To the Editor:
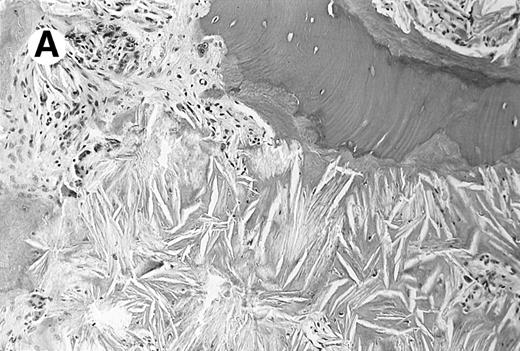
A 23-year-old woman described left-sided abdominal discomfort and stomach bloating for 4 months. Her past medical history was significant for recurrent kidney stones, which began at the age of 9 years. She developed progressive renal failure secondary to nephrolithiasis and for the past 3 years had required hemodialysis. Five months ago she developed a painless hard mass on the distal phalanx of her left fifth finger; the mass was biopsied. The biopsy specimen was consistent with granulomatous dermatitis with birefringent crystals in the dermis. The finger mass continued to enlarge, and a repeat biopsy was taken. On physical examination there was massive hepatosplenomegaly with no lymphadenopathy or peripheral edema. There was a nontender, firm, flesh-colored nodule on her left fifth distal phalanx. Laboratory studies showed pancytopenia with a hemoglobin level of 6.3/dL, a white blood cell count of 2,600/μL, and a platelet count of 106,000/μL. Values for serum aspartate transaminase, alanine transaminase, and bilirubin were normal. Her peripheral-blood film had numerous teardrop forms compatible with myelophthisis. A bone marrow biopsy revealed extensive crystal deposition with almost complete obliteration of hematopoietic cells (Fig1). Infrared spectroscopy performed on the bone marrow biopsy and finger mass identified calcium oxalate monohydrate crystals.
Photomicrograph of the bone marrow biopsy. (A) Oxalate crystal deposition seen adjacent to hematopoietic cells. (B) Same biopsy section viewed under polarized light showing the birefringent property of oxalate crystals.
Photomicrograph of the bone marrow biopsy. (A) Oxalate crystal deposition seen adjacent to hematopoietic cells. (B) Same biopsy section viewed under polarized light showing the birefringent property of oxalate crystals.
Conditions associated with calcium oxalate crystal deposition include primary and familial hyperoxaluria, a diet rich in oxalate, increased absorption or production of oxalate, and decreased excretion of oxalate as seen in renal failure. The most likely cause of calcium oxalate deposition in this patient was primary hyperoxaluria. To confirm the diagnosis a liver biopsy was obtained and showed markedly reduced alanine:glyoxylate aminotransferase (AGT) activity consistent with primary hyperoxaluria type 1 (PH1). PH1 is inherited as an autosomal recessive disorder characterized by hyperoxaluria, calcium oxalate urinary lithiasis in childhood, nephrocalcinosis, and renal failure.1 Once renal failure occurs, blood oxalate concentrations rise and precipitation occurs throughout the body. This stage is termed “oxalosis.” Extrarenal manifestations of oxalosis are becoming more apparent as hemodialysis is being used to treat end-stage renal failure in PH1. We corroborate that pancytopenia is a complication of oxalosis, as manifested by this patient, and believe it indicates the need for aggressive therapeutic intervention.2
Therapy for oxalosis should be pursued early in disease because oxalate deposited in tissues can be resorbed once normal production and urinary excretion are maintained.1 Exogenous oxalate ingestion should be avoided in PH1, although only 10% of excreted oxalate is from dietary origin. The remainder of excreted oxalate comes from endogenous production. Definitive treatment of PH1 requires AGT enzyme replacement, which can be achieved with liver transplantation.1,3 This ultimately reduces endogenous production of oxalate and in combination with renal transplantation has been successful in treating PH1 patients with irreversible kidney failure.3 The patient was referred for evaluation of a kidney-liver transplant and remains on hemodialysis.