Abstract
Positron emission tomography (PET) using F-18 fluorodeoxyglucose (FDG) was performed in non-Hodgkin's lymphoma (NHL), which is known to be highly responsive to chemotherapy, but also yields variable treatment results to answer the following questions: (1) What is the extent and time course of changes in FDG utilization in response to chemotherapy? (2) Are the changes of FDG uptake at early time points of chemotherapy predictive for therapy outcome? (3) Which quantitative FDG parameter provides the most sensitive measures of initial tumor response? Dynamic PET scans were performed in 11 patients at baseline and 1 and 6 weeks after initiation of chemotherapy. Based on attenuation corrected images acquired 30 to 60 minutes postinjection, standardized uptake values (SUV) were determined. Arterial input functions were estimated from vascular F-18 activity and the metabolic rates for FDG (MRFDG) were calculated using Patlak analysis. Before chemotherapy, high FDG uptake was found in all lesions (SUV[max] 13.3 ± 4.2). Seven days after initiation of chemotherapy, tumor FDG uptake decreased 60% (SUV[max]). A further decrease of 42% was seen at day 42 resulting in a total decrease of 79% from baseline to day 42. During a follow-up of 16.0 ± 4.2 months, six of the 11 patients continued to show complete remission. Seven days after initiation of chemotherapy, this group of patients displayed significantly lower mean MRFDG than the group of patients with relapse. At day 42, all parameters of FDG uptake showed a significant difference for both patient groups. The relative change of MRFDG from baseline to day 42, as well as from day 7 to day 42, was significantly larger as compared with SUV parameters. Standard chemotherapy of patients with NHL causes rapid decrease of tumor FDG uptake as early as 7 days after treatment, which continues to decline during therapy, indicating the sensitivity of metabolic signals to chemotherapeutic interventions. FDG uptake at 42 days after therapy was superior in prediction of long-term outcome over day 7 parameters. Dynamic data acquisition combined with Patlak analysis of FDG kinetics may provide superior information in therapy monitoring.
MALIGNANT TUMORS HAVE been shown to display increased glucose metabolism.1 Positron emission tomography (PET) with the radiolabeled glucose analog fluorine-18-deoxyglucose (FDG) allows quantitative assessment of glucose utilization in tumor tissue. The mechanism of cellular uptake of FDG reflects both transport and phosphorylation of glucose. FDG is phosphorylated by hexokinase and trapped intracellulary as FDG-6-PO4 with a slow rate of dephosphorylation in most tissues. Because the majority of tissue activity represents FDG-6-phosphate at the time of PET imaging, regional radioactivity will correlate with FDG metabolism.2 The ability of FDG-PET to localize primary carcinomas, regional lymph nodes, and distant metastatic sites is well documented in the literature.3 4
Several studies on FDG uptake after radiotherapy, as well as after chemotherapy in miscellaneous tumors, suggest the use of FDG for assessment of therapeutic effects.5-8 Reduced numbers of viable cells or reduced metabolism of the damaged cells might be associated with decrease of tumor glucose utilization.9,10Few data exist defining the time course and extent of changes in tumor FDG uptake as response to chemotherapy. Data from cell culture and animal studies suggest an initial enhancement of glucose uptake induced by chemotherapy11,12 or radiotherapy.13 PET imaging in patients with breast cancer showed that chemotherapy leads to a decrease of the glucose metabolism in solid tumor within 1 or 2 weeks.9,14 15
The purpose of this study was (1) to assess the incidence and time course of changes of glucose utilization in response to chemotherapy with FDG-PET; (2) to investigate the value of FDG uptake during chemotherapy at early (day 7) and late (day 42) time points in prediction of therapy outcome; and (3) to identify quantitative PET parameters, which may provide sensitive measures of initial tumor response.
To evaluate these questions, non-Hodgkin's lymphoma (NHL) has been chosen as the tumor model because this tumor displays enhanced FDG uptake.16-23 In addition, NHL is known to be highly responsive to chemotherapy and shows a high variability of responses to chemotherapy.24 25 A baseline PET was performed before chemotherapy and was repeated 1 and 6 weeks after initiation of chemotherapy.
MATERIALS AND METHODS
Patients.
Eleven patients with newly diagnosed high-grade NHL, who were scheduled to undergo chemotherapy, were included in this study between July 1995 and July 1996. Mean age of the patients was 50 years, ranging from 21 to 77 years. Six patients were men and five were women. Patient characteristics are listed in Table 1.
The lymphatic malignancy was histologically verified in all patients. Exclusion criteria were pregnancy, known diabetes, or age younger than 18 years. No patient received prior therapy for NHL.
Details of the study were explained by a physician and written informed consent was obtained from all patients. The study protocol was approved by the institutional review board at the Technische Universität München.
Histologic classification.
Histology of lymphomas was classified according to the updated Kiel classification.26
Treatment protocol.
The patients underwent chemotherapy either with a standard protocol27 using a combination of cyclophosphamide, doxorubicin, vincristin, (etoposid), and prednisone (CHO(E)P) (CHOP: n = 4; CHOEP: n = 2) or were included in a phase 1 trial evaluating the polyamine pool depleting compound mitoguazone (MGBG, NSC 32946) in combination with CHOP (sequence MGBG − CHOP: n = 2; CHOP − MGBG: n = 3).
A baseline PET examination was performed within 1 week before chemotherapy, together with other staging modalities (clinical examination, computed tomography [CT], or magnetic resonance imaging [MRI]). PET imaging was repeated at day 7 (range, day 6 to day 8) after the first course of chemotherapy (day 1 to 5). A third PET study was performed after completion of an additional course (day 22 to 26) at day 42 (range, day 39 to day 47). At this time, restaging procedures including morphologic imaging (CT or MRI) were scheduled. In one case (patient no. 8), chemotherapy was repeated in 2-week intervals (day 15 to 19 and day 29 to 33).
PET imaging.
Patients were fasted at least 8 hours before PET imaging. The serum glucose level was measured before each PET examination with blood glucose reagent strips and photometric measurement (Glucometer II, Glucostix, Bayer Diagnostics, Munich, Germany). Mean blood glucose level summarizing all PET studies was 104.2 ± 14.2 mg/100 mL (mean ± standard deviation [SD]; range, 83 to 139).
F-18 was produced with a self-shielded 11 MeV cyclotron RDS 112 (Siemens/CTI, Knoxville, TN) by the acceleration of protons onto an O-18 H2O target. FDG of high specific activity was produced with a standard technique by means of nucleophilic fluorination using a method modified from the synthesis of Hamacher.28
PET scans were performed using a whole body PET scanner (ECAT 951R/31 or ECAT EXACT, Siemens CTI) over the largest known lesion. The ECAT 951R/31 scanner consists of 16 rings of bismuth germanate detectors (BGO) yielding 31 transverse slices, 3.375 mm apart (axial field-of-view 10.8 cm). The 24-ring ECAT EXACT yields 47 slices, 3.375 mm apart (axial field-of-view 16 cm). Emission data corrected for randoms, dead time, and attenuation were reconstructed with filtered back-projection (Hanning filter with cut-off frequency of 0.4 cycles/bin). Transmission measurements were acquired before tracer injection with Germanium-68 rod sources yielding approximately 4 million counts per slice. The resulting in-plane image resolution of transaxial images of both scanners was approximately 8 mm full width half maximum (FWHM) with an axial width of approximately 5 mm FWHM.
In all patients, dynamic data acquisition was performed up to 60 minutes after intravenous bolus injection of 370 MBq F-18 FDG (30 frames: 12 × 10 seconds, 6 × 30 seconds, 5 × 60 seconds, 4 × 5 minutes, 3 × 10 minutes).
Data analysis.
Regional FDG uptake in tumor tissue from the PET image data was quantified using the following methods. (1) Standardized uptake value (SUV): SUV were calculated using the sum of the three last frames of dynamic imaging (30 to 60 minutes postinjection [p.i.]). Standardized circular regions of interest (ROI) with a diameter of 12 mm were drawn on the slice displaying highest tumor activity concentration and the neighboring planes. SUV values represent the average (SUV[avg]) or maximum (SUV[max]) radioactivity concentration of each set of three ROIs normalized for injected dose and body weight.3 (2) Patlak analysis: dynamic PET studies allow the assessment of a FDG influx constant using the Patlak analysis approach29 if unidirectional transport between two compartments can be assumed. This requirement is fulfilled during approximately the first hour after FDG injection as long as dephosphorylation is negligible. A graphical analysis of tissue-time-activity curve and arterial blood activity curve results in a measure for tracer uptake rate. The arterial input function was obtained using 90% isocontour ROIs placed in a large vessel (aorta, arteria carotis) in four consecutive planes in frames 3 or 4. During follow-up, the ROIs were repositioned using the same vascular structure. Tissue-time-activity curves were derived from standardized circular ROIs with a diameter of 5 mm, which were drawn over the tumor in the plane displaying highest activity concentration and the neighboring planes. The tissue radioactivity concentration divided by plasma activity was plotted against cumulative plasma radioactivity divided by plasma activity for each time point. The slope of this linear plot from 20 to 60 min p.i. determined the utilization constant of FDG (influx constant K), expressed as mL/100g/minute. To indicate a parameter of the quality of the influx constant K, the r value of the linear regression was used. Furthermore, the metabolic rate of FDG (MRFDG) in tumor was calculated by multiplication of the influx constant K and the basic blood glucose concentration. Because the lumped constant accounting for differences in the transport and phosphorylation of FDG and glucose has not yet been determined in tumors, the local glucose metabolic rate could not be indicated.2
Clinical evaluation.
CT and MRI were performed as part of the routine clinical management of the patients. The decision for CT and/or MRI was made by the clinician depending on tumor locations.
Baseline CT and/or MRI of head and neck, thorax, abdomen, and pelvis were performed in all patients before chemotherapy. After the therapy protocols, the patients were reevaluated by means of CT and/or MRI after two courses of chemotherapy around day 42. The treatment response was classified as complete response (CR), partial response (PR), no change (NC), or progressive disease (PD) according to the criteria stated by Miller et al30 based on the bidimensional diameters of corresponding tumor lesions measured by ruler or caliper. Further evaluations of treatment response were done according to standard protocols every 3 months. The patient management was not influenced by the results of PET studies. Actual patient status was recorded in all remaining patients between April and August 1997.
Statistical analysis.
Data were expressed as the mean ± SD. For intraindividual comparison of the various FDG parameters at different time points, two sided paired t-test was used. The Mann-Whitney U test was used to evaluate the differences between the decreases from baseline to day 7 and 42 and from day 7 to day 42 and to test the differences between the FDG parameters of patients with long-term response and relapsed patients. All statistical tests were performed at the 5% level of significance.
RESULTS
Responses to chemotherapy.
We studied 11 patients at baseline and 7 and 42 days after initiation of chemotherapy. Conventional morphologic imaging after two courses of chemotherapy showed partial (n = 8) or complete (n = 3) response in all patients. As indicated in Table 1, at the end of first-line chemotherapy, seven patients showed complete remission. Four of these (no. 2, 3, 7, and 10) received radiotherapy of the main-bulk area after completion of chemotherapy. Over a follow-up period of 16.0 ± 4.2 months (range, 10.9 to 23.2 months), six of 11 patients remained in complete remission based on clinical and radiographic data. Patient no. 4 initially showed a local partial response, but during the third course, this patient developed tumor progression with additional new lesions in the central nervous system (CNS). Histopathologic reevaluation showed that this patient had a Burkitt's lymphoma. Patients no. 1 and no. 6 developed a relapse including CNS involvement during the sixth and fourth course of chemotherapy, respectively. Patient no. 11 was progressive at the end of six courses of CHOP. One patient (no. 9), who showed CR after two courses of chemotherapy, relapsed after a disease-free interval of 12 months. Three patients died of relapse 8 months (no. 1), 12 months (no. 4), and 10 months (no. 11) after the start of therapy.
Time course of FDG-uptake during treatment.
Statistical comparisons of blood glucose levels at the time of PET studies before chemotherapy (105.1 ± 16.0 mg/100 mL) and during chemotherapy at day 7 (101.9 ± 9.9 mg/100 mL) and day 42 (105.8 ± 16.2 mg/100 mL) showed no significant differences.
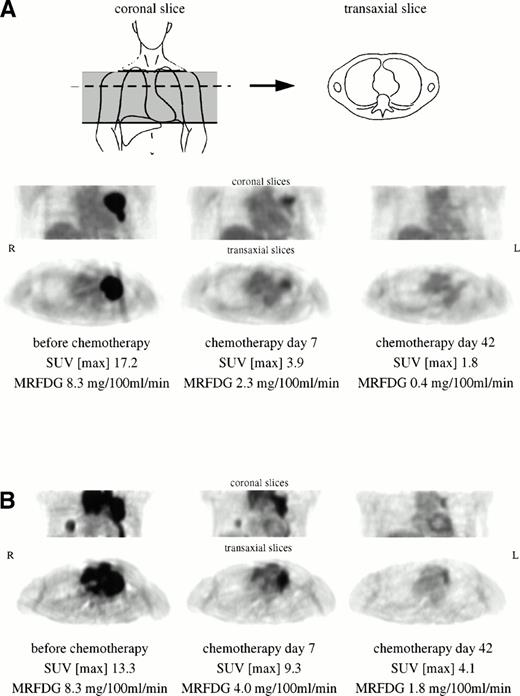
Figure 1A (patient no. 5) shows an example of bulky tumor in the left parahilar region with prominent FDG uptake. Seven days after initiation of chemotherapy, PET imaging showed a decrease of 77% of maximum SUV and 72% of MRFDG. On day 42, the tumor region can hardly be differentiated from background activity. This patient displayed CR over a follow-up period of 15 months. In contrast, the patient shown in Fig 1B (no. 6) exhibited partial remission at day 42, but relapsed during the third course of chemotherapy at multiple locations including CNS. FDG-PET at day 42 showed residual viable tumor mass in the left mediastinum and right lower lobe. The myocardial FDG uptake at day 7 and day 42 does not reflect tumor involvement.
(A) Bulky high-grade NHL in the left parahilar region with prominent FDG uptake. The patient (no. 5) showed CR over a follow-up period of 15 months. (B) Extensive mediastinal tumor mass of a high-grade NHL with an additional focal tumor lesion in the right lower lobe. The patient (no. 6) exhibited partial remission at day 42, but relapsed during the third course of chemotherapy. Myocardial FDG uptake at day 7 and day 42 does not reflect tumor involvement.
(A) Bulky high-grade NHL in the left parahilar region with prominent FDG uptake. The patient (no. 5) showed CR over a follow-up period of 15 months. (B) Extensive mediastinal tumor mass of a high-grade NHL with an additional focal tumor lesion in the right lower lobe. The patient (no. 6) exhibited partial remission at day 42, but relapsed during the third course of chemotherapy. Myocardial FDG uptake at day 7 and day 42 does not reflect tumor involvement.
All tumor lesions imaged before chemotherapy had increased FDG uptake as compared with surrounding normal tissue (SUV[max] 13.3 ± 4.2). In Table 1, the results of quantitative analysis of FDG uptake are summarized. There was no significant difference notable between SUV[max] and SUV[avg]. The metabolic rate MRFDG was 6.8 ± 2.8 mg/100 mL/minute. The linear fit of dynamic FDG data from 20 to 60 minutes p.i. at baseline showed an r value of 0.988 ± 0.021 (range, 0.927 to 0.999).
Seven days after initiation of chemotherapy, parameters of tumor FDG uptake decreased by 60% (SUV[max]) and 67% (MRFDG) of initial values. From day 7 to 42, all lesions exhibited a further decrease of tracer uptake of 42% (SUV[max]) and 71% (MRFDG). The total decrease from baseline to day 42 was 79% (SUV[max]) and 89% (MRFDG). Statistical comparison of all baseline and day 7 parameters, as well as day 7 and day 42 parameters, showed significant differences (P< .001). The relative decrease of FDG uptake did not correlate with the initial uptake values either for SUV values or for accumulation rates. No correlation was found concerning either patients' age or tumor stage.
In five patients, there were two evaluable lesions within the field-of-view. We correlated the decrease of SUV[max] of these two lesions, intraindividually, from baseline to day 7, as well as from baseline to day 42. The SUV decrease from baseline to day 7 (r = 0.563) did not correlate as well, as compared with the decrease from baseline to day 42 (r = 0.981). The intraindividual comparison of SUV[max] at day 7 (lesion 1, 5.7 ± 2.0; lesion 2, 3.7 ± 1.9) and day 42 (lesion 1, 2.6 ± 1.1; lesion 2, 2.3 ± 1.3) showed no statistically significant difference.
Correlation of FDG-PET data with therapy outcome.
FDG uptake parameters at day 7 and day 42 were correlated with long-term clinical outcome (Fig 2). The analysis of the parameters of FDG uptake at day 7 showed that the group of patients with long-term remission had significantly lower mean values of the MRFDG (1.46 ± 0.75 mg/100 mL/minute) as compared with the group of patients with disease relapse (2.70 ± 1.10 mg/100 mL/minute; P = .045). Using the SUV parameters, there was no notable difference between both groups (SUV[max] P= .235; SUV[avg] P = .201).
Comparison of SUV[max] (⧫) and the metabolic rate of FDG (MRFDG) (▪) at (A) day 7 and (B) day 42 in patients with relapse (n = 5) and complete response (CR; n = 6).
Comparison of SUV[max] (⧫) and the metabolic rate of FDG (MRFDG) (▪) at (A) day 7 and (B) day 42 in patients with relapse (n = 5) and complete response (CR; n = 6).
At day 42, we also found significantly lower mean values of MRFDG in the group of patients with complete remission (0.24 ± 0.22 mg/100 mL/minute) as compared with the group of relapsed patients (1.15 ± 0.62 mg/100 mL/minute; P = .018) and also the mean values of maximum (2.1 ± 0.3 v. 3.3 ± 1.0;P = .018) and average (1.5 ± 0.4 v 2.7 ± 0.9; P = .029) SUV showed significant differences for both groups. Neither baseline parameters nor percentage decrease from baseline to day 42 was significantly different in the two groups.
Comparison of various quantitative FDG parameters in therapy monitoring.
Comparing the percentage change from baseline to day 7, there was no statistically significant difference between different parameters of FDG uptake. Considering the percentage decrease of FDG uptake parameters from baseline to day 42, as well as from day 7 to day 42, the accumulation rates (influx constant K and MRFDG) showed the largest decrease as compared with SUV parameters (P < .05). However, the r value of linear regression at day 42 (0.628 ± 0.494; range, −0.720 to 0.983) was significantly lower than r values at baseline (0.989 ± 0.020; range, 0.927 to 0.999; P= .012) and day 7 (0.950 ± 0.086; range, 0.685 to 0.996; P= .018).
DISCUSSION
Changes in FDG uptake have been correlated with response to antitumor therapy by several investigators.31-33 To establish the extent and time course of changes in metabolic parameters that occur during therapy, high-grade NHL was used as a tumor model to evaluate FDG uptake in this study. As anticipated, these tumors displayed a high FDG uptake as assessed with the SUV method in agreement with previous studies.16 All patients responded to chemotherapy by partial or complete remission after two courses of chemotherapy. This response rate is in accordance with data from the literature.25 34 A rapid and marked decrease of FDG uptake paralleled this therapy response. However, patients with relapse displayed higher residual FDG uptake after therapy, suggesting the sensitivity of FDG to detect residual tumor tissue.
FDG-PET in lymphomas.
NHL are known to have increased FDG uptake before therapeutic intervention indicating high metabolic activity.18-21Rodriguez et al23 found an average SUV of 11.8 ± 4.7 (mean ± SD) in 10 patients with high-grade NHL in good agreement with the average SUV found at the baseline in our patient population (11.1 ± 3.5). In contrast, Lapela et al16 reported a significantly higher average SUV in a group of 10 high-grade NHL (17.2 ± 8.2; P = .032), which may reflect methodologic differences.
Time course of FDG uptake during treatment.
The immediate changes of glucose metabolism after initiation of antitumor chemotherapy are not completely understood. In a homogeneous population of 11 patients with high-grade NHL, we observed for the first time that tumor FDG uptake as a marker of glucose metabolism showed a marked decrease of 60% (SUV[max]) to 67% (MRFDG) as early as 7 days after initiation of chemotherapy compared with baseline. All lesions displayed further decrease of FDG uptake from day 7 to day 42 resulting in a total decrease of 79% (SUV[max]) to 90% (MRFDG) from baseline to day 42. The observed changes of FDG parameters cannot be explained by statistical variations. Recent data from our laboratory showed that repeated measurements of FDG uptake in different solid tumors are associated with variations of 8% to 14%.35 Minn et al36 found only 10% variation of FDG uptake in 10 untreated lung cancer patients who were studied two times within 1 week. Therefore, changes in FDG-parameters of more than 25% cannot be explained by statistical variations, but represent definite change in metabolic parameters as a consequence of therapeutic interventions.
In view of the rapid response to chemotherapy, the question may arise whether the pronounced decrease of FDG uptake is a consequence of rapid decrease of tumor size resulting in partial volume effects. Recovery of radioactivity concentration in structures less than twice the spatial resolution will be decreased (partial volume effect). For the scanner system used in this study, the spatial resolution is 8 mm. However, only two lesions displayed a diameter less than 16 mm at day 7, estimated from transaxial PET images. Therefore, it appears unlikely that partial volume effects are responsible for the observed changes of tumor FDG uptake.
It was shown by Lindholm et al,37 as well as by Langen et al,38 that the FDG uptake is influenced by plasma glucose levels. Significantly higher FDG uptake was measured in fasting conditions as compared with oral glucose loading. Chemotherapeutic agents may affect blood glucose level. However, comparison of blood glucose levels before and during chemotherapy showed no difference. Therefore, changes in FDG uptake are not correlated with therapy-induced changes in blood glucose levels.
In 1993, Wahl et al14 described successful early tumor response monitoring of chemohormonotherapy with FDG-PET in breast cancer. At day 8 of the first course of chemohormonotherapy, a significant decrease of 22% of maximum FDG tumor uptake was shown in eight therapy responding patients with large primary breast cancer. Three nonresponding tumors did not show significant decrease in FDG uptake. Decreases of metabolic parameters preceded changes in tumor size. Jones et al10 examined patients with soft tissue and musculoskeletal sarcomas 1 to 3 weeks after initiation of chemotherapy. In two responsive tumors, FDG uptake decreased by 38% and 36%, whereas in a tumor progressive under therapy, FDG uptake increased by 71% within some weeks.
Hoekstra et al21 reported similar results in patients with Hodgkin's disease (n = 3) and NHL (n = 8). In nine of 11 patients, they described a reduction of FDG uptake studied with planar scintigraphy during the first cycle of chemotherapy. In five patients, the second study was performed within 1 week after initiation of treatment. Two patients who failed to respond to therapy exhibited persistent high FDG uptake. These results were confirmed by Dimitrakopoulou-Strauss et al18 in a group of 10 patients with Hodgkin's disease and NHL who were studied at baseline and around day 30 of chemotherapy.
In summarizing the indicated studies, we conclude that the decrease of FDG-PET in NHL does reflect treatment-induced metabolic changes.
Correlation of functional and morphologic data with therapy outcome.
It was of particular interest that FDG uptake at day 42 correlated closely with the patients' disease status. The group of patients with stable, complete remission even 10 months or more after completion of therapy exhibited significantly lower mean SUV values, as well as mean FDG accumulation rates after two courses of chemotherapy compared with the group of patients with transient tumor response followed by tumor relapse. Furthermore, already at day 7, the group of patients with long-term remission had significantly lower mean values of the metabolic rate MRFDG than the group of patients with disease relapse. In contrast, SUV parameters did not show significant differences. Although more studies in larger patient populations are required, one may speculate that PET represents a sensitive method to detect not only early tumor response to therapy, but also predict long-term outcome.
Monitoring of antitumor chemotherapy is generally performed by sequential determinations of tumor size using morphologic imaging modalities (ultrasound, CT, MRI). These assessments do not necessarily reflect the quantity of remaining viable tumor cells. Based on morphologic criteria alone, residual tumor mass cannot be differentiated from scar. In addition, several courses of chemotherapy must be administered before treatment effectiveness can be reliably determined.39 40 However, there are no data showing that cross-sectional imaging with CT or MRI, after completing one course of chemotherapy in a highly responsive tumor like NHL, would predict the result of treatment. Further studies comparing PET data and morphologic data are required to define the relative benefit of each modality in early therapy monitoring.
In eight of 11 patients in this investigation, morphologic imaging after two courses of chemotherapy showed residual tumor mass. Four of these eight patients showed recurrence at later examinations. In three patients, there was no evidence of residual tumor mass. One of these patients relapsed after a disease-free interval of 12 months. Therefore, in this case, morphologic imaging after two courses was not predictive for long-term outcome. In contrast, using a maximum SUV of 2.5 as a cut-off value for differentiating viable tumor from scar, FDG PET indicated no evidence of residual viable tumor cells in seven of 11 patients at day 42. This cut-off value was defined in previous studies to be well-suited for differentiation of benign and malignant breast tumors.41Only one of these seven patients relapsed. This was the same patient who had shown CR by morphologic criteria after two courses of chemotherapy. All patients displaying a maximum SUV = 2.5 (n = 4) relapsed.
Our data are in accordance with the results recently published by Janicek et al42 who studied the value of early restaging gallium-67 scans in predicting chemotherapy outcome in NHL. They found that Ga-67 scans after two courses of chemotherapy accurately distinguished long-term complete responders from patients with relapsed disease. At a median follow-up of 31 months, 94% of patients who had negative Ga-67 scans after two courses of chemotherapy remained free from progression, whereas only 18% of patients who had positive early restaging Ga-67 scans remained in complete remission. Similar to FDG, the tumor seeking radionuclide Ga-67 concentrates in viable tumor, but lacks to accumulate in fibrotic and necrotic tissue. However, the use of Ga-67 is limited by its unfavorable radiation properties. The long half-life of 78 hours results in a relatively high radiation burden of 44 mSv (120 μSv/MBq) per examination using a standard dose of 370 MBq.43 This is in contrast to FDG-PET, which results in a lower radiation burden of about 10 mSv per study (27 μSv/MBq). A second disadvantage of Ga-67 is the length of time required for the examination. Imaging is usually performed 48 to 96 hours after administration to allow time for clearance of background activity. In contrast, a FDG-PET examination takes a maximum of 90 minutes scanning time. Finally, imaging with Ga-67 does not enable absolute quantification of tracer uptake, allowing only qualitative assessment of residual tumor mass. Therefore, FDG-PET seems to be the favorable technique, as long as these first promising data can be confirmed in larger patient populations.
Based on the data of this study, we postulate that a single PET study after two courses of chemotherapy may be sufficient to separate patient subgroups with good prognosis who can be cured with standard chemotherapy from those with much poorer survival in whom more aggressive approaches may be required. More aggressive, but also more toxic regimens, are currently available, but clear criteria to define the best suited treatment protocol are lacking.27,44 45 The prognostic value of FDG-PET imaging in this clinical situation needs to be further investigated.
Comparison of various quantitative FDG parameters in therapy monitoring.
Several scintigraphic parameters have been suggested for the analysis of FDG uptake in tumor tissue with respect to their value in monitoring therapy effects.29 46-48 Dose uptake ratios have been introduced to normalize tracer uptake to injected dose and body weight. These simple methods allow comparison of regional tracer uptake in sequential studies. We calculated maximum and average standardized uptake values (SUV) of the tumor lesion.
SUV parameters only reflect the concentration of FDG in tumors at the time of data acquisition, ie, from 30 to 60 minutes postinjection, without indicating changes in the level of FDG accumulation during this period. Accumulation parameters expressed as the influx constant K or the MRFDG indicate the net rate of FDG transport and phosphorylation in tumor tissue. In contrast to SUV parameters, these parameters include information on the kinetics of FDG uptake and may possibly be more sensitive to changes during therapy.
Comparison of SUV values and accumulation parameters suggested that the influx constant K and MRFDG may be more sensitive in predicting the tumor response to treatment. In contrast to SUV parameters, at day 7 the influx constant K and MRFDG were already significantly lower for long-term responding patients as compared with patients with disease relapse. The percentage decrease of the accumulation rates from baseline to day 42, as well as from day 7 to day 42, was more pronounced as compared with SUV parameters. However, because all tumors showed effective response to therapy at day 42, the activity in the lesions was low. Therefore, the Patlak graphical analysis at this time resulted in very shallow curves causing large uncertainties in K, as shown by the significantly lower r values. In reviewing our data, only influx constants K of more than 1.4 were associated with r values greater than 0.9. Thus, the accumulation rates at baseline and day 7 are more reliable than those at day 42. However, this does not affect the principle applicability of Patlak analysis in therapy monitoring. In contrast, SUV parameters may be more stable in areas with low FDG uptake and may represent the preferable method to differentiate long-term responders from patients with disease relapse. This hypothesis has to be tested prospectively in a larger patient population.
From our data, we conclude that a more demanding data acquisition including the determination of the blood input curve may yield additional information for therapeutic monitoring. On the other hand it has to be taken into account that in lesions with very low FDG uptake, the reliability of Patlak analysis will be limited. It needs to be addressed in the future whether this conclusion is also valid for other tumor types.
CONCLUSION
In conclusion, FDG uptake in successfully treated NHLs decreased by 60% to 67% from baseline to 7 days after the initiation of chemotherapy. These results indicate that two thirds of the metabolic effect of chemotherapy, as assessed by FDG uptake, occur within the first 7 days of therapy. In addition, FDG uptake at day 42 showed significantly lower values for long-term responders. Based on these results, we hypothesize that primary outcome of chemotherapy may be predicted using FDG-PET as early as 7 days after beginning of treatment, while long-term prognosis is best correlated with the results after completion of two courses of chemotherapy.
In NHL, dynamic data acquisition combined with Patlak analysis of FDG kinetics may provide superior information in therapy monitoring. Similar studies in solid tumors are needed to determine whether more dynamic acquisition methods will improve the predictive value of FDG-PET.
ACKNOWLEDGMENT
We thank Sylvia Fürst, Claudia Kolligs, and Coletta Kruschke for their excellent performance of the PET scans and Jodi Neverve for her review of this manuscript. Furthermore, we gratefully acknowledge the support of the cyclotron and radiochemistry staff. The authors appreciate the assistance of Cornelia Pankalla in preparation of the figures.
Address reprint requests to Wolfgang Römer, MD, Nuklearmedizinische Klinik und Poliklinik, Klinikum rechts der Isar, Technische Universität München, Ismaninger Straβe 22, 81675 München, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 2. Comparison of SUV[max] (⧫) and the metabolic rate of FDG (MRFDG) (▪) at (A) day 7 and (B) day 42 in patients with relapse (n = 5) and complete response (CR; n = 6).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4464/4/m_blod41208002ax.jpeg?Expires=1766000853&Signature=0NNYofHW2oUI1VQV2ibPDQHoDC1mF4U~31Svl51qzDdNOXS0w~-6eXRtsQayX5IMex4XMndJFk~RtS1H8CrWylVNCw8QvCvTAEGmAA3rn0Fw-UUljQHiUvkZhw-WOnqRGfWFbJsYeuNy1cSqNziFUjjfch-WYWgL3TR4IZUOSifa3gDukZQF4Z1UpCiNp2mmp484nbaMqj1nM8gVPx~HqAW8cd3zfIMBnD1O0-cayDZYC7cAa5C6jLYmJNIodbPAPj39QWPM2d0o1FSNVduEmm2PfyUW~RZpnzMSijVVbFyviXRveSM7m2pOkMxYXzbRjWMEwcUHQIORJ9WSBwBKiQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Comparison of SUV[max] (⧫) and the metabolic rate of FDG (MRFDG) (▪) at (A) day 7 and (B) day 42 in patients with relapse (n = 5) and complete response (CR; n = 6).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4464/4/m_blod41208002bx.jpeg?Expires=1766000853&Signature=xQCpNLhsvzDPEuLrbNUqi7wCCGYt6WRrHZ5W4upGrguMxv5TCWzEOWLaRW5dv2EUzOzFpfP08Pk-fDForgpQu8yIxFulxVFE987dxBEHKwww9EIRpwhVIdMPKYSQIKcE26AoU7SImVKQvug75f7S2QezGk7lY2ctdUykiX7EVpauAj9vLQOdB8mCc~mvZvgKeSO-1sLB4ujjE5zr7hUQs8CGQMrD1omxXgSTVafkR-0ldBzoq5LMHhq9LpF~qik8eOzFLGdoSfNmXZFE7F1M~ZG2baHxYMoAUhnmqFEcwHqHjCHnh-xgwEm1gpIzOksQJr0-VSXOdGJjX1na2hED0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 2. Comparison of SUV[max] (⧫) and the metabolic rate of FDG (MRFDG) (▪) at (A) day 7 and (B) day 42 in patients with relapse (n = 5) and complete response (CR; n = 6).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4464/4/m_blod41208002ax.jpeg?Expires=1766081138&Signature=prA-401bQJlmzjheFd7Fe5FFxIo3ojADSq53rJwoR7p-y9wZEgeTg9w4z4MJudk3s2QHIvJIQPT2UdaLNpiUP7nQsqAvG~z6Ya~m7gMIYser~KUOZoBBrP32IH4N31~z2ZVWBn3QJUOY8dlFgg~Erz5EaZRTRHmo~b~F0R5UmBLVltUG0x25J5Lb0KOWOtbXkL4GXvzOzgA~8PvApBLjVARvLjl3gMYzVgHIVIsavN3AtVaDLucCkINsfM2cqm8u3XyX-ItgzF2xfP3xusVbj8pBSDXAFvrgiSvsbxfqpXKWGoaPg6v7dRG3l-E03WsyQDBaCFtz33NEXSSsJyW~zA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Comparison of SUV[max] (⧫) and the metabolic rate of FDG (MRFDG) (▪) at (A) day 7 and (B) day 42 in patients with relapse (n = 5) and complete response (CR; n = 6).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4464/4/m_blod41208002bx.jpeg?Expires=1766081138&Signature=j4HJIfb6gvF2e8Xu1CPw13R3Y7vESsQDqGy3EH3-pRYUuzVNaHkXyeBzgcolN-645RQA1Az12AYe0TQsuHuxPYtBooM3O0KLGqLeGPQjN9W2qNo3x716qFpqQZNURr86befpWYNbXSsCcTA0NIfl12aPblfVK5aTpHT-Ik3mAJH1oM~Iwh2YbXAF1s8pz7UXCplVikKJZw9aAAy358e55SCxyWJH6l~~pa38ugRui3paf742tT9WfeQa7PeMl8GGzzYXWoSzizIkDh-zGRxyIywTiNQ1RjSbVSPyWn11JHR6UUYjmy6APtAzxPN4aeHSdFZ4Zxu8oIqxfjEdQMgCyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)