-CH intron. The transcriptional start site is indicated by an arrow. The EcoRI (R) and Xba I (X) sites were used for the S1 protections and primer extensions on the β-actin promoter.
Cell transfection and selection of stable integrants.
Transfection of human and murine cell lines was performed by electroporation with a 1.9-mm gap cuvette electrode and a Transfector 300 (Biotechnologies & Experimental Research, Inc, San Diego, CA). Log phase cells (1 × 107) were washed and resuspended in 400 μL of serum-free RPMI 1640 (GIBCO). They were mixed with 100 μL RPMI 1640 containing 5 μg of RSVneo36and a 2 molar excess of linearized construct DNA together with 125 μg of salmon sperm DNA. Transfection of the various cell lines was performed at the optimal voltage and capacitance (200 to 250 V and 450 to 800 μF). After 24 to 48 hours, cells were selected in 1 g/L of G418 (Geneticin; GIBCO). Whereas half of each transfection was selected as a bulk, the other half was plated in 2-mL tissue culture wells at a density of 103 to 104 cells to obtain oligoclonal subpopulations. Transfectants were harvested after 10 to 14 days and assayed for construct expression by S1 nuclease protection.
RNA preparation.
For the preparation of nuclear and cytoplasmic RNA, cells were lysed in a hypotonic buffer containing 0.5% NP-40 and separated on a sucrose gradient.37 Cytoplasmic RNA from the supernatant was prepared by this standard protocol, including DNaseI digestion. The nuclear pellet was resuspended in 4 mol/L guanidine thiocyanate and nuclear RNA was purified on a 7.5 mol/L CsCl2gradient.38 Total RNA from transfected cells for run-on assays was prepared by a guanidine-thiocyanate-acid-phenol miniprep method.39 S1 probes that can discriminate between RNA and DNA detected no DNA contamination in these minipreps.
DNA probes for S1 nuclease protection.
A 406-nt HindIII/HincII fragment across the Bcl-2 intron II/exon III acceptor splice site and a 410-nt SstI/Bgl II fragment across the Bcl-2 exon II/intron II donor splice site were subcloned into m13 to generate single-stranded, synthetically labeled probes. A 183-nt BamHI/Sal I human β-actin cDNA fragment40 was protected as a control for RNA amount within the same tube.
The probes for the protection assay on RNA from transfected cell lines were derived from p229.6. This plasmid was end labeled at theEcoRI or Xba I site and used to detect the correct β-actin initiation site. A 145-nt Bal I/EcoRI fragment spanning the acceptor splice site in the polylinker was subcloned into Bluescript pKS and end labeled at the EcoRI site, giving a probe length of 2.9 kb, a precursor protection of 145 nt, and an exon protection of 95 nt.
S1 nuclease protection assay.
S1 protection with single-stranded reverse complementary DNA probes was performed as described.6 5′ end labeled probes were generated using 1 μg of linearized and dephosphorylated plasmid, 60 U of T4-polynucleotide kinase (US Biochemicals, Cleveland, OH), and 100 μCi of [γ-32 P] ATP (Amersham, Arlington Heights, IL).41 RNAs were hybridized overnight with 2 × 105 cpm at the appropriate temperature (52°C) in 15 μL of a juice containing 80% formamide, 40 mmol/L PIPES (pH 6.4), 400 mmol/L NaCl, and 1 mmol/L EDTA. Samples were digested with 200 U S1 nuclease (Boehringer Mannheim, Indianapolis, IN) and analyzed on 6% polyacrylamide gels.
Nuclear run-on and primer extension assays.
Log phase cells (5 × 107) were washed in RPMI 1640 and resuspended in 10 mL ice-cold hypotonic lysis buffer (10 mmol/L HEPES, pH 8.0, 1.5 mmol/L MgCl2, 10 mmol/L KCl). After 10 minutes on ice, cells were lysed by two to three passages through a 22G needle. Nuclei were washed and resuspended in 220 μL of transcription buffer containing 20 mmol/L Tris (pH 8.0), 6 mmol/L Mg (C2H3O2)2, 84 mmol/L KCl, 10 mmol/L NH4Cl, 0.3 mol/L EDTA, and 10% glycerol. The run-on assay was performed as described6 at 30°C for 30 minutes using 250 μCi of [α-32 P] GTP as label. After the reaction was completed, RNA was extracted, ethanol-precipitated, and resuspended in hybridization buffer (50% formamide, 4× SSC, 2× Denhardt's solution, 20 μg/mL tRNA [Escherichia coli], 50 mmol/L NaPO4 [pH 7.4], and 0.1% sodium dodecyl sulfate [SDS]). Equal counts (2 × 106 cpm) were hybridized to 2 μg of slot-blotted single (histone H4) or double-stranded (β-actin, RSVneo) DNA template for 36 hours at 42°C. Membranes were washed three times for 20 minutes at room temperature with 2× SSC, 0.1% SDS and twice for 20 minutes at 63°C with 0.1% SSC, 0.1% SDS.
Primer extensions were performed as described6 using RNAs from transfected cell lines as template and a 20-nt oligonucleotide primer starting from the EcoRI site in the polylinker of p229.6.
RNA half-life experiments.
Actinomycin D (Sigma, St Louis, MO) was added to cell lines growing in log-phase at a concentration of 10 μg/mL. RNA was extracted after 0, 1, 2, 4, 8, and 24 hours and analyzed by S1-nuclease protection.
RESULTS
Altered posttranscriptional processing of Bcl-2–Ig fusion RNAs in t(14;18) cell lines.
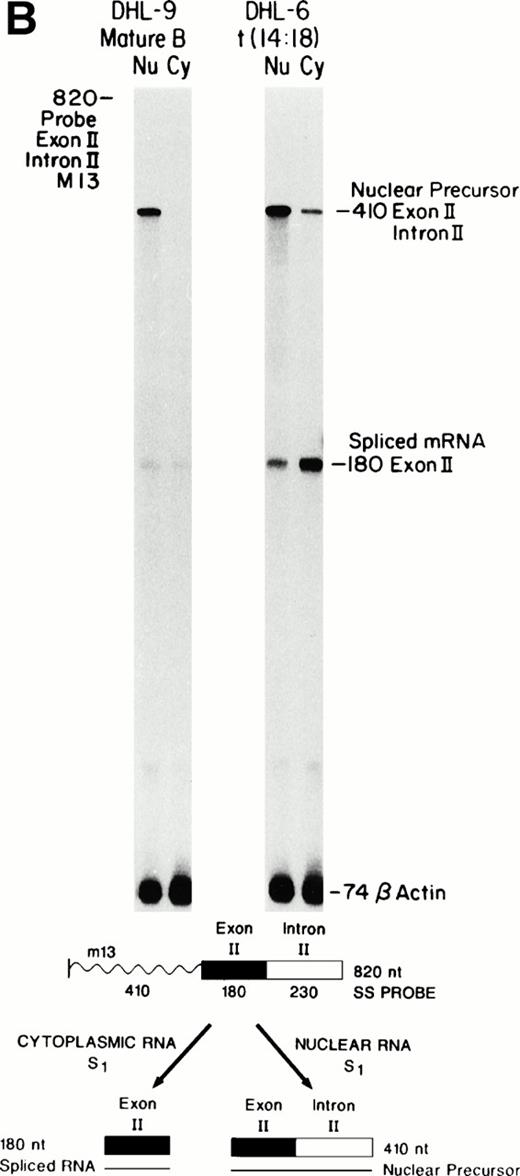
S1 nuclease protection assays that cross the Bcl-2 exon II-intron II or intron II-exon III borders were developed to determine the relationship between the levels of Bcl-2 nuclear precursors, nuclear spliced message, and final cytoplasmic mRNA in mature B-cell lines with or without the translocation (Fig 1A and B). This assay uses probes across the intron-exon borders, thereby hybridizing to the long, unspliced precursor as 406 nt (Fig 1A) or 410 nt (Fig 1B) fragments as well as the shorter spliced mRNA as 140-nt (Fig 1A) or 180-nt (Fig 1B) fragments. Precursors should only be visible in the nuclear lanes, whereas the spliced message is detected in the nucleus as well as cytoplasm. Bcl-2 transcription has previously been shown to be increased in the t(14;18)-bearing cell line SU-DHL-6, when compared with the nontranslocated SU-DHL-9 line.6 As shown in Fig 1A and B, the steady-state level of nuclear precursors was only slightly greater in SU-DHL-6 compared with SU-DHL-9 as detected by S1 nuclease protection. However, the amount of spliced cytoplasmic message was fivefold to 10-fold higher in SU-DHL-6 compared with SU-DHL-9. This occurred even though the Bcl-2–Ig RNA half-life remained unchanged after translocation.6 An approximately threefold increase in spliced nuclear message was noted in SU-DHL-6 versus SU-DHL-9. In addition, a twofold to threefold increase in the ratio of cytoplasmic to nuclear spliced RNA was noted in SU-DHL-6 compared with SU-DHL-9. These values were measured densitometrically and corrected to an equalized β-actin signal. Using a separate S1 nuclease protection assay for β-actin, which detected β-actin precursors, we verified that both cell lines processed β-actin pre-mRNA in an equivalent fashion (data not shown). These observations suggest a distinct posttranscriptional advantage for the Bcl-2–Ig fusion transcripts compared with the normal Bcl-2 transcripts at the level of splicing and/or nucleocytoplasmic transport.
Comparison of nuclear precursors, spliced nuclear mRNA, and cytoplasmic RNA. Single-stranded S1 nuclease protection probes and protected fragments are shown at the bottom. The m13-probes were uniformly labeled. The lack of visible bands at nt 735 (Bcl-2) and nt 183 (β-actin) indicates that nuclease digestion is complete and that only RNA is detected. Cohybridization of the same sample with a β-actin probe controls for equal amounts of RNA. Note that the β-actin probe used here contains only exon sequences (no precursor). (A) Bcl-2 intron II-exon III border. (B) Exon II-intron II border. Precursor protection of the upstream exon is much stronger and probably reflects increased polymerase loading at the 5′ end.
Comparison of nuclear precursors, spliced nuclear mRNA, and cytoplasmic RNA. Single-stranded S1 nuclease protection probes and protected fragments are shown at the bottom. The m13-probes were uniformly labeled. The lack of visible bands at nt 735 (Bcl-2) and nt 183 (β-actin) indicates that nuclease digestion is complete and that only RNA is detected. Cohybridization of the same sample with a β-actin probe controls for equal amounts of RNA. Note that the β-actin probe used here contains only exon sequences (no precursor). (A) Bcl-2 intron II-exon III border. (B) Exon II-intron II border. Precursor protection of the upstream exon is much stronger and probably reflects increased polymerase loading at the 5′ end.
Processing constructs identify a posttranscriptional RNA processing advantage for an Ig versus Bcl-2 3′ end.
A series of constructs was designed to test the influence of various Bcl-2 or Bcl-2–Ig molecules on posttranscriptional processing (Fig 2). To eliminate any differential influence from Bcl-2 promoter activity, all constructs used an identical strong promoter/enhancer system, human β-actin.34 Because β-actin, Bcl-2, and Ig genes are well conserved and function cross-species,7 33 we generated human based processing constructs and transfected them into murine target cells. All constructs contain an intron (IVS I of β-actin) to insure that each transcript is targeted to a spliceosome for processing. βAPr-Bcl-2 or βAPr-Ig constructs were cotransfected with RSV Neo R vector into the S194 murine plasmacytoma cell line by electroporation. Stable transfectants were selected with G418 and demonstrated comparable rates of transcription from either integrated construct when assessed by nuclear run-on analysis (Fig 3). The presence of the Ig heavy chain enhancer did not further augment newly initiated transcription in this system.
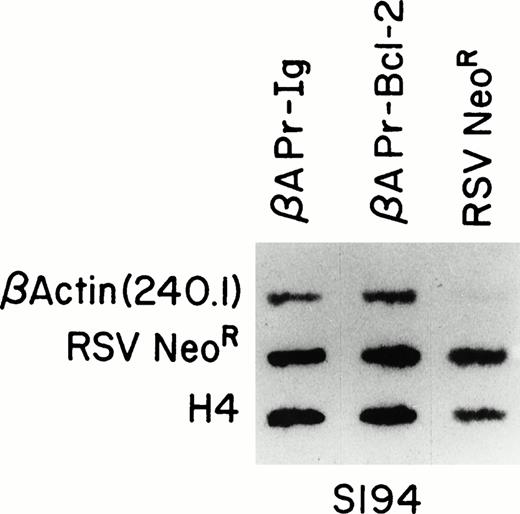
Run-on assays showing equal transcriptional activity ofβAPr-Bcl-2 and βAPr-Ig constructs in stable transfectants of a mouse B-cell line. Nascent, labeled RNA extracted from S194 cell lines transfected with βAPr-Bcl-2, βAPr-Ig,or only the RSVneo vector was hybridized to DNA probes detecting the human β-actin RNA produced by the Bcl-2 and Ig constructs (upper row), the RSVneo RNA produced by all three cell lines transfected with the neomycine resistance gene (middle row), and the mouse histone H4 gene integral to the S194 cell line (lower row). The 145-nt β-actin fragment (p240.1) is specific for the βAPr-constructs and is therefore only seen in lanes 1 and 2.
Run-on assays showing equal transcriptional activity ofβAPr-Bcl-2 and βAPr-Ig constructs in stable transfectants of a mouse B-cell line. Nascent, labeled RNA extracted from S194 cell lines transfected with βAPr-Bcl-2, βAPr-Ig,or only the RSVneo vector was hybridized to DNA probes detecting the human β-actin RNA produced by the Bcl-2 and Ig constructs (upper row), the RSVneo RNA produced by all three cell lines transfected with the neomycine resistance gene (middle row), and the mouse histone H4 gene integral to the S194 cell line (lower row). The 145-nt β-actin fragment (p240.1) is specific for the βAPr-constructs and is therefore only seen in lanes 1 and 2.
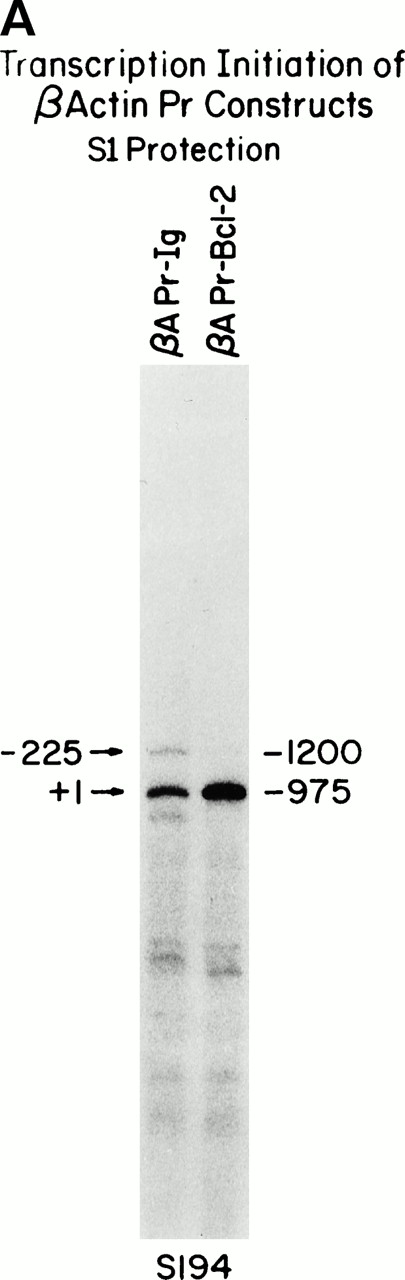
An S1 protection assay designed to assess the 5′ region of human β-actin showed that both the βAPr-Bcl-2 andβAPr-Ig constructs demonstrated correctly initiated transcription (Fig 4A). A few minor initiation sites were noted surrounding the β-actin promoter in the β-APr-Ig cell lines (Fig 4A). Two additional minor start sites were mapped by primer extension analysis to IVS I, immediately upstream of the acceptor splice site (Fig 4B). S1 analysis indicated that these intron-initiated sites (denoted as 117 and 125) were slightly augmented by the Ig enhancer (Fig 5). However, the vast majority of transcripts were still correctly initiated upstream.
Mapping construct RNA initiation sites. (A) S1 protections showing comparable amounts of transcripts correctly initiated at the human β-actin promoter start site (nt +1 corresponding to a protection of 975 nt). A few minor start sites upstream of the promoter and in the IVS-I are flushed on in theβAPr-Ig cell line. The 1.4-kb human β-actin promoter fragment (34) in bluescript (p229.6, see the Materials and Methods) was end-labeled at the EcoRI site and used for S1-protection of the transfected human allele. (B) The primer extension assay verifies the location of additional intron start sites in the βAPr-Ig cell lines, which are also seen as the middle bands on the S1 protection in Fig 5 (βAPr-Ig). A 20-nt primer starting from theEcoRI site of the β-APr plasmid (p229.6) was used.
Mapping construct RNA initiation sites. (A) S1 protections showing comparable amounts of transcripts correctly initiated at the human β-actin promoter start site (nt +1 corresponding to a protection of 975 nt). A few minor start sites upstream of the promoter and in the IVS-I are flushed on in theβAPr-Ig cell line. The 1.4-kb human β-actin promoter fragment (34) in bluescript (p229.6, see the Materials and Methods) was end-labeled at the EcoRI site and used for S1-protection of the transfected human allele. (B) The primer extension assay verifies the location of additional intron start sites in the βAPr-Ig cell lines, which are also seen as the middle bands on the S1 protection in Fig 5 (βAPr-Ig). A 20-nt primer starting from theEcoRI site of the β-APr plasmid (p229.6) was used.
Lineage specificity and intron dependency. The fragments protected by the end-labeled S1 probe are shown at the bottom. Total RNA from transfected cell lines was used. (A) Mature B-cell line; (B) pre-B–cell line; (C) non–B-cell (myeloid) line; (D) mature B-cell transfected with the βAPr-Ig▵Introns andβAPr-IgςIntron constructs.
Lineage specificity and intron dependency. The fragments protected by the end-labeled S1 probe are shown at the bottom. Total RNA from transfected cell lines was used. (A) Mature B-cell line; (B) pre-B–cell line; (C) non–B-cell (myeloid) line; (D) mature B-cell transfected with the βAPr-Ig▵Introns andβAPr-IgςIntron constructs.
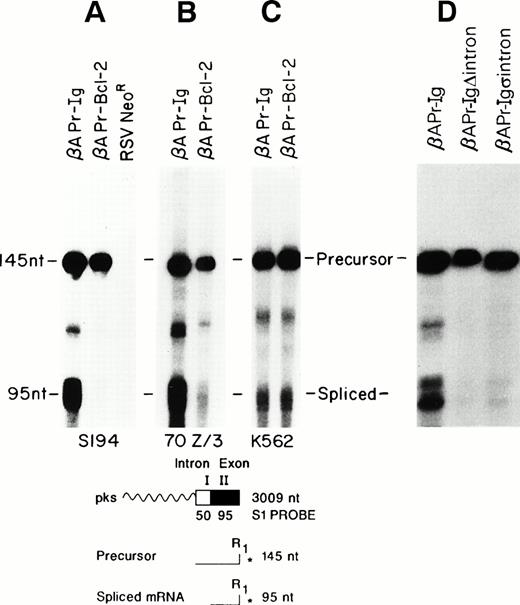
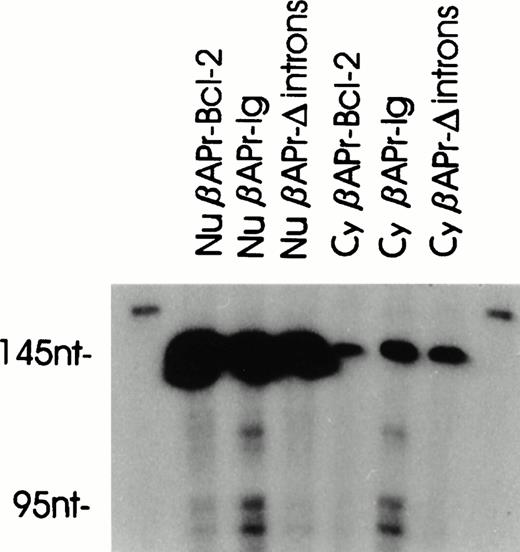
Stable integrants of the βAPr-Bcl-2 or βAPr-Igconstructs within the S194 plasmacytoma cell line were assessed for their efficiency of RNA processing. The inclusion of the β-actin intron within these constructs enabled the creation of a generic S1 protection assay that could distinguish nuclear precursors from spliced RNA (Fig 5). Both populations of stably transfected S194 cells showed comparable amounts of nuclear precursor RNA from eitherβAPr-Ig or βAPr-Bcl-2 constructs. Yet, the constructs with an Ig 3′ end had a marked processing advantage. The βAPr-Ig demonstrated log-fold greater amounts of spliced RNA product compared with the βAPr-Bcl-2 construct (Fig 5A). To insure that this difference in RNA processing was evident at a single-cell level, the stably transfected S194 bulk cell lines were cloned by limiting dilution. All subclones of S194 examined confirmed the same dichotomy in processing between constructs bearing Ig versus Bcl-2 3′ ends (Fig 6). Thus, the posttranscriptional advantage noted for the Bcl-2–Ig fusion RNA in t(14;18)-bearing cells was reproduced in these processing constructs.
RNA processing in oligoclonal subpopulations. S1 protection on total RNA from transfected cell lines. The probe (for schematic, see Fig 5) is specific for the transfected exon II-intron I sequences. The middle bands in the βAPr-Ig lanes correspond to additional IVS-I start sites.
RNA processing in oligoclonal subpopulations. S1 protection on total RNA from transfected cell lines. The probe (for schematic, see Fig 5) is specific for the transfected exon II-intron I sequences. The middle bands in the βAPr-Ig lanes correspond to additional IVS-I start sites.
Lineage-specific processing of the Ig 3′ end.
We wished to assess whether the processing advantage conferred by the Ig sequences was restricted to highly differentiated B-cell lineages such as S194. Consequently, stable transfectants of the pre-B–cell line 70z/3 were generated with both constructs. The amount of 95-nt spliced RNA detected by the S1 assay was markedly greater for theβAPr-Ig compared with the βAPr-Bcl-2 construct in this pre-B–cell line as well (Fig 5B). In contrast, no considerable processing advantage was noted for the Ig 3′ end when the constructs were introduced into a non–B-lineage cell, the K562 erythroleukemia cell line (Fig 5C).
The processing advantage is conferred by the introns of the Ig heavy chain gene.
As a first step to define the location of the processing effect conferred by the Ig 3′ end, a construct was generated substituting an Ig heavy chain cDNA for the 3′ end,βAPr-IgΔIntrons (Fig 2). Removal of the Ig introns eliminated the posttranscriptional advantage conferred by the complete Ig 3′ tail when assessed in S194 (Fig 5D). Moreover, substitution of the JH6-C-γ1 intron by an unrelated, non-Ig intron (the IVS II of the human β-actin gene, βAPr-Igs intron; Fig2) did not significantly improve processing of the fusion RNA, arguing that the responsible mechanism is directly related to the presence of Ig introns on the construct (Fig 5D).
To prove the validity of the constructs in reflecting the processing of the cellular Bcl-2–IgH fusion genes, we determined the half-life of the precursors as well as the spliced messages of the constructs in S194. The actinomycin D half-lives of all precursors were between 1.5 and 2.5 hours (Table 1). The half-life of the spliced RNA could only be determined in the case ofβAPr-Ig, whereas the basal levels of the other RNAs were too low. However, the half-life of βAPr-Ig was identical to that of the cellular Bcl-2–IgH RNA previously measured in t(14;18) cell lines.6 We further investigated nuclear and cytoplasmic RNA from S194 cells transfected with βAPr-Bcl-2, βAPr-Ig, orβAPr-IgΔintrons (Fig 7). Whereas the amounts of precursor in the nucleus were similar, the level of spliced RNA was higher in βAPr-Ig. Consequently, the cytoplasmic spliced message was predominantly present in the cell line containing this construct. The pattern of nuclear and cytoplasmic RNAs was very similar to that of the cellular counterparts shown in Fig 1B, suggesting that the constructs closely reflect the processing of Bcl-2 or Ig RNA. Interestingly, the βAPr-IgΔintrons construct produced much less spliced message than βAPr-Ig, despite having the same amount of nuclear precursor, suggesting again a processing advantage for the intron-containing construct.
Distribution of precursor and spliced construct RNA in the nucleus and cytoplasm of S194. A high but equal amount of precursors is present in nuclear RNA for all three constructs (βAPr-Bcl-2, βAPr-Ig, and βAPr-Ig▵introns), whereas spliced RNA is predominantly present in βAPr-Ig(nucleus and cytoplasm). Precursor bands in the cytoplasm represent low-level contamination with nuclear RNA.
Distribution of precursor and spliced construct RNA in the nucleus and cytoplasm of S194. A high but equal amount of precursors is present in nuclear RNA for all three constructs (βAPr-Bcl-2, βAPr-Ig, and βAPr-Ig▵introns), whereas spliced RNA is predominantly present in βAPr-Ig(nucleus and cytoplasm). Precursor bands in the cytoplasm represent low-level contamination with nuclear RNA.
DISCUSSION
Posttranscriptional processing as a novel mechanism of oncogene deregulation.
Chromosomal translocations frequently result in the deregulation of oncogenes through increased mRNA production. Transcriptional activation after juxtaposition with the Ig enhancer has been described for the c-myc and Bcl-2 proto-oncogenes.6,42 In the case of c-myc, abrogation of an elongation block as well as a promotor shift have also been noted.43 However the alteration of posttranscriptional processing of the Bcl-2–Ig fusion RNAs represent a novel mechanism of oncogene deregulation.
The Bcl-2–Ig RNA product of the t(14;18) demonstrated a posttranscriptional processing advantage when compared with normal Bcl-2 RNA. This marked difference was first noted by comparing Bcl-2 precursor RNA with final spliced mRNA products in mature B-cell lines with and without the t(14;18). This processing difference appeared to be specific for the Bcl-2–Ig fusion RNA, because both cell lines processed endogenous β-actin RNA in an equivalent manner. An increased rate of newly initiated transcription of the Bcl-2–Ig fusion gene in t(14;18) cells compared with the normal Bcl-2 gene in mature B-cell lines lacking the translocation has been documented.6 11 To eliminate the influence of transcriptional differences, we generated a series of constructs to further evaluate the processing advantage of Bcl-2–Ig fusion RNAs. These used a heterologous β-actin promoter-enhancer that conferred equivalent rates of transcription with either the Bcl-2 or Ig 3′ end. The analysis of precursor and spliced RNA products derived from the constructs in stable cell lines confirmed the processing advantage conferred by Ig versus Bcl-2 sequences. We believe that the constructs closely reflect the real processing mechanism, because theβAPr-Bcl-2 and βAPr-Ig constructs repeat the pattern of nuclear and spliced Bcl-2 versus Bcl-2–IgH RNA in Figs 1B and 7.
Posttranscriptional processing at the level of splicing or nucleo-cytoplasmic transport is an established mechanism of normal gene regulation. Examples include the regulation of HIV-1 expression by the rev or rex proteins,23,44-46 the influenza virus NS1,26 the polyoma early-late switch,47 the ribosomal L1 protein of Xenopus laevis,48 and the human c-fgr proto-oncogene.49 The ratios of Bcl-2 RNA species in nucleus and cytoplasm indicated that splicing and/or nucleocytoplasmic transport are enhanced in the t(14;18) cell lines. The latter mechanism modulates expression in a number of genes.50-53 Splicing and transport may be closely linked to each other by the association of the ribonucleoprotein complexes with the nuclear matrix.54-56 There is evidence that nuclear architecture and the organization of chromosomes are tissue specific57,58 and that even two copies of the same gene can be processed differently in the same nucleus.59
Implications on Ig regulation.
In 1985, Grosschedl and Baltimore29 showed that Ig RNA expression is regulated by at least three regions, including the VH promoter, the Ig enhancer, and intragenic sequences lacking the enhancer. Our experiments provide evidence that intragenic sequences located downstream of the JH regions play a role in splicing and transport. The regulated production of secretory versus membrane forms of Ig mRNAs has also been attributed to splicing60 or the choice of polyadenylation sites.61 We have tested both the Bcl-2 and Cγ membrane polyadenylation sites with the β-actin promoter in B cells and found no difference in their processing efficiency (U.J., unpublished results). Milcarek et al62 have shown that changes in the nuclear to cytoplasmic ratio are associated with differential expression of secretory to membrane-specific Igγ 2a heavy chain RNA. This suggests that RNA processing may play an important role in Ig regulation. Our data argue that these differences in processing are directly related to the presence of the Ig introns. It has previously been shown that insertion of a part of the Cγ1 switch region resulted in high-level expression of human IgH in transgenic mice, but not in transfected cell lines.63Moreover, a splicing enhancer for Cμ has been identified in the IgM M2 exon sequence.64 Removal of the JH-CH as well as the Cγ introns from our constructs (βAPr-IgΔintrons) resulted in a dramatic decrease in the spliced RNA species, despite the fact that the β-actin intron I is still retained to avoid completely intronless constructs that may not express RNA at all.65 Neuberger and Williams66 have shown that Ig expression increases with the addition of more Ig introns, yet no specific intron was solely required. Substitution of the JH-CH intron by a nonlymphoid intron (as in βApr-Igςintron) had no significant effect in our experiments. However, it is still possible that substitution of all Cγ introns will improve expression. In addition, a complex interaction between the Ig introns may be necessary for efficient expression, as is the case in the human triosephosphate isomerase gene, in which upstream introns have an effect on RNA 3′ end formation.67
It remains to be determined whether the presence of introns affects only splicing or also RNA stability. Unfortunately, it was impossible to determine the half-lives of the spliced messages except forβAPr-Ig, which was identical to that of its cellular Bcl-2–IgH counterpart. However, the fact that the intronless construct (βAPr-IgΔintrons) had almost no spliced nuclear message while having the same precursor half-life as βAPr-Ig argues strongly in favor of a processing effect, because both spliced messages should look identical and have the same decay rates.
Efficient processing of the βAPr-Ig constructs was seen in pre-B as well as mature B cells, indicating that this mechanism functions in various stages of B-cell differentiation.68However, the lack of significant processing differences in the nonlymphoid cell line K562 argues for a tissue specificity of this mechanism.
In conclusion, posttranscriptional processing constitutes a novel mechanism of activation that may contribute to the deregulation of oncogenes that produce fusion mRNAs after chromosomal translocations. In particular, fusion messages that contain Ig information, such as the LYT-10-Ca1 transcripts in the t(10;14)(q24;q32), could also be affected. In addition, any fusion RNA that juxtaposes messages with distinct lineage specificity may prove to have altered processing. Moreover, our findings may have implications for the regulation of normal Ig production, indicating a B-cell–specific, efficient pathway for the posttranscriptional handling of Ig RNA.
ACKNOWLEDGMENT
The authors thank M. Bergmann and E. Roth for their help, C. Milliman and T. Carlisle for expert technical assistance, T. Ley for stimulating discussions, and Roche Serena for support.
Supported by a grant of the “Max Kade Foundation,” by Grant No. P-7565 of the Austrian “Fonds zur Foerderung der wissenschaftlichen Forschung,” by grant “Kommission Onkologie“ of the University of Vienna, and by National Institutes of Health Grant No. P01 CA49712-05.
Presented in part at the Second Annual Meeting of the European Haematology Association, May 29, to June 1, 1996, in Paris, France.
Address reprint requests to Ulrich Jaeger, MD, Klinik fuer Innere Medizin I, Haematologie 6I, Waehringer Guertel 18-20, A-1090 Vienna, Austria.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.