IN 1948, BERNARD AND SOULIER described a young male patient with a severe bleeding disorder that was characterized by a prolonged bleeding time, thrombocytopenia, and extremely large platelets.1 They termed the disorder “la dystrophie thrombocytaire-hémorragipare congénitale.” Since then, an identical or similar disorder has been described in a large number of individuals, virtually always transmitted in an autosomal recessive manner and often occurring in persons whose parents are close relatives.
The first clue to the molecular abnormality affecting the platelets of patients with this disorder (now known as the Bernard-Soulier syndrome [BSS]) came in 1969 from the work of Gröttum and Solum,2 who noted reduced electrophoretic mobility of the platelets due to a marked decrease in the concentration of sialic acid on their membranes. Subsequently, Howard et al3 and Caen and Levy-Toledano4 found that the platelets of BSS patients failed to aggregate to ristocetin, a peptide antibiotic known to aggregate normal platelets but not the platelets of patients suffering from von Willebrand disease. Weiss et al5 in 1974 extended this observation by demonstrating a defect in the ability of BSS platelets to adhere to rabbit aortic subendothelium. They also suggested that the defect resulted from absence of a receptor for von Willebrand factor (vWF) on the platelet surface. Numerous other phenotypic abnormalities have been described in BSS, including defective platelet aggregation to bovine vWF,3,6abnormalities of membrane phospholipid content7,8 and coagulant activity,6,8 and morphological characteristics that include large size and disordered cytoskeletal structure.9 10
The nature of the missing vWF receptor was suggested in 1975 when Nurden and Caen11 demonstrated that 1 of the 3 major carbohydrate-containing proteins on the platelet surface, glycoprotein I, was virtually absent in the platelets of BSS patients. The biochemical defect was defined further in the laboratories of Clemetson et al12 and Berndt et al,13 when they demonstrated, in unrelated patients with BSS, deficiencies of 4 polypeptides: glycoproteins (GP) Ibα, Ibβ, IX, and V. These polypeptides all associate on the platelet surface to form a receptor called the GP Ib-IX-V complex.
The importance of this receptor for normal hemostasis is perhaps best illustrated by the clinical history of the original patient described by Bernard and Soulier.14 As both a child and a young man, this patient suffered numerous bleeding problems, including prolonged bleeding after tooth extraction, life-threatening cerebrospinal hemorrhage, and orbital and periorbital hematomas after an accident. He died at 28 years of age of intracranial hemorrhage after a barroom brawl.
THE GP Ib-IX-V COMPLEX: STRUCTURE AND FUNCTION
The GP Ib-IX-V complex has two important roles in platelet function that explain the often severe bleeding observed in BSS: it mediates adhesion to the blood vessel wall at sites of injury by binding vWF and it facilitates the ability of thrombin at low concentrations to activate platelets.15 The interaction with vWF underlies another potentially important function that may be more relevant to thrombosis than to hemostasis: shear-induced platelet aggregation.16 Furthermore, the GP Ib-IX-V complex may have important roles in the process by which platelets are generated and possibly in platelet turnover, as evidenced by the decreased number and abnormal size of platelets from BSS patients.
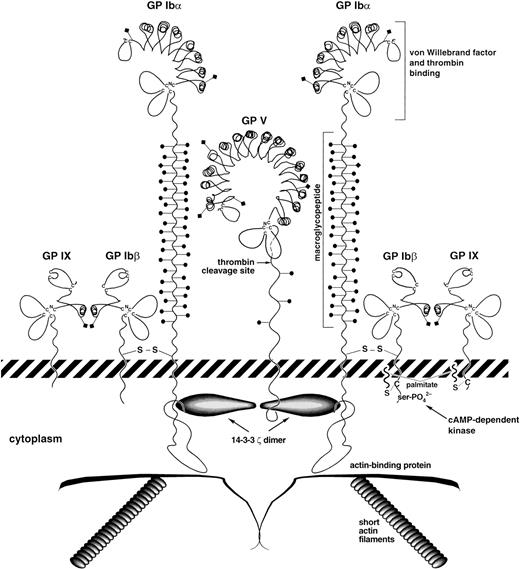
The key structural features of the GP Ib-IX-V complex are depicted schematically in Fig 1. The complex comprises 4 distinct transmembrane polypeptide subunits, GP Ibα, GP Ibβ, GP IX, and GP V, with a stoichiometry based on monoclonal antibody binding of 2:2:2:1, respectively.17-20 Each of the 4 subunits is a member of the leucine-rich repeat motif superfamily, members of which are involved in such diverse processes as cell signaling, cell adhesion, and development.21,22 In the polypeptides of the GP Ib-IX-V complex, the leucine-rich repeat sequences are approximately 24 amino acids in length, occur singly or in tandem repeats, and are flanked by conserved N- and C-terminal disulfide loop structures.22 However, despite these structural similarities, the polypeptides comprising the GP Ib-IX-V complex all arise from distinct genes residing in different regions of the genome.23-27
Schematic view of the platelet GP Ib-IX-V complex. Key structural features of the complex are shown. The leucine-rich repeats of the four polypeptides are drawn based on the structure determined for the porcine ribonuclease inhibitor, a protein made up entirely of leucine-rich repeats.32 The depicted polypeptide arrangement is based on the published stoichiometry determined by monoclonal antibody binding17-19 and on the associations determined for the polypeptides.47,112 A caveat about this depiction: the quantity of GP V on the platelet surface has only been determined using 2 GP V monoclonal antibodies,18,20 which could lead to overestimates or underestimates of true polypeptide number. In addition, no quantitation has ever been performed to indicate that every GP V molecule on the platelet surface is associated with the complex. Complexes of greater complexity having the same stoichiometry are also possible.22 82 Diamonds on stalks represent N-linked carbohydrates and circles on stalks represent O-linked carbohydrate.
Schematic view of the platelet GP Ib-IX-V complex. Key structural features of the complex are shown. The leucine-rich repeats of the four polypeptides are drawn based on the structure determined for the porcine ribonuclease inhibitor, a protein made up entirely of leucine-rich repeats.32 The depicted polypeptide arrangement is based on the published stoichiometry determined by monoclonal antibody binding17-19 and on the associations determined for the polypeptides.47,112 A caveat about this depiction: the quantity of GP V on the platelet surface has only been determined using 2 GP V monoclonal antibodies,18,20 which could lead to overestimates or underestimates of true polypeptide number. In addition, no quantitation has ever been performed to indicate that every GP V molecule on the platelet surface is associated with the complex. Complexes of greater complexity having the same stoichiometry are also possible.22 82 Diamonds on stalks represent N-linked carbohydrates and circles on stalks represent O-linked carbohydrate.
GP Ibα (135 kD, 610 amino acids) consists of an N-terminal globular domain28 that contains 7 tandem leucine-rich repeats and their flanking sequences, a 19-amino acid sequence rich in negatively charged aspartate and glutamate residues, and 3 sulfated tyrosines,29,30 a highly glycosylated, macroglycopeptide mucin core, a single transmembrane sequence, and a cytoplasmic tail of 96 amino acid residues.31 The structure of the leucine-rich repeats depicted in Fig 1 is based on the x-ray crystal structure of porcine ribonuclease inhibitor, a protein made up entirely of leucine-rich repeats.32 In this structure, each repeat forms a β-α structural unit (a short β-strand parallel to an α-helix), resulting in a horseshoe-shaped molecule in which the helices form the outer circumference and the β-strands form the inner surface. If the GP Ib-IX-V leucine-rich repeats adopt a similar structure, this produces a fan-shaped surface with most of the amino acid side chains exposed to solvent, a property that may maximize surface interactions with target proteins and that also has the effect of bringing the flanking sequences into proximity. The macroglycopeptide contains an O-linked, sialylated hexasaccharide on average every 3 to 4 amino acids,33-35creating a scaffold that extends the N-terminal globular domain and vWF binding site approximately 45 nm from the surface of the platelet plasma membrane.28 This region is highly polymorphic. In any individual, its length depends on which combination of 4 possible alleles is inherited. The products of these alleles differ in having 1, 2, 3, or 4 tandemly repeated copies of a 13-amino acid sequence,36,37 each of which has been predicted to add about 32 Å to the length of the macroglycopeptide.36
GP Ibβ (25 kD, 181 amino acids) has a single leucine-rich repeat and is disulfide-linked to GP Ibα immediately proximal to the platelet plasma membrane.38 The cytoplasmic sequence of 34 amino acids contains a protein kinase A phosphorylation site at Ser16639 that appears to regulate platelet cytoskeletal rearrangement in response to agonist stimulation.40
GP IX (22 kD, 160 amino acids), like GP Ibβ, has a single leucine-rich repeat motif41 and remains associated with GP Ib as a 1:1 complex when purified in Triton X-100.42 It has a short cytoplasmic tail of 5 amino acids. The cytoplasmic sequences of GP Ibβ and GP IX both have a membrane-proximal cysteinyl residue that can be palmitoylated in vitro, a modification that may provide additional anchorage for the complex in the platelet membrane.43 Analysis of guinea pig megakaryocyte proteins suggests that GP IX is primarily myristoylated rather than palmitoylated.44
GP V (82 kD, 544 amino acids) has 15 leucine-rich repeats and a short cytoplasmic tail of 16 amino acids.45,46 It is thought to bridge adjacent GP Ib-IX complexes through an interaction with GP Ibα.47 The other feature of GP V is that it is one of a limited set of thrombin substrates on the platelet plasma membrane, with a major fragment, GP Vf1 (69.5 kD), released from the surface of thrombin-treated platelets.48 The functional significance of this cleavage in platelet physiology remains unclear.
The principal function of the GP Ib-IX-V complex in hemostasis is to initiate the arrest of platelets at sites of vascular injury. Like other adhesion receptors, ligation of the GP Ib-IX-V complex by vWF can transduce signals to the platelet cytoplasm, initiating the cascade of events that leads to the formation of a hemostatic platelet plug. However, unlike other adhesion receptors, the GP Ib-IX-V complex is a unique adhesive system unrelated in structure to members of the integrin, selectin, or Ig superfamilies, which mediate other aspects of blood cell–vessel wall interaction.49 The binding site for the GP Ib-IX-V complex resides within the A1 domain of vWF,50,51 included within residues 480-718 of the mature sequence.52 Mature vWF has a subunit molecular weight of 230,000 (2,050 amino acids)53 and circulates in a nonadhesive form consisting of disulfide-linked multimers of up to 20 × 106 in molecular weight.54 vWF bound to the subendothelial matrix is believed to undergo a conformational change that reveals a normally cryptic binding site for the GP Ib-IX-V complex within its A1 domain.55 vWF also binds to the GP Ib-IX-V complex under the influence of high shear forces16by induction of conformational changes in either the receptor or vWF or in both.56,57 Consistent with this finding, gain-of-function mutations occur in both the receptor and in vWF that enhance the receptor-ligand interaction. In platelet-type (or pseudo) von Willebrand disease, mutations of GP Ibα (Met239→Val58 or Gly233→Val59) result in a receptor complex with higher affinity for circulating vWF.60,61 In type 2B von Willebrand disease, point mutations in the vWF A1 domain clustered around the Cys509-Cys695 disulfide bond and between Met540 and Arg578 yield a form of vWF with enhanced avidity for the native GP Ib-IX-V receptor on platelets.62 A number of modulators have been identified that also enhance the interaction between vWF and the GP Ib-IX-V complex.63 These include the antibiotic ristocetin, from the gram-negative bacterium Nocardia lurida, which appears to function, at least in part, by binding to proline-rich sequences flanking the disulfide bond between Cys509 and Cys695 in the vWF A1 domain.64-66 A second modulator, botrocetin (a disulfide-linked heterodimer of 28 kD from the venom of the South American pit viper, Botrops jararaca) activates vWF adhesive function towards platelets by binding to noncontiguous sequences within the A1 domain loop.66 67
The regions involved in the binding of vWF to GP Ibα have only been partially defined and appear to be dependent, in part, on conformational structure in both the ligand and receptor. In vWF, both the peptide sequence, Asp514 to Glu542,66 and the region encompassing Glu596 and Lys59968 have been proposed as receptor recognition sites. In GP Ibα, the vWF binding site is located within the N-terminal approximately 300 amino acids.30,69,70 Three regions within this domain appear to be important for vWF binding (Fig 2). One corresponds to the anionic sulfated-tyrosine sequence,29,30,71,72 which appears to be preferentially involved in botrocetin-dependent binding of vWF.30,71Sulfation of tyrosine residues in this sequence is more critical for botrocetin-dependent than for ristocetin-dependent binding of vWF,72 but both modulators require the modification for optimum effect. An Escherichia coli-produced GP Ibα fragment containing the sequence encompassing Gln221-Leu318 has been reported to contain the ristocetin-dependent binding site for vWF, with a disulfide-bond between Cys248 and Cys264 critical for function.73 Because Cys248 and Cys264 are normally disulfide-bonded to Cys209 and Cys211, respectively,74 the significance of this finding is not clear. The leucine-rich repeats also appear to have an important role in vWF binding, as suggested by studies of BSS patients who express mutant GP Ib-IX-V complexes on their platelets. Platelets expressing these mutant complexes, which both result from mutations in the GP Ibα leucine-rich repeats (Leu47→Phe75 and Ala156→Val76), bound vWF less efficiently than did normal platelets. Finally, two gain-of-function mutations (Gly 233→Val59 and Met 239→Val58) in platelet-type von Willebrand disease are located in the flanking sequence C-terminal to the leucine-rich repeats.22 Both of these mutants spontaneously bind vWF in the absence of ristocetin, botrocetin, or shear, implying that this domain may be directly involved in vWF binding or could regulate that function.
The GP Ibα N-terminus with the regions shown to be important for vWF binding. Asterisks indicate that the tyrosines are sulfated.
The GP Ibα N-terminus with the regions shown to be important for vWF binding. Asterisks indicate that the tyrosines are sulfated.
Available evidence indicates that the GP Ib-IX-V–vWF interaction may in many ways be similar to the interaction between selectins and their ligands. Similar to the rolling of leukocytes mediated by selectins, recent observations indicate that the GP Ib-IX-V complex can mediate translocation of platelets along a surface coated with vWF. Such a phenomenon requires that the bonds be able to form and break rapidly. In the studies of Savage et al,77 the vWF–GP Ib-IX-V interaction could slow the platelets in this way, but a further interaction between vWF and the GP IIb-IIIa complex was required to fully arrest the platelets. As yet, it is unclear how this in vitro phenomenon relates to the situation in vivo, because vWF in the environment of the subendothelium may adapt a different conformation than when immobilized on glass. The influence of vWF conformation on platelet translocation was nicely demonstrated in the studies of Moroi et al78 (in a system similar to that of Savage et al77), who demonstrated that addition of botrocetin to vWF immobilized on glass markedly decreased platelet translocation, presumably because it increased the affinity of the interaction.
Thrombin also binds within the N-terminal sequence, His1-Glu282, of GP Ibα, specifically to the anionic sulfated-tyrosine sequence.30,79 Thrombin recognition, in contrast to the binding of vWF, has a greater stringency requirement for tyrosine sulfation in that all 3 tyrosine residues must be sulfated for effective binding of thrombin to GP Ibα.72 High-affinity binding to the GP Ib-IX-V complex may also involve recognition of segments of the leucine-rich repeat C-terminal flanking sequence80,81 and GP V.82 Although the agonist action of thrombin towards platelets primarily involves signaling through the 7-transmembrane PAR-1 and/or PAR-3 thrombin receptors,83,84 binding of thrombin to the GP Ib-IX-V complex facilitates the platelet response to low concentrations of thrombin.85-87 A defective response to thrombin undoubtedly contributes to the bleeding diathesis of patients with BSS. A detailed discussion of the nuances of thrombin's association with the GP Ib-IX-V complex is beyond the scope of this review. The interested reader is referred to the recent review of Jamieson.88
Although it is unknown how thrombin signals through the GP Ib-IX-V complex, there is increasing evidence that ligation of vWF initiates signaling events that result ultimately in inside-out activation of the integrin, GP IIb-IIIa, and platelet aggregation.16,89Signaling by other adhesion receptors can be initiated by receptor cross-linking90; recent evidence suggests that a similar mechanism may be operative in GP Ib-IX-V–dependent signaling in platelets. First, a monomeric 39/34-kD proteolytic fragment of vWF is able to bind to the GP Ib-IX-V complex and inhibit binding of multimeric native vWF, but does not activate platelets.52Second, GP Ibα is arranged on the cell surface as part of a larger receptor complex, with two or more GP Ibα subunits forming a cluster with the other glycoproteins of the complex.22 Third, GP Ibα is associated via its cytoplasmic region with actin-binding protein and 14-3-3ζ protein (see below and Fig 1), both of which form noncovalent dimers. Finally, the 50-kD (presumably bivalent) viper venom protein, alboaggregin, binds to GP Ibα and activates platelets, whereas structurally related monomeric 25-kD venom proteins bind to the same domain on GP Ibα, but do not activate platelets.91
The signaling events induced by vWF binding to GP Ib-IX-V in the presence of shear, ristocetin, or botrocetin include elevation of cytosolic Ca2+ and activation of protein kinases.92-95 Ser/Thr protein kinases become activated, as 2 of their substrates, pleckstrin and the myosin light chain, are rapidly phosphorylated.92 Two major tyrosine kinase substrates (∼76 and ∼36 kD) also become phosphorylated,92 but the identity of neither is known.95 Interestingly, both species are also phosphorylated in response to 50-kD alboaggregin.91 Other consequences of vWF binding to GP Ib-IX-V include association of activated phosphatidylinositol 3-kinase (PI 3-kinase) and src with the cytoskeleton,94 breakdown of phosphatidylinositol 4,5-bisphosphate, generation of phosphatidic acid, activation of phospholipase A2, and synthesis of arachidonic acid and thromboxane A2.92
One of the interesting features of the GP Ib-IX-V complex is that none of the cytoplasmic sequences of its 4 constituent polypeptides contains motifs known to interact with signaling proteins. Nevertheless, these regions do interact with proteins of the platelet membrane cytoskeleton, providing a potential means for the complex to transduce activation signals. The cytoplasmic domain of GP Ibα contains a binding site for actin-binding protein within the sequence Thr536 to Leu554.96 This association with actin-binding protein links the complex with a network of short submembranous actin filaments.97,98 This membrane skeleton of quiescent platelets contains other cytoskeletal proteins, including spectrin, dystrophin, talin, vinculin, and protein 4.1, and several signaling proteins, including the tyrosine kinases src, yes, and syk, the small G protein, p21 ras, and the tyrosine phosphatase, SHP 1.99-101 In unstimulated platelets, much of the GP IIb-IIIa complex is also attached to the membrane skeleton,99suggesting that one of the functions of this structure may be to preassemble key signaling elements, allowing transmission of signals after GP Ib-IX-V ligation, eventually leading to GP IIb-IIIa activation. Consistent with a role for cytoskeletal attachment in GP Ib-IX-V functions, recent studies show that even small C-terminal truncations of GP Ibα greatly increase the mobility of the complex within the plane of the plasma membrane and decrease its ability to bind vWF.102
A second possible mechanism by which the GP Ib-IX-V complex may transmit signals derives from the recent finding that its cytoplasmic domain contains binding sites for the ζ isoform of 14-3-3.103,104 Although platelet 14-3-3ζ was originally reported to have phospholipase A2 activity,105 this enzymatic activity was not found in other studies.106Rather, 14-3-3 proteins have recently been shown to regulate the activity and assembly of key signaling molecules that, in turn, regulate such diverse processes as mitogenesis, cell cycling, vesicular transport, and apoptosis. Proteins reported to bind 14-3-3 include the cell death agonist BAD, raf-1, bcr, cbl, PKCε, PKCγ, the cdc25a and cdc25b phosphatases, the p85 subunit of PI 3-kinase, tyrosine hydroxylase, tryptophan hydroxylase, and ADP ribosyltransferase.107-109 The 14-3-3 protein family consists of a number of closely related isoforms with subunit molecular weights of approximately 30 kD that form highly stable homodimers and heterodimers.107 This latter property allows them to bridge and assemble cytoplasmic proteins containing 14-3-3 recognition motifs. The 14-3-3 isoform most commonly identified as binding signaling molecules is 14-3-3ζ.107
Recent analysis of 14-3-3 binding to raf-1 has identified 2 nonoverlapping binding sites for 14-3-3 within raf-1.108Both sites contain serines within a conserved R S X S X P motif, a motif also found in other 14-3-3 binding proteins, including PKCε, cdc25b, bcr, and BAD. The binding of 14-3-3 to these sites is regulated by phosphorylation, with the presence of phosphate on the serine favoring binding. Within the GP Ib-IX-V complex, a major binding site for 14-3-3ζ corresponds to the 4 C-terminal amino acids of GP Ibα, Gly-His-Ser-Leu.104,110 Additional binding sites have been identified by analysis of overlapping peptides corresponding to the cytoplasmic sequences of GP Ibα, GP Ibβ, GP IX, and GP V.110 These include the central region of the GP Ibα cytoplasmic domain (Arg557-Gly575) and the entire cytoplasmic tail of GP V. Another binding site for 14-3-3ζ encompasses the PKA phosphorylation site in GP Ibβ. Serine phosphorylation of a synthetic peptide containing this sequence increased its affinity for 14-3-3ζ. This effect of phosphorylation on a 14-3-3ζ–binding sequence in GP Ibβ suggests an additional effect of PKA-dependent phosphorylation on regulating platelet activation. Because GP Ibβ phosphorylation specifically inhibits actin polymerization,40 the increased affinity for 14-3-3ζ is consistent with a role for this protein in the control of this process. Whether 14-3-3ζ is involved in mediating the assembly of signaling complexes in response to vWF ligation of the GP Ib-IX-V complex remains to be determined.
SYNTHESIS OF THE GP Ib-IX-V COMPLEX
GP Ibα, Ibβ, and IX exist in equal numbers on the surfaces of platelets17 and cells transfected with the cDNAs encoding the 3 polypeptides.111 Only half as many molecules of GP V are found on platelets,18,19 although the preciseness of this molar relationship with the rest of the complex requires further characterization. Based on studies using both transfected cells47,111-113 and the platelets of BSS patients with different mutations,114-116 it appears that maintenance of this stoichiometry relies primarily on the relative instability of partial complexes and single polypeptides. For example, in studies of GP Ibα surface expression in transfected cells, it was shown that this polypeptide is expressed on the surface of the cells most efficiently when both GP Ibβ and GP IX were cotransfected.111 Cotransfection with GP Ibα of less than the full complement of the other 2 polypeptides did not completely prevent GP Ibα expression, but did decrease it substantially. None of these 3 polypeptides is expressed efficiently on the cell surface unless expression in the cells is increased by manipulations such as gene amplification.112,117 Combinations of 2 polypeptides are more efficient in reaching the cell surface than single polypeptides if the 2 polypeptides interact with each other directly.112 GP V is not necessary for efficient expression of the rest of the complex and has only a minor effect, at most, on the expression of GP Ib-IX.47,118,119 It is the only 1 of the 4 complex polypeptides that can be efficiently expressed alone on the surfaces of transfected cells, although its surface expression is increased in the presence of the rest of the complex.47 From these studies, it was suggested that BSS could be caused by mutations of either GP Ibα, GP Ibβ, or GP IX, but the typical syndrome was unlikely to be caused by mutations of GP V.47,111 112
The molecular defects characterized thus far in patients with BSS support the findings from these in vitro studies in that mutations responsible for BSS have only been shown to involve the genes for GP Ibα, GP Ibβ, and GP IX (Table1). Mutations of the latter 2 polypeptides apparently cause the disorder by decreasing surface expression of GP Ibα.114,115,121 In several of the cases described, residual quantities of the unaffected polypeptides are still found in the platelets.114,116 122
Studies in transfected cells have also proved useful for determining how the polypeptides interact with each other. From such studies, it has been demonstrated that GP Ibα and GP Ibβ are able to interact in the absence of the other polypeptides, as are GP Ibβ and GP IX.112 Thus, GP Ibβ is the polypeptide bridging the interaction between GP Ibα and GP IX, at least initially, because no interaction between the later 2 polypeptides could be detected in the absence of GP Ibβ. In contrast, antibody inhibition studies of platelet lysates and purified GP Ib-IX complex suggest that GP IX is more strongly associated with GP Ibα than with GP Ibβ.123 Confocal microscopy and expression studies indicate that the interaction of GP V with GP Ib-IX is through a direct link with GP Ibα.47 This association has a direct functional consequence, because expression of GP V in cultured cells is required for the complex to bind thrombin with high affinity, even though the site of thrombin binding is on GP Ibα.82
The polypeptides of the complex all associate soon after their synthesis and insertion into the membrane of the endoplasmic reticulum.124 Before the complex reaches the cell surface, which in cultured cells takes approximately 3 hours,124 its polypeptides undergo a number of posttranslational modifications, including the addition of both N- and O-linked carbohydrate, modification of the intracytoplasmic cysteines of GP Ibβ and GP IX by acylation with fatty acids, and sulfation of tyrosines in the ligand-binding domain of GP Ibα. These modifications are all likely to influence the functions of the complex, and it is probable that mutations that disrupt any of the posttranslational modifications in vivo will result in variant forms of BSS.
GENES ENCODING THE GP Ib-IX-V POLYPEPTIDES
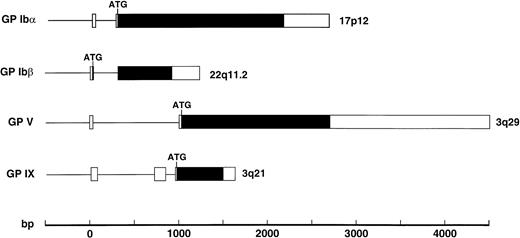
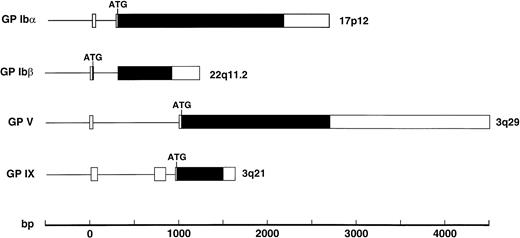
A separate gene encodes each component of the GP Ib-IX-V complex receptor. Like the polypeptides of this complex, the genes share a number of structural features (Fig 3). All except the gene for GP Ibβ contain the entire coding sequence within one exon45,125,126; the GP Ibβ gene contains an intron 10 bases after the start of the coding sequence.25 All are also relatively devoid of introns, with only the GP IX gene containing more than 1 (it contains 2).126 These genes share this compact structure and paucity of introns with other genes of the leucine-rich repeat family, the best example being the gene for oligodendrocyte-myelin glycoprotein, which contains one small intron in its 5′ untranslated region and the entire coding region in 1 exon.127 Despite their structural similarity, the genes encoding the GP Ib-IX-V polypeptides are not clustered in 1 region of the human genome. The GP Ibα gene is located on the short arm of chromosome 17,23 the GP Ibβ gene is on the long arm of chromosome 22,24 and the GP IX and GP V genes are located on the long arm of chromosome 327 (3q21 and 3q29, respectively; Fig 3).
Structures of the genes encoding the 4 polypeptides of the GP Ib-IX-V complex with exons shown as boxes, introns as the lines between boxes, and open reading frames in black. The position of the ATG start codon is also indicated.
Structures of the genes encoding the 4 polypeptides of the GP Ib-IX-V complex with exons shown as boxes, introns as the lines between boxes, and open reading frames in black. The position of the ATG start codon is also indicated.
Expression of the GP Ib-IX-V complex is limited to a very small number of tissues, the only major constitutive expression being in megakaryocytes and platelets. This complex may also be expressed in endothelial cells, although this is a matter of controversy. There have been reports of low level expression of GP Ibα in endothelial cells,128,129 expression that can be enhanced by the inflammatory cytokine, tumor necrosis factor-α.130,131Further evidence for expression of GP Ibα in endothelium was obtained by the cloning of a GP Ibα cDNA from an endothelial cell library.131 This cDNA was virtually identical to the original GP Ibα cDNA cloned from a HEL cell library.31More recently, Wu et al20 have provided evidence that endothelial cells, in culture and in vivo, express the full GP Ib-IX-V complex. One difference with the platelet complex is in the nature of GP Ibβ. Kelly et al24 found a polypeptide in endothelial cells that reacted with GP Ibβ antisera, but that migrated at a higher molecular mass (∼50 kD) than the platelet polypeptide (∼25 kD). They also cloned a cDNA that encoded a polypeptide with an amino terminus unrelated to platelet GP Ibβ but fused in frame with the platelet sequence such that the new polypeptide also contained essentially all of the platelet sequence. This interpretation of the data has since been challenged by Zieger et al,132 who also cloned a cDNA containing the GP Ibβ sequence. They identified a new gene immediately 5′ to the GP Ibβ gene that produced 2 transcripts, 1 containing the GP Ibβ sequence. The latter transcript presumably arose because the 5′ gene contains a suboptimal polyadenylation sequence. Hence, the transcription machinery sometimes reads through it and into the GP Ibβ gene, eventually using the GP Ibβ polyadenylation sequence. The resulting transcript thus also contains the GP Ibβ sequence, albiet out of frame, a finding at odds with that of Kelly et al.24
One potential function for the complex expressed in endothelial cells derives from the work of Beacham et al,133 who suggested that the complex can mediate attachment of endothelial cells to vWF. Bombeli et al134 also recently proposed a role for endothelial cell GP Ibα in adhesion of activated platelets to umbilical vein endothelial cells. Others have not been able to demonstrate GP Ib-IX-V–mediated attachment of endothelial cells to vWF and have even called into question whether these cells have significant levels of complex expression.135 If, how, and when the GP Ib-IX-V complex is expressed in endothelial cells are thus still open questions in need of more investigation. Such expression of the GP Ib-IX-V complex in endothelium in vivo may depend on such variables as regional shear stresses, the presence of inflammatory cytokines, and the particular vascular bed from which the cells are derived.
At least part of the restricted expression of the GP Ib-IX-V complex can be ascribed to the unusual structure of the promoter regions of its genes. None of the promoters contain functional TATA or CAAT boxes, consensus transcription factor-binding sequences found in a high percentage of eukaryotic genes (GP V does contain 2 potential TATA boxes, but primer-elongation studies did not show transcripts of the expected sizes45). Instead, these promoters contain binding sites for the GATA and ETS families of transcription factors, a feature shared with other genes expressed in cells of megakaryocytic and erythroid lineages.136-142 Neither GATA nor ETS is specific for megakaryocytes; it has been suggested that particular combinations and relative levels of the GATA and ETS families are what determine megakaryocyte specificity.138,140 This specificity may also be related to transcriptional cofactors. Recently, a transcription factor named FOG (Friend of GATA-1) was described, which acts as a cofactor for GATA-1 during both erythroid and megakaryocytic cell differentiation.143 Together, the 2 transcription factors may stimulate transcription in a context-specific manner.144
The importance of these factors for transcription of the GP Ib-IX-V genes is demonstrated by both synthetic and natural mutations. Mutations of both the GATA and ETS binding sequences in the promoters of GP Ibα and GP IX have been shown to reduce or abolish reporter gene expression in human erythroleukemia cells.141,142Likewise, a single-base mutation of the GATA-1 site in the GP Ibβ promoter markedly reduced expression of GP Ibβ and caused BSS in a patient with deletion of the other GP Ibβ allele and velo-cardio-facial syndrome.115
POLYMORPHISMS AFFECTING THE GENES AND POLYPEPTIDES OF THE GP Ib-IX-V COMPLEX
Several polymorphisms of the GP Ib-IX-V complex have been described, affecting primarily the GP Ibα gene. In addition to potentially affecting the structure and functions of the complex, these polymorphisms serve as useful linkage markers for the genes affected.
The first described polymorphism of the complex was a variable number of tandem repeats (VNTR) polymorphism affecting the region encoding the GP Ibα macroglycopeptide.36,37,145,146 The 4 alleles vary in the number of tandem repeats of a 39-nucleotide sequence, which is present either 1, 2, 3, or 4 times in the different alleles.36,37 The resulting polypeptides specified by these alleles contain different numbers of 13-amino acid repeats in their macroglycopeptide region. Each repeat contains 5 potential sites forO-glycosylation, a modification predicted to add approximately 6 kD to the mass of the macroglycopeptide and 32 Å to its length.36 This VNTR polymorphism is the most informative as a genetic marker because of the high frequency of heterozygosity at this locus (25% to 30% in most populations).36 The frequencies of the different alleles vary widely in different ethnic populations, although the variant with 2 repeats (C variant) is the most common in all populations studied.22
Another polymorphism of GP Ibα results in dimorphism at residue 145, with either Thr or Met occupying this position. The allele frequencies have been reported to be 90% and 10%, respectively, for the Thr and Met codons in both European and Japanese populations.147,148 This marker is closely linked to the VNTR polymorphism, with Met at position 145 being found only associated with the 3 largest size variants.37,149,150 Thus, this marker might be of use in determining heterozygosity in someone homozygous for the larger VNTR alleles. This marker has the additional advantage that the products of its alleles can be recognized on platelets with antisera, because this polymorphism accounts for the HPA-2 (or Ko) alloantigen system.147 148
Recently, 2 more polymorphisms of the GP Ibα locus were described, the RS system, its alleles specifying either C or T at position −5 from the ATG start codon,151 and a nucleotide dimorphism (A or G) of the third base of the codon for Arg358.151 152 The degree of association between these markers and the other GP Ibα polymorphisms has yet to be determined.
To analyze for possible linkage of the BSS phenotype with the GP Ibβ locus, markers used in the analysis of the Di George and velo-cardio-facial syndromes can be used.153-156 As yet, no markers are available for the GP IX or GP V genes.
BSS: CLINICAL MANIFESTATIONS, DIAGNOSIS, AND THERAPY
BSS is extremely rare. In the populations of Europe, North America, and Japan, which have been studied most intensively, a prevalence of less than 1 in 1,000,000 can be estimated from cases reported in the literature. No doubt, this is an underestimate due to misdiagnosis and underreporting, but the low frequency of reported cases nevertheless is an indication of the rarity of the disorder. Perhaps one reason for this low prevalence is that, despite the potential for the disorder to be caused by mutation of any of 3 genes (and perhaps 4), the compactness of these genes decreases the frequency at which they are subject to random mutation. The lack of introns interrupting the coding sequence also greatly decreases the possibility that missplicing will cause deficiency of the encoded polypeptides. The low frequency of mutation at these loci is reflected also in the fact that the majority of the reported cases are homozygous for the same allele, having inherited 2 mutant alleles from parents who are blood relatives. The clinical features of the BSS patients reported to date are summarized in Table 1. Based on this relatively small number of reported cases, there appears to be no gender preference for BSS (47 of 88 patients described in Table 1 are female), as one would expect from an autosomal disorder. Of the patients in Table 1 for whom ethnicity was reported, 49 are Caucasian, 13 are Japanese, and 4 are of other ethnic groups.
Inheritance.
Inheritance of the BSS is usually autosomal recessive and is often associated with consanguinity (Table 1). Heterozygous family members may show about half the normal levels of platelet GP Ib-IX-V expression, but with no bleeding diatheses or only mild bleeding. Autosomal dominant inheritance has been reported in only 1 family.75
Clinical manifestations.
BSS is characterized clinically by a prolonged skin bleeding time, morphologically enlarged platelets, and thrombocytopenia (Table 1 and reviewed in Dunlop et al157). Clinical manifestations commonly include frequent episodes of epistaxis, gingival and cutaneous bleeding, and hemorrhage associated with trauma. Although these characteristics are typical, comparisons of the clinical profiles of BSS patients reveal considerable variation between individuals. Platelet counts may range from very low (<30,000/μL) to marginally low or normal (∼200,000/μL) and in individual patients may fluctuate considerably over a period of years. Skin bleeding times may range from only marginally prolonged (5 to 10 minutes) to greater than 20 minutes. Bleeding tendencies associated with BSS are usually evident from early childhood. However, the severity of symptoms may progressively worsen or become alleviated throughout puberty and adult life. Most often, severe bleeding episodes are associated with tonsillectomy, appendectomy, splenectomy, other surgical procedures, dental extractions, menses and pregnancies, or accidents. Ecchymoses without significant trauma are relatively common, as are episodes of spontaneous epistaxis and gingival and gastrointestinal bleeding. Menorrhagia in premenopausal women is of variable severity and may be controlled in some cases by oral contraceptives.13,158,159Pregnancy in BSS patients may be relatively uneventful or may present complications of varying severity.116,121,122,158,160-166Bleeding associated with childbirth is generally supported by blood and/or platelet transfusions and may necessitate hysterectomy to control bleeding.161 Multiple childbirth is not uncommon.116 121
Diagnosis.
Congenital platelet disorders related to platelet adhesion, activation, secretion, aggregation, or number and various coagulopathies are often not distinguishable from their clinical manifestations alone, presenting a challenge to diagnosis that often requires specialized tests or biochemical analyses. For example, BSS has frequently been misdiagnosed as idiopathic thrombocytopenic purpura (ITP),116,121,167-170 based on a prolonged bleeding time and thrombocytopenia, and often is treated unsuccessfully with steroids or splenectomy. The initial laboratory assessment of BSS should involve measurement of blood cell counts and examination of a blood smear for thrombocytopenia and morphological abnormalities of platelets. BSS can usually be differentiated experimentally from other bleeding disorders by functional analysis of stirred platelet suspensions in an aggregometer. The characteristic abnormality in BSS is an isolated defect in ristocetin-induced agglutination. Unlike the defect in von Willebrand disease, this abnormality is not corrected by the addition of normal plasma. Platelet aggregation in response to other agonists, such as collagen and ADP, as well as clot retraction, is usually normal. The provisional diagnosis based on aggregometry should be confirmed biochemically (reviewed in Dunlop et al157). This may involve assessment of platelet surface glycoprotein expression by flow cytometry, surface-labeling of washed platelets followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography, or immunoblotting of platelet lysates with specific antiplatelet glycoprotein antibodies. Finally, establishing an abnormal genotype by molecular studies may allow precise definition of the abnormality causing the platelet defect, as discussed below.
Therapy.
The therapeutic approaches to the management of patients with BSS involve both general supportive measures and specific treatment of bleeding episodes. General measures include educating the patients about their bleeding diathesis and the importance of avoiding even relatively minor trauma and advising them against the use of antiplatelet medications such as aspirin. Adequate dental hygiene should be maintained to prevent gingival disease and to minimize dental procedures. Iron deficiency may result from chronic gingival bleeding or menorrhagia and should be treated. In some cases, splenectomy has apparently been beneficial in moderating thrombocytopenia and the severity of clinical symptoms,158,168,171,172 although this treatment should be avoided because of the high risk for perisurgical hemorrhage and the lack of controlled data to support its use. Control of bleeding episodes or prophylaxis for prevention of bleeding during surgical procedures usually requires transfusion of blood and/or platelets, despite the risk that these patients will develop antiplatelet and/or antierythrocyte alloantibodies.158,168,171,172 General anesthesia has been successful in a BSS patient, although anesthetics such as halothane or dibucaine that compromise platelet reactivity should be avoided.173 The use of antifibrinolytic drugs, such as ε-aminocaproic acid or tranexamic acid, may or may not be beneficial.169,170,172 DDAVP may shorten the bleeding time in some168,171,174 but not all159 169 BSS patients. The different responses of individual patients to these latter measures may reflect differences in the underlying disease, with those with milder forms of the disease more likely to respond to these therapies.
Because of the relative ease with which the molecular lesions can be determined and given the simplicity of the affected genes, BSS seems an ideal candidate disease for gene therapy. Such therapy would presumably involve transduction of a hematopoietic stem cell with a working copy of the defective gene, under the control of its own promoter or of another platelet-specific promoter. Among the questions to be answered before such therapy becomes reality is whether reconstituting the blood with only a relatively small proportion of normal platelets will be sufficient to ameliorate the bleeding diathesis associated with BSS.
BSS: CLASSIFICATION
The genetic defects underlying BSS so far determined (Table 1) are clearly heterogeneous, but may be broadly categorized in 2 ways. First, the abnormality may be either (1) a biosynthetic defect affecting synthesis, processing, or expression of the GP Ib-IX-V complex; or (2) a functional defect in which GP Ibα is expressed in a dysfunctional form that fails to bind ligand. Second, the genetic lesion may be localized to (1) the GP Ibα gene (chromosome 17pter-p12), (2) the GP Ibβ gene (chromosome 22q11.2), (3) the GP IX gene (chromosome 3q21), or possibly (4) the GP V gene (chromosome 3q29). The syndrome may conveniently be classified, therefore, as type 1a to indicate a defect of the GP Ibα gene that results in a biosynthetic defect, type 1b for a synthetic defect of the GP Ibβ gene, etc. The molecular defects thus far reported arise from missense, nonsense, or deletion mutations of the GP Ibα, GP Ibβ, or GP IX genes (Table 1) that produce truncated, unstable, or dysfunctional polypeptides.
INFORMATIVE MUTATIONS IN BSS AND PLATELET-TYPE VON WILLEBRAND DISEASE
Clearly, much remains to be learned about the cause of several phenotypic features of the BSS. Nevertheless, elucidation of the molecular basis of BSS and platelet-type von Willebrand disease has provided several very valuable insights into the synthesis and functions of the complex. A few of the more informative mutations will be reviewed in this section.
GP Ibα mutations.
Several mutations of GP Ibα provide interesting information regarding the functions of the GP Ib-IX-V complex. The Bolzano variant of BSS, caused by a substitution of Val for Ala at position 156 of GP Ibα, produces mutant complexes that appear on the cell surface essentially at normal levels.76 This mutant is unable to bind vWF normally, but binds thrombin with a similar affinity to that of the wild-type complex.175 The platelets of the patient with the Bolzano variant of BSS thus do not have the defect in the response to low concentrations of thrombin that most BSS platelets do. This finding suggests that the leucine-rich repeats have an important role in binding vWF, but not thrombin. Another interesting feature of this mutant is that it has no mutations of its cytoplasmic region that would be predicted to influence the association of the complex with the platelet cytoskeleton, yet the platelets of the affected patient are much larger than normal, indicating that the large platelets in BSS cannot be explained simply by a defective membrane-cytoskeletal association.
Only one instance of autosomal-dominant transmission of BSS has been described. The responsible mutation, Leu57→Phe, like the Bolzano mutation, affects the leucine-rich repeat region of GP Ibα and encodes a mutant polypeptide that appears on the cell surface, but, once there, is apparently abnormally susceptible to cleavage by plasma proteases.75 The dominant nature of this mutation suggests that the product of the mutant allele interferes with the functions of the wild-type polypeptide, giving further support for the existence of a vWF receptor containing more than 1 GP Ibα polypeptide.
Also of interest is the recently described deletion of the last 2 bases of GP Ibα codon 492, which results in a reading frame-shift within the region encoding the GP Ibα membrane-spanning segment, with the addition of 81 novel amino acids before the polypeptide reaches a premature stop. Two unrelated patients homozygous for this mutation were described simultaneously; both had a considerable amount of GP Ibα or a degradation product in their plasma, indicating that GP Ibα was synthesized normally but failed to be anchored in the plasma membrane.120,176 In addition to carrying the same mutation, these 2 patients had an identical haplotype, with identical sequences at 3 other polymorphic sites. The ancestors of both of these patients emigrated to the United States from Germany. Interestingly, a Finnish patient carrying an identical mutant haplotype was recently reported in abstract form.177 This mutant haplotype probably arose at least several centuries ago in the northern European population and will likely be a common mutation associated with the disorder in patients of northern European ancestry.
Platelet-type von Willebrand disease is another bleeding disorder caused by mutations affecting the GP Ib-IX-V complex, but in this case resulting in a dominant gain-of-function phenotype.60,61The resultant mutants bind vWF with high affinity and the paradoxical presence of a bleeding predisposition is due to clearance of the hemostatically most active large vWF multimers. The mutations described in this disorder (Gly233→Val59 and Met239→Val58,178,179) are found within a short linear sequence encompassing residues 233 to 239 of GP Ibα, a region that lies within the loop formed by a disulfide bond between Cys209 and Cys248 of GP Ibα (Fig 2). Molecular modeling studies of this region suggest that the mutations produce an active conformation of GP Ibα that is competent to bind vWF in the absence of modulators.180 181 These mutants thus provide clues as to changes that the receptor undergoes when platelets are exposed to shear or ristocetin.
GP Ibβ.
Two BSS variants that result from mutations of GP Ibβ are particularly informative. The first was described in a patient with velo-cardio-facial syndrome, a developmental disorder caused by deletion of the chromosomal region 22.11.2, which contains the gene for GP Ibβ.182 This patient's remaining GP Ibβ gene did not contain any mutations in the polypeptide coding region, but a single point mutation was found in the promoter region within a binding sequence for the GATA-1 transcription factor.115Transcription studies performed in cell lines demonstrated that the mutation decreased the transcription of a reporter gene sixfold. So far, this is the only reported case of BSS not caused by mutations of polypeptide coding regions.
Another interesting variant of BSS caused by mutations of the GP Ibβ gene was described in a Japanese patient with a very mild propensity for bleeding.183 This patient was a compound heterozygote for 2 mutations (Tyr88→Cys and Ala108→Pro). The platelets were not defective for agglutination by either ristocetin or botrocetin and had only slightly decreased surface levels of the GP Ib-IX-V complex. This patient's platelets were very large, and the only finding that could explain this abnormality was the failure of the normal disulfide linkage between GP Ibα and GP Ibβ, suggesting that the mutant complexes may have a signalling defect.
GP IX.
BSS caused by mutations in the gene for GP IX emphasize the importance of this polypeptide in the synthesis and surface expression of the entire GP Ib-IX-V complex. The first case of BSS reported to be due to such mutations was described by Wright et al114 in 3 siblings who were compound heterozygotes for mutations in the GP IX gene. Both alleles were affected by missense mutations of the GP IX leucine-rich repeat region (Asp21→Gly and Asn45→Ser), and the expression of the entire complex on the surfaces of their platelets was minimal. Expression of the mutants in cultured cells showed that both mutant polypeptides failed to associate with GP Ibβ or augment expression of GP Ib (α-β) on the cell surface, suggesting a role for the GP IX leucine-rich motif in polypeptide associations.184
Bernard-Soulier variants.
In addition to the disorder produced by germline mutations, acquired BSS from somatic mutation of bone marrow stem cells has also been described. Two cases have been described in association with myelodysplasia (both in children),185,186 and 1 case was described in a patient with acute myelogenous leukemia (M6).186 In 1 patient with a myelodysplastic syndrome and monosomy 7, 2 populations of platelets were identified in her blood, 1 population of normal-sized platelets with normal levels of the GP Ib-IX-V complex on their surfaces and 1 of large platelets lacking the complex.185 The latter population was apparently produced by the abnormal marrow clone. This patient died of acute leukemia shortly after acquiring the disease.
BSS-like defects are also rarely seen associated with immune thrombocytopenia when the offending antibody interferes with vWF binding to the GP Ib-IX-V complex.187-189 In this situation, the bleeding risk is much greater than usually seen in immune thrombocytopenia because the hemostatic defect resulting from the low platelet count is compounded by the severe adhesive defect of the platelets that remain in circulation.
UNEXPLAINED PHENOMENA IN BSS
BSS platelets have several phenotypic features that remain poorly understood. One is their large size. An obvious explanation for this feature is provided by the altered plasma membrane–cytoskeletal interaction due to the absence of the GP Ib-IX-V complex on the platelet surface. Although this defect undoubtedly explains the increased deformability of the Bernard-Soulier platelet membrane,190 it may not be the cause of the abnormally large platelets in this syndrome. Several BSS patients have been described whose platelets are large but contain relatively normal surface levels of the complex. In 1 of these, the Bolzano variant, the GP Ibα polypeptide contains only a single amino acid substitution in its extracellular domain that affects vWF binding but not surface expression of the complex. These platelets presumably have a normal linkage with the cytoskeleton, although the possibility remains that the mutation changes the conformation of the cytoplasmic domain and influences that association.
These data would also seem to suggest a role for vWF binding in platelet production from megakaryocytes; however, such a role is ruled out by the observation that patients with severe (type 3) von Willebrand disease have normal-sized platelets. Consistent with this, another BSS variant with defective GP Ibα–GP Ibβ chain association produces large platelets and has relatively normal expression of the complex and vWF binding.183 These data suggest that the abnormality in BSS platelets that leads to their abnormal size may either be the failure of the GP Ib-IX-V complex to recognize a novel bone marrow ligand involved in platelet shedding or a disruption of the signalling pathways involved in this process. In this regard, BSS platelets have been reported to have decreased levels of phospholipase C activity.191
The second phenotypic characteristic of BSS that has yet to be fully explained is the abnormality of the prothrombin consumption test in BSS platelets.192 The prothrombin consumption test detects deficiencies of factors V, VIII, IX, XI, or XII,193 more than 1 of which could contribute to the abnormality of this test in BSS. Accordingly, BSS platelets have been reported to be deficient in collagen-induced coagulant activity and to be unable to bind factor XI.6 In addition, the GP Ib-IX-V complex has recently been reported to be the platelet binding site for high molecular weight kininogen194 and for factor XII.195 Finally, the abnormal prothrombin consumption test with BSS platelets has been reported to be correctable by the addition of factor VIII. This latter finding is consistent with the GP Ib-IX-V complex providing a low-affinity receptor on platelets for vWF/factor VIII, a conjecture consistent with the observation that treating normal platelets with an anti-GP Ibα antibody that blocks vWF binding mimics the abnormality in the prothrombin consumption test seen in BSS platelets.196 In contrast to the abnormality in the early stages of contact activation, resting BSS platelets show enhanced prothrombinase activity8 due to increased levels of phosphatidylserine on their surfaces.
STRATEGIES FOR CHARACTERIZING MUTATIONS CAUSING BSS
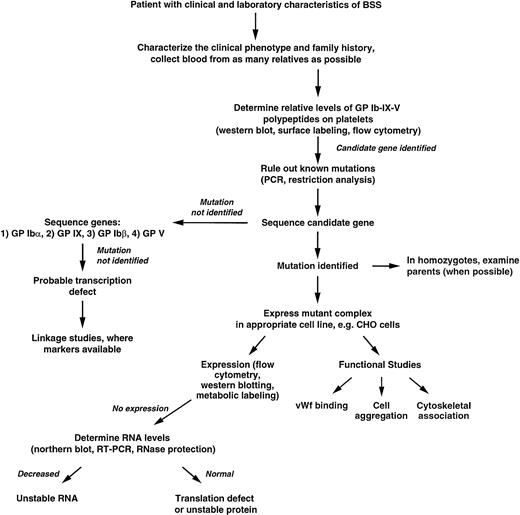
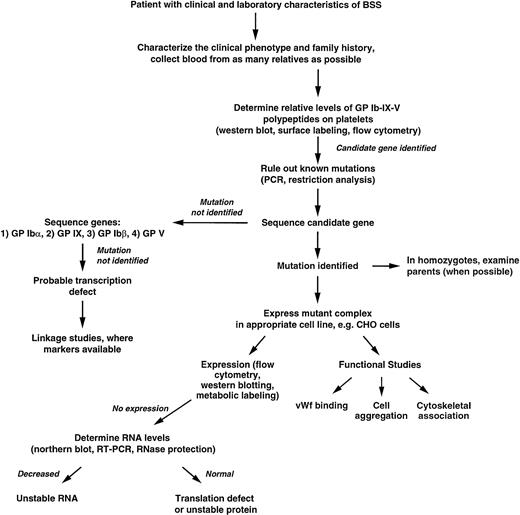
Figure 4 presents an algorithm that can be used as a guide for the rational exploration of the molecular basis of BSS. Several features of this strategy will be discussed briefly here.
Algorithm for determining the genetic basis of BSS. Details are given in the text.
Algorithm for determining the genetic basis of BSS. Details are given in the text.
After a patient with the clinical characteristics of BSS has been identified, it is important to take as complete a medical history and family history as possible. Particularly important is a history of consanguinity in the parents. It is also vital to collect blood from as many of the relatives as possible, so as to be able to identify the affected gene and verify the mode of inheritance. Biochemical studies of the parents' platelets will also be useful in identifying the affected gene (discussed below).
The levels of the individual polypeptides on the patient's platelets should be determined by biochemical means to identify a candidate gene for sequencing. Quite often residual quantities of some of the polypeptides appear in the platelets of patients with a severe deficiency of one polypeptide. For example, in the GP IX mutants described by Wright et al,114 flow cytometry showed residual amounts of GP Ib in a subset of platelets, whereas GP IX was virtually undetectable; in BSS Kagoshima, due to truncation of GP Ιbα, residual GP IX and GP Ibβ were detected on the platelet surface.197 The polypeptide found to be most deficient by these assays will indicate which gene should be studied first by sequencing. Here too, studies of the parents' platelets may be useful. For example, in a patient severely deficient in all of the GP Ib-IX-V complex polypeptides whose parents are blood relatives, the finding of 2 bands for GP Ibα on immunoblots of platelets from 1 of the parents (indicating heterozygosity for the VNTR polymorphism) will rule out GP Ibα as the affected gene because the patient could not have inherited a defective GP Ibα allele from this parent. Similarly, if more than 1 patient in a family is affected, it is a relatively simple matter to rule out GP Ibα as the affected gene if any 1 of the affected individuals is heterozygous for the VNTR, or if any 2 are homozygous for different alleles. Homozygosity for this marker is not as informative; its predictive value is increased if the affected siblings are homozygous for all of the other GP Ibα polymorphisms, indicating that they inherited the same GP Ibα allele from both parents, an event with a probability of only 6.3% (for 2 siblings of heterozygous parents carrying the same mutant allele).
Several of the previously described mutations can be identified by restriction analysis of polymerase chain reaction (PCR)-amplified genomic DNA. One nonsense mutation of GP Ibα that produces a truncated polypeptide also ablates an Ava II site,198 a change that can be used as a marker for the presence or absence of the mutation. The C to T mutation that causes the Ala156→Val substitution responsible for the Bolzano variant of BSS introduces a new Hpa I site into the coding region of the GP Ibα gene,76 the Cys209→Ser mutation produces a new Mse I site,199 and the Leu129→Pro mutation eliminates a Sac I site.200 Both of the mutations of GP IX described by Wright et al114 (Asp21→Gly and Asn45→Ser) create new recognition sites for the enzyme Fnu4H1. Thus, several of the mutations known to be associated with BSS can be easily identified by restriction enzyme digestion of PCR-amplified DNA. If the restriction pattern indicates that the patient is heterozygous for 1 of the previously identified mutations, the other allele should be sequenced to identify the second mutation.
If this strategy fails to identify a candidate gene or no mutation is found in the suspected gene, the genes encoding all of the GP Ib-IX-V polypeptides can be sequenced. The relative simplicity of the gene structures makes direct sequencing the most straightforward approach for identifying mutations, particularly with the availability of automated sequencing. In addition, the paucity of intervening sequences obviates the need for reverse transcription of mRNAs, because the uninterrupted coding sequence can be obtained directly from genomic DNA (except in the GP Ibβ gene, which has only 1 small intron interrupting the coding region). The order of priority shown (GP Ibα > GP IX > GP Ibβ > GP V) is based on the relative frequency that the genes for the individual polypeptides have been found to be affected in reported cases of BSS. The GP V gene is given the lowest priority because it has not been reported as the affected gene causing BSS and because of the likelihood that the clinical sequelae of its absence will be less severe than those of the others. Failure to identify a mutation in any of the polypeptide coding regions suggests the possibility that the disorder is due to decreased transcription of 1 of the genes. The affected gene can be identified by the use of linkage markers to determine which of the genes is homozygous (in the offspring of a consanguineous union). The promoter regions of the gene identified by linkage markers can then be sequenced.
When an apparently homozygous mutation is identified in patients in whom the parents are not known to be consanguineous, it will be necessary to rule out a hemizygous situation in which the other allele has been deleted, rendering impossible its amplification by PCR. This is a particularly important concern with apparently homozygous mutations of the GP Ibβ gene, because this gene usually undergoes hemizygous deletion in velo-cardio-facial syndrome,182 a condition whose manifestations can be subtle.201
Finally, when mutations are identified whose consequences are not obvious (eg, missense mutations), it is important that the causative nature of the mutation be demonstrated by expression and functional studies. Examples of some characterizations are given in the algorithm. Similarly, if a mutation is identified within 1 of the promoters that is believed to be responsible for decreased transcription, this should be determined by expression of the mutant promoter, perhaps directing the expression of a reporter gene in a megakaryocytic cell line.115,142 202
CONCLUSIONS
BSS is a rare but fascinating disorder. Since its clinical description in 1948, analysis of its functional and molecular defects has spawned much of our current understanding of the role of the GP Ib-IX-V complex as a key receptor in initiating hemostasis. The continued analysis of the molecular and genetic defects in BSS should provide further information on the topography and assembly of the GP Ib-IX-V complex, on the role of this complex in the interactions of vWF and thrombin with platelets, on how its ligation activates platelets, on its role in coagulation, and on its participation in regulating platelet size and turnover.
ACKNOWLEDGMENT
It is a pleasure to acknowledge the assistance of David Smith with the preparation of this review.
Supported by grants from the National Institutes of Health, the American Heart Association, and the National Health and Research Council of Australia.
Address reprint requests to José A. López, MD, Veterans Affairs Medical Center, Hematology/Oncology (111H), 2002 Holcombe Blvd, Houston, TX 77030; e-mail: josel@bcm.tmc.edu; or Michael C. Berndt, PhD, Baker Medical Research Institute, Commercial Road, Prahran, VIC 3181, Australia; e-mail: michael.berndt@baker.edu.au.