Abstract
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by the accumulation of resting lymphocytes. The identification of p27kip1, a cyclin-dependent kinase inhibitor that contributes to cell cycle arrest and represents a link between extracellular signals and cell cycle, prompted us to study p27 protein in the lymphocytes from 88 patients with B-CLL and 32 patients with other chronic B-lymphoproliferative disorders. The expression of p27 protein was higher in B-CLL samples with variations among them. In B-CLL, p27 levels were independent of absolute number of circulating lymphocytes, but strongly correlated with both lymphocyte and total tumor mass (TTM) doubling time. High p27 expression was associated with a poorer overall prognosis. In vitro, there was an increased spontaneous survival of B-CLL cells expressing high p27 levels. Interleukin-4 (IL-4) upregulated p27 levels in B-CLL cells, while fludarabine decreased p27 levels. Thus, our results indicate that p27 may be a valuable kinetic marker in B-CLL by providing instantaneous estimation of the disease doubling time. In addition, these results suggest that there is a link between p27 expression and the ability of CLL cells to undergo apoptosis.
B-CELL CHRONIC LYMPHOCYTIC leukemia (B-CLL), the most frequent form of leukemia in Western countries, is a disease with a low proliferative activity characterized by the progressive accumulation of clonal B lymphocytes in early phases (G0/G1) of the cell cycle.1,2 The pathogenesis of the disease remains largely unknown, but recent investigations indicate that defective apoptosis (programmed cell death) may play an important role in clonal expansion.3 Previous studies have suggested that the deregulation of some cell cycle regulatory genes may contribute to the malignant process of chronic B-lymphoproliferative disorders.4-6
In the past few years, major insights have been accomplished in the understanding of the complex mechanisms that regulate the eukaryotic cell cycle.7,8 The progression through the different phases of the cell cycle is governed by the cyclin-dependent kinase (cdk) complexes consisting of a positive regulatory cyclin subunit and a catalytic serine/threonine kinase subunit. Several cyclin-cdk complexes are sequentially required at different checkpoints of the cell cycle. More recently, negative regulatory elements called cyclin-dependent kinase inhibitors (CKI) have been identified.9 10 CKI may be divided into two groups that differ in sequence homology and mechanism of action. Members of the KIP/CIP family including p21Cip1/WAF1, p27Kip1, and p57Kip2, inhibit all cyclin-dependent kinases by direct binding to cdk complexes, while the members of the INK4 family (p15INK4B, p16INK4A, p18INK4C, and p19INK4D) interact exclusively with cdk4 and cdk6 by competing with the cyclin for the association with the kinase.
p27Kip1 (p27) is mainly involved in growth inhibitory processes. It is highly expressed when cells are arrested in G0/G1 and its expression declines as cells progress toward S phase.11-13 In vitro, the overexpression of p27 has been shown to arrest cells in G0/G1, suggesting that this CKI has a pivotal role in cell cycle exit.14 p27 expression is upregulated by cell to cell contact and growth inhibitory cytokines such as transforming growth factor-β (TGF-β) or interferon gamma, showing that p27 is an important link between extracellular signals and the cell cycle machinery.15-18
The key role of p27 in the early phases of the cell cycle led us to study its expression in accumulative disorders such as B-CLL. We have therefore investigated the expression of p27 protein in B-CLL and other chronic B-cell malignancies and correlated its level of expression with clinical data. In addition, we have also studied both the influence of p27 level on the in vitro survival of B-CLL cells and the behavior of p27 expression after exposure to apoptosis modulating agents.
MATERIALS AND METHODS
Patients.
Peripheral blood samples from 88 patients with chronic B-CLL obtained between 1993 and 1997 were studied. Apart from appropriate clinical and cytological features, the diagnosis of B-CLL relied on a characteristic phenotype with CD5 positivity, weak expression of surface immunoglobulins, and for the 56 most recent cases, CD23 positivity and lack of reactivity with FMC7 antibody, as assessed by flow cytometry analysis.19 Additional samples from 32 patients with other leukemic B-lymphoproliferative disorders (14 lymphoplasmacytic lymphomas [LPL], 13 mantle cell lymphomas [MCL], and five prolymphocytic leukemias [PLL]) were also studied. All of the patients included in this study were followed in our institution.
Clinical charts of all patients were reviewed and the following data recorded: white blood cell (WBC) and lymphocyte counts, clinical stage according to Binet classification,20 as well as total tumor mass (TTM) score.21 Both lymphocyte and TTM doubling time (DT) (defined as the time needed to reach twice the value of initial observation) were calculated for the untreated patients.
Cell isolation and culture.
Peripheral blood from patients with leukemic chronic B-cell malignancies was obtained after informed consent. Blood mononuclear cells were isolated by Ficoll-Hypaque density gradient and monocytes removed by plastic adherence. B and T lymphocytes from normal healthy donors were separated by sheep erythrocyte rosetting.
For some experiments, freshly isolated cells were cultured at a final concentration of 2 × 106/mL in RPMI 1640 containing 10% heat-inactivated fetal calf serum (FCS), glutamine 2 mmol/L, penicillin 50 U/mL, and streptomycin 50 μg/mL at 37°C in a 5% CO2 humidified atmosphere. Normal T lymphocytes were stimulated with phorbol myristate acetate (PMA) 1 ng/mL (Sigma, St Quentin, France) and phytohemagglutinin (PHA) 10 μg/mL (Sigma). B-CLL cells were cultured for 5 days in medium alone or added with fludarabine (5 × 10-6 mol/L) or interleukin-4 (IL-4; 10 ng/mL).
Protein extracts.
A total of 1 × 107 cells were lysed in buffer (Tris 20 mmol/L pH8, NaCl 150 mmol/L, Triton-X 100 1%, EDTA 5 mmol/L, Na orthovanate 1 mmol/L, NaF 10 mmol/L, leupeptin 10 μg/mL, aprotinin 10 μg/mL, phenylmethyl sulfonyl fluoride [PMSF] 100 μg/mL) for 20 minutes at +4°C. Lysates were spun at 12,000 rpm for 15 minutes and supernatant collected. Protein concentration was assessed by the Bio-Rad assay method (Bio-Rad Laboratories, Richmond, CA). Protein extracts were either used immediately for Western blotting or frozen at −70°C.
Western blotting.
A total of 100 μg of protein extracts was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon-P membranes (Millipore, Bedford, MA). Membranes were blocked for 1 hour in 5% nonfat dry milk phosphate-buffered saline (PBS) and incubated with purified rabbit anti-p27 antibody at 1 μg/mL (kind gift from G. Ponzio, INSERM U364, Nice, France). Subsequently, filters were washed twice in Tris 10 mmol/L pH7.5, NaCl 200 mmol/L, and Tween 0.05% and incubated with an appropriate horseradish peroxidase-linked secondary antibody at a 1:10,000 dilution for 1 hour. Membranes were washed three times in the same rinsing buffer, and detection was performed by enhanced chemoluminescence protocol (ECL, Amersham, Buckinghamshire, UK). Control of protein deposition was assessed by subsequent hybridization of membranes with an anti-HSP 72/73 antibody at 0.5 μg/mL (Oncogene Research Products, Cambridge, MA).
Signal quantitation on Western blots.
Films were scanned on a densitometer and signals quantitated using the Bioprofil software (Vilber Lourmat, Marne la Vallée, France). For each sample, the p27 value was expressed as the ratio of the signal obtained to that of a positive control. The latter originated from a B-CLL patient whose lymphoid cells expressed high level of p27 protein. The cell lysates of this control were obtained from one sampling and contained enough proteins to be loaded on each gel. To make sure the Western blot assay and signal quantitation were accurate throughout the range of protein found in the samples, we generated a standard curve by serial dilution of recombinant p27 protein (Santa Cruz Biotechnology Inc, Santa Cruz, CA) and compared it with serial dilutions of our positive control and to representative samples.
RNA extraction and Northern blot.
RNA was extracted by the guanidium thiocyanate-phenol-chloroform method as described by Chomczynski and Sacchi.22 Briefly, 10 μg of total RNA were subjected to 1% 3-N-morpholino propane sulfonic acid (MOPS)/formaldehyde agarose gel electrophoresis and blotted onto nitrocellulose membranes. The blots were hybridized overnight with a 32P-labeled human 5′ cDNA p27 probe (obtained by reverse transcriptase-polymerase chain reaction [RT-PCR]). Filters were washed twice in 2X SSC, 0.1% SDS at 50°C for 15 minutes and then twice in 0.2X SSC, 0.1% SDS at 50°C for 15 minutes. Autoradiography was performed for 3 days at −80°C using intensifying screens.
Cell viability and apoptosis.
Percentage of viable B-CLL cells in culture was determined by the trypan blue dye exclusion test. Apoptosis of cells in culture, either in medium alone or after exposure to fludarabine or IL-4, was assessed by immunocytochemistry using an in situ cell death detection kit (Boehringer Mannheim, Meylan, France). Briefly, cells were fixed in 10% neutral buffered formalin for 15 minutes before being spun down on slides. Slides were incubated with methanol at −20°C for 5 minutes, then with 0.25% Triton-X 100 in TBS at room temperature for 5 minutes and TUNEL reaction mixture was added according to manufacturer's instructions. Revelation was made with alkaline phosphatase substrate solution (Vector Laboratories, Burlingame, CA). The percentage of apoptotic cells was determined on 500 cells.
Statistical methods.
Standard regression analysis, t-test, and the nonparametric Mann-Whitney test were used to determine possible correlations between p27 levels and corresponding data. Survival curves were plotted according to the Kaplan-Meier method and differences between curves estimated by means of log-rank test. Length of overall survival was measured from the date of sample collection to the date of last follow-up or death.
RESULTS
Expression of p27 protein in normal lymphocytes and tumor cells from B-CLL and other chronic B-cell disorders.
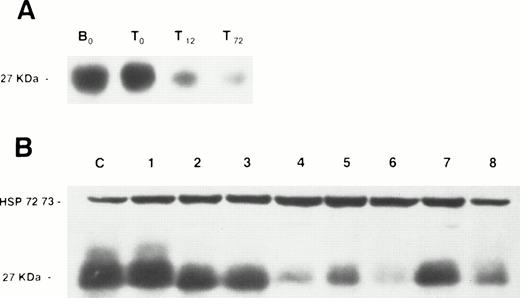
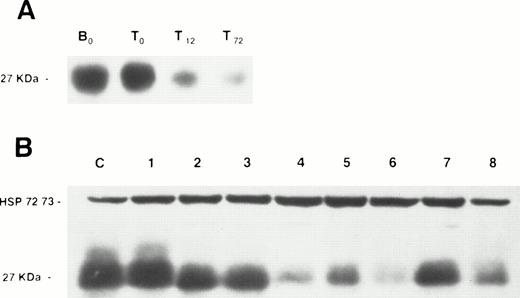
We first examined p27 protein expression in normal resting B and T lymphocytes from peripheral blood of healthy donors and found p27 to be consistently highly expressed. When T lymphocytes were exposed to mitogens (PMA and PHA), p27 expression decreased within 12 hours of culture (Fig 1), a time point corresponding to mid G1.23
Western blot analysis of p27 protein expression in normal lymphocytes and lymphoproliferative disorders. (A) A membrane containing protein extracts from normal resting B lymphocytes (B0), normal resting T lymphocytes (T0), PMA + PHA-stimulated T lymphocytes after 12 (T12) and 72 (T72) hours of culture. (B) A membrane containing protein extracts from peripheral blood lymphocytes of the B-CLL patient serving as positive control (C), and peripheral blood lymphocytes of five B-CLL patients (no. 1 through 5), a patient with MCL (no. 6), and two LPL patients (no. 7 and 8). Each lane was loaded with 100 μg of total protein. Blots were exposed to anti-p27 antibody and anti-HSP 72/73 as protein deposit control.
Western blot analysis of p27 protein expression in normal lymphocytes and lymphoproliferative disorders. (A) A membrane containing protein extracts from normal resting B lymphocytes (B0), normal resting T lymphocytes (T0), PMA + PHA-stimulated T lymphocytes after 12 (T12) and 72 (T72) hours of culture. (B) A membrane containing protein extracts from peripheral blood lymphocytes of the B-CLL patient serving as positive control (C), and peripheral blood lymphocytes of five B-CLL patients (no. 1 through 5), a patient with MCL (no. 6), and two LPL patients (no. 7 and 8). Each lane was loaded with 100 μg of total protein. Blots were exposed to anti-p27 antibody and anti-HSP 72/73 as protein deposit control.
All B-CLL patients whose tumor cells were analyzed in this study had more than 4 × 109/L circulating lymphocytes at the time of collection. There were 33 female and 55 male patients, and their mean age was 65.5 years (range, 35.7 to 84.5). Blood samples were collected at the time of diagnosis in 21 patients and later in the course of the disease in 67 patients, among whom 39 had received prior treatment and 28 patients had not.
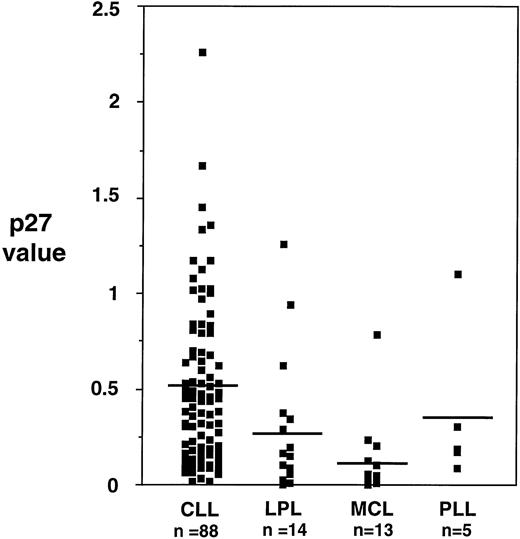
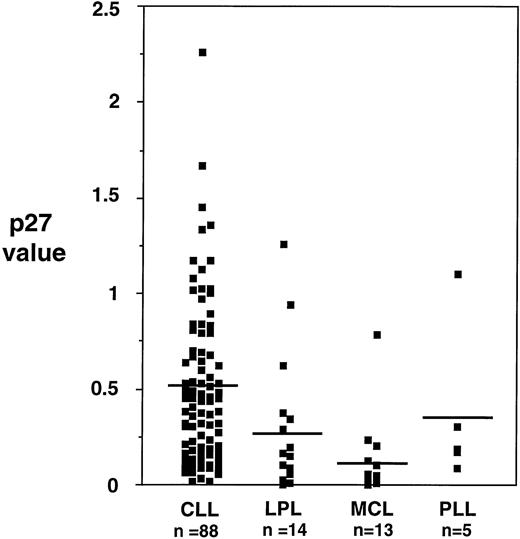
p27 protein was expressed in all the 88 B-CLL samples examined. However, the level of expression was variable among samples, with a ratio to control (as defined in the Materials and Methods section) ranging from 0.1 to 2.3 (mean, 0.51 ± 0.42) (Fig 1). Overall, the expression of p27 in samples from other chronic B-cell malignancies was significantly lower than the one observed in B-CLL: PLL 0.37 ± 0.42; LPL 0.27 ± 0.38; MCL 0.12 ± 0.21 (Fig 2).
Levels of p27 protein expression in different chronic B-lymphoproliferative disorders. Mean levels of p27 in B-CLL are higher than in other lymphoproliferative disorders, reaching statistical significance (Kruskal and Wallis, P < .001).
Levels of p27 protein expression in different chronic B-lymphoproliferative disorders. Mean levels of p27 in B-CLL are higher than in other lymphoproliferative disorders, reaching statistical significance (Kruskal and Wallis, P < .001).
Expression of p27 mRNA.
Thirteen representative B-CLL samples expressing various p27 protein levels and normal resting T lymphocytes were examined by Northern blot. In all of these cases, only the expected 2.5-kb normal transcript was detected (data not shown). p27 mRNA expression was roughly identical in all samples and there was no correlation between the intensity of mRNA signal and protein expression.
Correlation between p27 protein expression and clinical data in B-CLL.
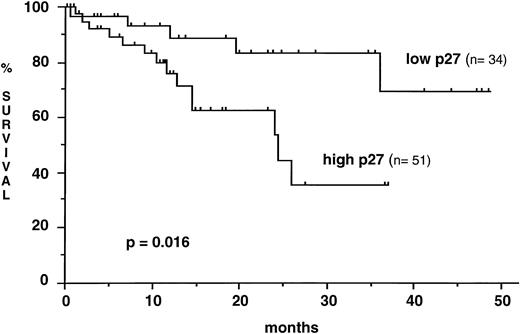
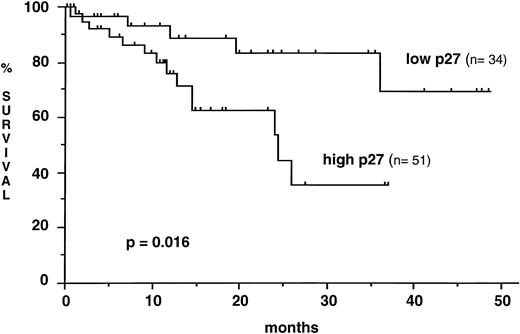
p27 levels in samples collected at diagnosis did not differ significantly from p27 levels in samples taken later during the course of the disease (mean, 0.47 ± 0.42 v 0.52 ± 0.43). There was no significant difference of p27 expression as well between samples from untreated and from previously treated patients (mean, 0.46 ± 0.38 v 0.57 ± 0.47). No statistically significant correlation was found between the level of p27 expression and both the absolute number of circulating lymphocytes or the TTM score. In contrast, p27 expression was strongly correlated to the lymphocyte DT and the TTM DT in the 57 patients for whom the follow-up without treatment was sufficient for these parameters to be calculated. Lymphoid cells from patients with a short lymphocyte DT (≤ 12 months) exhibited higher p27 expression than those from patients with a longer lymphocyte DT (0.66 ± .52 v 0.35 ± 0.33, P = .005). Similar results were observed in patients with a relatively short TTM DT (≤ 24 months) when compared with those with a more prolonged TTM DT. p27 expression was also sequentially analyzed during the course of the disease in 10 patients. The interval between two p27 determinations ranged from 3 to 48 months. In the three patients with unquestionable disease progression, there was a significant increase of p27 expression, whereas p27 remained roughly at the same level in five untreated patients with stable disease, whatever the blood lymphocyte count was (from 32 up to 248 × 109/L). In the two patients under cytostatic treatment, only a slight decrease of p27 expression was observed (data not shown). To analyze the prognostic significance of p27 expression on survival, CLL patients were stratified into low and high p27 expressors (ratio to control ≤ 0.31, n = 34 and ratio to control > 0.31, n = 51, respectively). Mean time elapsed from diagnosis to sample collection did not differ significantly between the two groups (low p27, 48.5 months ± 55.2; high p27, 53.8 ± 60.2). Mean observation period from sample collection was 14.1 months (range, 0.6 to 48.5). The overall survival from the date of sampling was shorter in patients with a high p27 expression (median survival, 24.4 months) than in patients with a low p27 expression (median not reached) (P = .016) (Fig 3). The number of patient samples tested was not sufficient to allow comparison through multivariate analysis of the independency of p27 expression level from other prognostic factors. It is noteworthy that there were more patients with advanced Binet's stage C disease among the high p27 expressors (37%) than among the low p27 expressors (14.7%) (P = .02). A contributive blood karyotype was available in 40 patients, and p27 expression was not correlated with the presence or the absence of cytogenetic abnormalities (mean, 0.58 ± 0.34 v 0.55 ± 0.61).
Kaplan-Meier curve for survival from the time of sampling according to p27 expression. High levels of p27 are associated with a shorter survival (Log rank test).
Kaplan-Meier curve for survival from the time of sampling according to p27 expression. High levels of p27 are associated with a shorter survival (Log rank test).
In vitro viability of B-CLL cells and p27 expression.
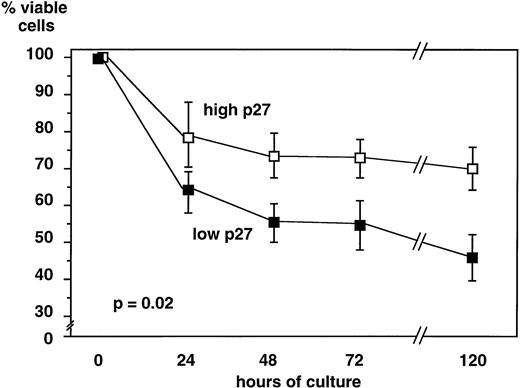
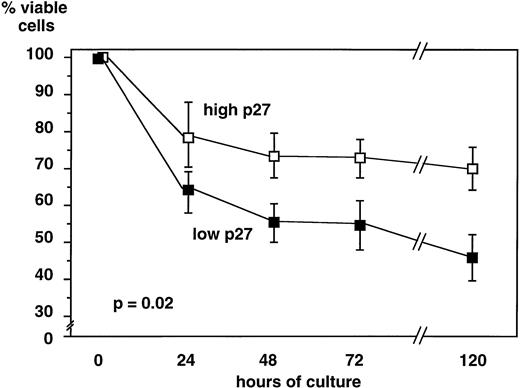
Lymphoid cells from 31 B-CLL patients were cultured in medium alone, and cell viability was assessed at 24 hours, 48 hours, 72 hours, and 5 days. Cells expressing a high level of p27 survived longer in culture (70% ± 21.2% viable cells at day 5) than cells with a low p27 expression (45.8 ± 17 viable cells at day 5,P = .02) (Fig 4). Because B-CLL cells in culture die spontaneously by apoptosis, these data show that high p27 expression is correlated with a decreased rate of apoptosis in vitro.
In vitro viability of B-CLL cells is related to p27 level of expression. Lymphoid cells from 31 B-CLL patients were cultured up to 5 days in RPMI+ 10% FCS. Viability was assessed by trypan blue exclusion. For each time point, the percentage of viable cells was calculated. Data are presented as means ± standard deviation comparing 19 samples of high and 12 samples of low p27 expressing cells. When cultured in vitro, B-CLL cells expressing higher p27 levels had sustained viability as compared with cells expressing low p27 levels.
In vitro viability of B-CLL cells is related to p27 level of expression. Lymphoid cells from 31 B-CLL patients were cultured up to 5 days in RPMI+ 10% FCS. Viability was assessed by trypan blue exclusion. For each time point, the percentage of viable cells was calculated. Data are presented as means ± standard deviation comparing 19 samples of high and 12 samples of low p27 expressing cells. When cultured in vitro, B-CLL cells expressing higher p27 levels had sustained viability as compared with cells expressing low p27 levels.
Effects of fludarabine and IL-4 exposure on p27 expression.
We assessed the variations of p27 protein expression in 14 B-CLL samples after a 5-day in vitro culture in the presence of apoptosis modulating agents.
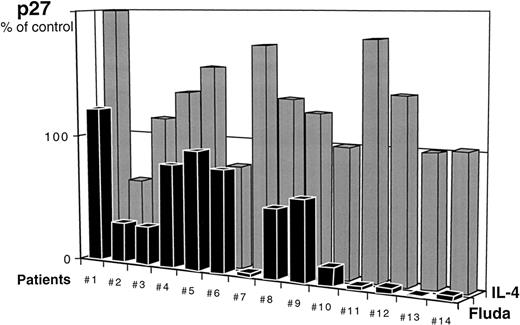
In all samples, decreased viability as measured by trypan blue dye exclusion test was closely related to increased apoptosis as assessed by TUNEL assay. For each experiment, control value was that of cells after 5 days of culture in medium alone. As expected, cell viability was increased in the presence of IL-4 compared with control and less apoptotic cells (19% ± 3% in medium + IL-4 v 29% ± 8% in medium alone) were observed at day 5. On the contrary, in all cases, cells exposed in vitro to fludarabine exhibited less viability and more apoptosis (83% ± 13%) . We studied p27 protein expression after 5 days of in vitro culture with and without modulating agents. When expressed as the ratio of p27 level after exposure to IL-4 to p27 level in medium alone (= control) at day 5, p27 expression was stable or increased in 12 of 14 cases (P = .004) (Fig 5). In 12 of 14 cases, p27 levels were significantly lower after exposure to fludarabine ranging from 1% to 80% of p27 expression in medium alone (P = .0001).
Evolution of p27 expression in B-CLL cells after 5 days of culture in the presence of IL-4 (10 ng/mL) or fludarabine (5 × 10-6 mol/L). For each patient, results are expressed as the ratio of p27 expression at day 5 of culture in medium + IL-4 or medium + fludarabine to p27 expression at day 5 in medium alone (control value = 100%).
Evolution of p27 expression in B-CLL cells after 5 days of culture in the presence of IL-4 (10 ng/mL) or fludarabine (5 × 10-6 mol/L). For each patient, results are expressed as the ratio of p27 expression at day 5 of culture in medium + IL-4 or medium + fludarabine to p27 expression at day 5 in medium alone (control value = 100%).
DISCUSSION
B-CLL results from the progressive accumulation of primarily noncycling CD5+ clonal B lymphocytes with abnormally prolonged survival. Because of this distinctive feature, conventional kinetic studies are irrelevant in B-CLL, for they mostly assess S and G2 + M phases. They do not provide appropriate information on the early phases of the cell cycle, while even in clinically aggressive disease, there is only a small percentage of cells out of G0/G1. These findings highlight the need for these early phases to be investigated in B-CLL. In the past few years, different cyclin-dependent kinase inhibitors have been identified and their role in the regulation of the cell cycle machinery is currently being elucidated.24-26 p27 plays a key role in maintaining cells arrested in a quiescent state.14,18 As we confirmed here in T lymphocytes from healthy donors, p27 protein is highly expressed in normal G0/G1 cells. Its level falls down rapidly after mitogenic stimulation and remains low thereafter in actively proliferating cells.27
We investigated p27 protein expression in the peripheral blood lymphoid cells from 88 patients with B-CLL and 32 patients with other leukemic chronic B-cell lymphoproliferative disorders. p27 protein was expressed in all cases, but its level appeared significantly higher in B-CLL than in other B-cell malignancies, namely MCL and PLL. There was no correlation between p27 mRNA and protein levels, and this is consistent with previous reports in other models showing that p27 regulation is posttranscriptional.28-30
The clinical course of B-CLL is highly variable and reliable individual prognostic tools are still limited.31 32 Although they have a powerful overall significance, the currently used staging systems do not allow prognostic discrimination within each group. Both the lymphocyte DT33 and the TTM DT21 were shown to provide useful prognostic information in B-CLL. They do reflect the kinetics of the disease, but they require several months of follow-up to be calculated. We found that the level of p27 protein expression was variable among B-CLL patients and correlated strongly with both the lymphocyte DT and the TTM DT. Patients with high p27 lymphocyte levels had shorter lymphocyte and TTM DT, as well as poorer clinical outcome and survival. It should be noted that there were significantly more patients with advanced Binet's stage C disease among the high p27 expressors. The levels of p27 were not linked to parameters, which are not related to the kinetic characteristics of the disease, such as the absolute lymphocyte count or the time elapsed since the diagnosis. They were not significantly different either in patients who received prior therapy and in those who did not. Therefore, the determination of p27 protein level could provide instantaneous information about the likelihood of a disease that might progress within a few months. The sequential analysis that we performed on 10 patients showed that the lymphocyte p27 levels remained stable in patients who had stable disease and increased in those who experienced disease progression. For example, we observed a dramatic increase (by 600%) of p27 protein level in one patient with nodal progression, but no significant modification of blood lymphocyte count. However, additional studies on a larger number of patients are warranted to confirm the prognostic value of p27 protein lymphocyte level.
The role of p27 in inhibiting proliferation is quite clearly established. The consequences of p27 inactivation have been studied in knock-out mice, which develop normally except for a significant increase in both the size of organs and the number of cells per organ, suggesting that p27 is necessary for physiologic growth control.34-36 On infection of human breast cancer cells with an adenoviral vector containing p27, a high level of p27 expression was observed, and this resulted in a marked decrease in the proportion of cells in S-phase.37 p27 gene alterations have been investigated in various human tumors, and unlike the INK4 family of kinase inhibitors, were found to be very uncommon.38-40Nevertheless, several groups have recently demonstrated an inverse correlation between the level of p27 protein expression and proliferative index, such as the staining with Ki67 antibody in human tumors.41-44 In breast or colorectal carcinomas, p27 expression was significantly lower in the tumor samples from patients with an aggressive disease and a short survival.45-48 Low p27 expression appeared therefore as an adverse prognostic parameter, which is in striking contrast to our findings in B-CLL. However, all of the malignancies in which p27 has been studied so far may be viewed as proliferative diseases, while B-CLL is an accumulative one in which progression does not result from excessive proliferation, but from defective apoptosis. This led us to consider that high p27 expression might contribute in this setting to inhibition of apoptosis.
Contrary to what is thought to occur in vivo, cultured B-CLL cells rapidly die from apoptosis in vitro.49,50 We found, as recently reported by Agular-Santelises et al,51 that B-CLL cells of patients with progressive disease have a longer spontaneous survival in vitro than those of patients with stable disease. The bcl2 family of apoptosis regulatory proteins have been largely investigated in B-CLL, but the results of these studies are controversial.52-55 Some of them suggested that bcl2 protein levels are not related to the extent of in vitro apoptosis or to clinical behavior. Bax, a bcl2 homologue known to promote cell death, is downregulated in B-CLL cells, as compared with normal B-cells and has been found to be lower in patients with progressive disease.56 However, some investigators proposed that high bcl2/bax ratio correlates with resistance to cytostatic agents rather than being indicative of advanced disease.57 58 We show here that cells expressing p27 at low levels died significantly more rapidly in culture than cells expressing p27 at high levels. These data support high p27 protein level as a possible protection of B-CLL cells against apoptosis.
Several recent publications have shown that IL-4 promotes in vitro survival of B-CLL cells by preventing spontaneous apoptosis, but without inducing proliferation.54,59-62 Accordingly, we observed less apoptotic cells in the samples cultured in the presence of IL-4 than in controls. In most of these cases, p27 level was higher after a 5-day exposure to IL-4, showing that prevention of apoptosis by IL-4 is associated with the increase or at least the maintenance of p27 level. This is in agreement with recent reports in other models pointing out p27 as one of the possible mediators of the action of IL-4.63,64 Conversely, we found that in vitro restoration of apoptosis after fludarabine65 66 exposure was associated in most cases with a marked decrease of p27 expression. This suggests that the decrease of p27 level is mandatory for apoptosis to occur and provides additional evidence that p27 might play a role in the apoptotic pathway.
Several investigators have studied the effects of transforming growth factor (TGF)-β on B-CLL cells, and most of them have found aberrant defective apoptotic responses to this growth inhibitory factor.67 68 Because p27 expression is upregulated by TGF-β in normal cells, we are currently investigating the links between TGF-β, p27, and apoptosis in B-CLL.
In this study, we have shown that p27 protein is variably expressed in B-CLL cells and its level is correlated with the kinetic features of the disease. Contrary to other human malignancies, high p27 level is associated with a poorer outcome in B-CLL. Our in vitro results suggest that p27 level might reflect the ability of CLL cells to undergo apoptosis. Additional observations and experiments are required to ascertain p27 as a possible component of the apoptotic pathway in this disease.
ACKNOWLEGMENT
The authors are very grateful to N. Rochet and G. Ponzio (INSERM U364, Nice, France) for helpful discussion and for providing p27 antibody and p27 cDNA probe.
Supported in part by grants from Ligue Nationale contre le Cancer (Comité de Paris).
Address reprint requests to Florence Ajchenbaum-Cymbalista, MD, Service d'Hématologie Biologique, Hôtel-Dieu, 1 Place du Parvis Notre-Dame, 75181 Paris Cedex 04, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.