Abstract
The sphingomyelin cycle, which plays an important role in regulation of cell growth, differentiation, and apoptosis, involves the formation of ceramide by the action of a membrane-associated, Mg2+-dependent, neutral sphingomyelinase and/or a lysosomal acid sphingomyelinase. In human polymorphonuclear leukocytes (PMNs), ceramide production correlates with and plays a role in the regulation of functional responses such as oxidant release and Fcγ receptor-mediated phagocytosis. To increase our understanding of the sphingomyelin cycle in human PMNs, the cellular location of neutral and acid sphingomyelinases was investigated in resting, formylmethionylleucylphenylalanine (FMLP)-activated, and FMLP-activated PMNs engaged in phagocytosis. In resting PMNs, a Mg2+-dependent, neutral sphingomyelinase was the predominant activity and was localized to the plasma membrane fractions along with the majority of ceramide. Upon FMLP-activation, there was a 1.9-fold increase in this neutral, Mg2+-dependent sphingomyelinase activity, which increased to 2.7-fold subsequent to phagocytosis of IgG opsonized targets. This increase in sphingomyelinase activity was restricted to the plasma membrane fractions, which were also the site of increased ceramide levels. Phospholipase D (PLD) activity, which is a target of ceramide action and is required for phagocytosis, was also found primarily in the plasma membrane fractions of FMLP-activated and phagocytosing PMNs. Our findings indicate that in human PMNs engaged in phagocytosis, the sphingomyelin cycle is restricted to the plasma membrane where intracellular targets of ceramide action, such as PLD, are localized.
POLYMORPHONUCLEAR leukocytes (PMNs) serve a crucial role in host defense by phagocytosing and killing invading microorganisms. An array of microbicidal activities are produced in human PMNs and include several acid hydrolases, natural antibiotics, and reactive oxygen species generated by assembly of the multicomponent NADPH oxidase.1 2 These substances are secreted by activated PMNs from at least three granule subsets either extracellularly or to the phagosome through fusion of granules with the plasma membrane or the phagosomal membrane, respectively. The phagosome contains the opsonized particle that has been engulfed through binding to Fcγ and/or complement receptors expressed on the PMN surface.
The metabolism of phospholipids has long been recognized as an important event in intracellular signal transduction. Recently, it has become evident that sphingolipid metabolism via the sphingomyelin cycle plays an important regulatory role in growth factor responses, such as proliferation, differentiation and apoptosis, gene regulation, and intracellular vesicle transport.3-15 The sphingomyelin cycle involves activation of sphingomyelinases that hydrolyze sphingomyelin to form ceramide, a key second messenger of this cycle. At least three distinct sphingomyelinase activities have been described in mammalian cells: (1) a lysosomal acid sphingomyelinase; (2) a cytosolic, Mg2+-independent, neutral sphingomyelinase; and (3) a membrane-associated, Mg2+-dependent, neutral sphingomyelinase.16,17 By crude subcellular fractionation the latter activity has been localized to membrane fractions in human brain,18 bovine adrenal medulla,19 rat liver,20 human renal proximal tubular cells,21and in cultured neuroblastoma cells, where it was suggested to be externally oriented.22,23 The intracellular targets of ceramide are not yet fully elucidated, but a recent report by Wiegmann et al11 indicated that in U937 cells stimulated with tumor necrosis factor (TNF), the subcellular topology of ceramide formation determined its action because the acidic, endosomal sphingomyelinase was involved in activation of the transcription factor NF-κB, whereas the neutral, plasma membrane-associated sphingomyelinase was involved in activation of a proline directed ser/thr protein kinase. Ceramide has also been shown to activate a cytosolic ser/thr protein phosphatase, and thus could potentially regulate a protein phosphorylation-dephosphorylation cascade.24 25
Recent data from this and other laboratories have indicated that PMN functional responses are regulated by the sphingomyelin cycle. Specifically, (1) preincubation of adherent PMNs with cell permeable short-chain ceramides results in diminished formylmethionylleucylphenylalanine (FMLP)-induced H2O2 production and inhibition of phospholipase D (PLD) activity.26 (2) Concomitantly, ceramide is formed in parallel with cessation of H2O2 production and specific granule release in FMLP-stimulated PMNs adherent to fibrinogen.26 (3) Exogenously added ceramide also inhibits FMLP-induced superoxide formation and calcium influx in PMNs held in suspension.27 (4) Exogenously added C2-ceramide inhibits PLD activity, MAP kinase activation, and IgG-dependent phagocytosis.28,29 Consistent with these findings, IgG-dependent phagocytosis is accompanied by activation of a neutral sphingomyelinase and generation of ceramide at a time when the rate of ingestion is declining.28 Thus, in both phagocytosing and adherent FMLP-activated PMNs, ceramides inhibit the activity of PLD and affect PMN functional responses.26 28 This suggests that both ceramide formation and PLD activation are occurring at the same intracellular sites.
The subcellular topology of sphingomyelinase activity and ceramide formation has not previously been addressed in human PMNs. It is generally believed from investigations in other cell types that acidic sphingomyelinase localizes to the lysosomal compartments, whereas neutral sphingomyelinase is associated with plasma membranes. However, PMNs are specialized cells with several intracellular compartments including at least three granule subtypes (of which the azurophil granules are believed to be the lysosomal compartment), and the highly mobilizable secretory vesicles. These granules and vesicles fuse with the plasma membrane during cell activation and introduce functional proteins and enzymes to the cell surface.1 To increase our understanding of the sphingomyelin cycle in PMNs, we investigated the subcellular localization of sphingomyelinase activity and ceramide formation in resting, FMLP-activated, and phagocytosing human PMNs.
EXPERIMENTAL PROCEDURES
Cells.
Human PMNs were isolated from peripheral blood donated by healthy volunteers, as previously described.26 In short, blood was anticoagulated in acid citrate dextrose and red blood cells (RBCs) sedimented by addition of dextran (Abbott Laboratories, Abbott Park, NC). Remaining RBCs were removed by hypotonic lysis, followed by centrifugation through Ficoll-Paque (Pharmacia LKB Biotechnology Inc, Piscataway, NJ) to remove mononuclear cells. Before activation of cells and subcellular fractionation, cells were incubated for 5 minutes on ice with diisopropylfluorophosphate (DFP, 5 mmol/L; Sigma Chemical Co, St Louis, MO), washed, and resuspended in the desired buffer.
Opsonization of sheep RBCs.
Sheep erythrocytes were purchased from Biowhittaker (Walkersville, MD). Five milliliters of stock erythrocytes were washed three times with buffer containing 2.5% dextrose, 0.05% gelatin, 2.5 mmol/L sodium barbital (pH 7.5), 75 mmol/L NaCl, 0.15 mmol/L CaCl2, and 0.5 mmol/L MgCl2. Erythrocytes (109/mL) were incubated for 30 minutes at 37°C with anti-sheep erythrocyte IgG (1:300 to 1:500 dilution; Cappel, Durham, NC), followed by a 30-minute incubation on ice. The dilution of antibody used was that which caused slight aggregation of erythrocytes as determined by titration. Antibody-treated erythrocytes (EIgG) were washed three times and suspended in the same buffer for incubation with PMNs in suspension.
Phagocytosis of EIgG.
Phagocytosis assays were conducted essentially as outlined by Pommier et al.30 PMNs, suspended at 2 × 106/mL in phosphate-buffered saline (PBS) with 1 mmol/L CaCl2, 1 mmol/L MgCl2, were warmed to room temperature for 1 hour. PMNs were activated with 100 nmol/L FMLP for 10 minutes at 37°C, then EIgG (at 1 × 108/mL, final concentration) were added and cells allowed to phagocytose for 30 minutes. The supernatant after the first spin was saved to measure the amount of marker proteins released during activation. EIgG that were not ingested were lysed twice in ice-cold water and tonicity restored by addition of 0.6 mol/L KCl. Phagocytosis was quantitated microscopically and expressed as the number of EIgG ingested per 100 PMNs (phagocytic index). Control cells (room temperature cells), FMLP-activated cells, and FMLP-activated, phagocytosing cells were pelleted by centrifugation and resupended in cold disruption buffer (see below) at 1.5 to 5 × 107/mL for subsequent subcellular fractionation.
Subcellular fractionation.
DFP-treated PMNs (4°C control cells, room temperature cells, FMLP-activated cells, or FMLP-activated, phagocytosing cells) were resuspended at 1.5 to 5 × 107/mL in disruption buffer (100 mmol/L KCl, 3 mmol/L NaCl, 1 mmol/L Na2ATP, 3.5 mmol/L MgCl2, 10 mmol/L piperazine N,N′-bis2{ethane-sulfonic acid}, pH 7.2, containing 0.5 mmol/L phenylmethylsulfonyl fluoride [PMSF]). Cells were disrupted by nitrogen cavitation as previously described.31 Nuclei and intact cells were pelleted by centrifugation at 400g for 15 minutes (P1). Ten milliliters of postnuclear supernatant (S1) was applied on top of either a three-layer Percoll (Pharmacia LKB Biotechnology) gradient (1.05/1.09/1.12 g/mL, 9 mL of each density32) or two-layer Percoll gradient (1.05/1.12 g/mL, 14 mL of each density31) containing 0.5 mmol/L PMSF. The gradient was centrifuged at 37,000g for 30 minutes. This resulted for the two-layer gradient in three visible bands from the bottom designated the α-band, containing azurophil granules, the β-band containing specific and gelatinase granules, and a γ-band containing light membranes including secretory vesicles and plasma membranes, with the clear cytosol on top. On the three-layer gradient, the β-band was separated into a lower β1-band containing specific granules and an upper β2-band containing gelatinase granules. The gradient was collected in fractions by aspiration from the bottom of the tube (25 fractions for two-layer gradient, 35 fractions for three-layer gradient). All fractions were assayed for myeloperoxidase (azurophil granule marker), lactoferrin (specific granule marker), gelatinase (gelatinase granule marker), HLA class I (plasma membrane marker), and latent alkaline phosphatase (alkaline phosphatase activity only measurable in the presence of a detergent, a marker for secretory vesicles). Except for alkaline phosphatase enzymatic assay,33 all marker proteins were measured by enzyme-linked immunosorbent assay (ELISA), as previously described.32 For ceramide and sphingomyelin analysis, Percoll was removed from fractions by ultracentrifugation, the fractions washed once in PBS and stored at −20°C until extracted for lipid analysis.
Assays for ceramide formation and sphingomyelinase activity.
Sphingomyelinase assays were always performed on the same day as cell fractionation. Acid and neutral sphingomyelinase assays were based on the method of Gatt et al34 using liposomes containing NBD-sphingomyelin (10 μmol/L substrate; Molecular Probes, Inc, Eugene, OR) and 30 μmol/L 1,2 dioleoyl-sn-glycerol-3 phosphocholine (Avanti, Alabastar, AL). The assay medium for the neutral enzyme contained 50 mmol/L Tris-HCl (pH 7.2), 25 mmol/L KCl, 5 mmol/L MgCl2, and 100 μL of the subcellular fraction in a total volume of 0.25 mL. Samples were probe sonicated and incubated at 37°C for 30 minutes in a temperature-regulated bath sonicator. The fluorescent product, NBD-ceramide, was isolated by partitioning the assay mixture with 0.45 mL 2-propanol, 1.5 mL heptane, and 0.2 mL water. Samples were centrifuged at 2,000g and 0.9 mL of the upper phase transferred to a clean tube containing 0.35 mL of water to remove traces of contaminating NBD-sphingomyelin. After repeat centrifugation, the upper layer was analyzed on a fluorimeter (460 nm excitation and 515 nm emission). Assays were performed in duplicate or triplicate. Sphingomyelinase activity was expressed as picomoles of sphingomyelin hydrolyzed per minute per milliliter fraction. The acid sphingomyelinase was assayed similarly in 125 mmol/L sodium acetate buffer, pH 5.0. To distinguish between the acid and neutral sphingomyelinase activity, the assays were performed in the presence of 0.05 mmol/L HgCl2, which augments the acid but inhibits the neutral activity, or 1 mmol/L dithiothreitol, which inhibits the acid but augments the neutral activity.18 Spingomyelinase assays were also performed in the presence of 0.1% Triton X-100 (Sigma Chemical Co) to rule out the presence of latent sphingomyelinase activity; ie, activity present in secretory vesicles or granules that is not “available” in the absence of detergent treatment. Although the enzyme activity was somewhat higher (50%) in the presence of Triton X-100, the overall distribution of sphingomyelinase activity in the gradient fractions was the same in the presence and absence of detergent. Unless indicated, the data represent experiments conducted in the absence of detergent. Some sphingomyelinase assays were performed in the presence of protease inhibitors (1 mmol/L PMSF, 10 μg/mL soybean trypsin inhibitor, 1 μg/mL leupeptin, 1 μg/mL aprotinin) to determine whether granule proteases affected sphingomyelinase activity.
Lipids were extracted by the method of Van Veldhoven and Bell35 as recently described.36 Specifically, material from subcellular fractions was pelleted at 100,000g, extracted with methanol, and each sample mixed with chloroform and water to obtain a chloroform:methanol:water ratio of 10:20:8. Samples were vortexed and incubated at room temperature for 1 hour, centrifuged for 10 minutes at 2,000g, and the supernatants transferred to clean tubes. The pellets were re-extracted and both supernatants combined. Chloroform and water were added to achieve a final ratio of 10:10:9 (chloroform:methanol:water). After vortexing and centrifugation, the lower phase was removed, washed with equal volume of 1 mol/L NaCl:methanol (9:1), and centrifuged. The lower phase was transferred to a clean tube, stored at −20°C, and analyzed within 48 hours. Total cellular ceramide was assayed by the method of Preiss et al37 as previously reported.26
Phosphatidic acid (PA) and phosphatidylethanol (PEt) formation.
PMNs were resuspended at 1 × 107/mL in PBS and labeled with 1-O-[3H]-octadecyl-sn-glycero-3-phosphocholine (108 mol/L; Amersham, Arlington Heights, IL) for 30 minutes at 37°C. The labeled cells were washed with PBS and resuspended at 2 × 106 cells/mL in PBS containing 1 mmol/L Ca2+ and 1 mmol/L Mg2+. EtOH (200 mmol/L) was added for 5 minutes at 37°C. PMNs were activated with FMLP and phagocytosis initiated as outlined above. Thirty minutes after the addition of opsonized targets, EIgG not internalized were lysed, and PMNs resuspended in cavitation buffer. Cells were fractionated as described above and the lipids from each fraction were extracted according to the method of Van Veldhoven and Bell.35 Assays for [3H]-labeled PEt and PA were performed as previously decribed.38
Protein measurement.
Protein was measured by the BCA protein kit from Pierce (Rockford, IL) using bovine serum albumin as a standard.
RESULTS
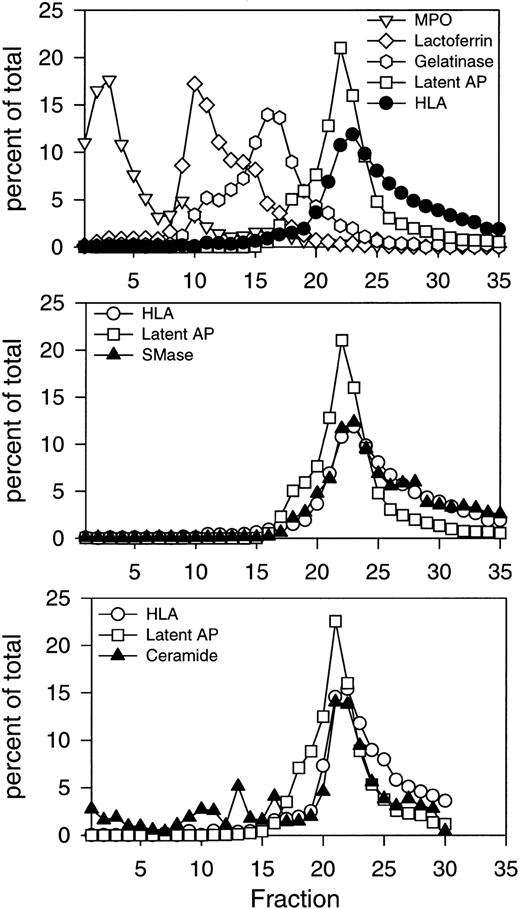
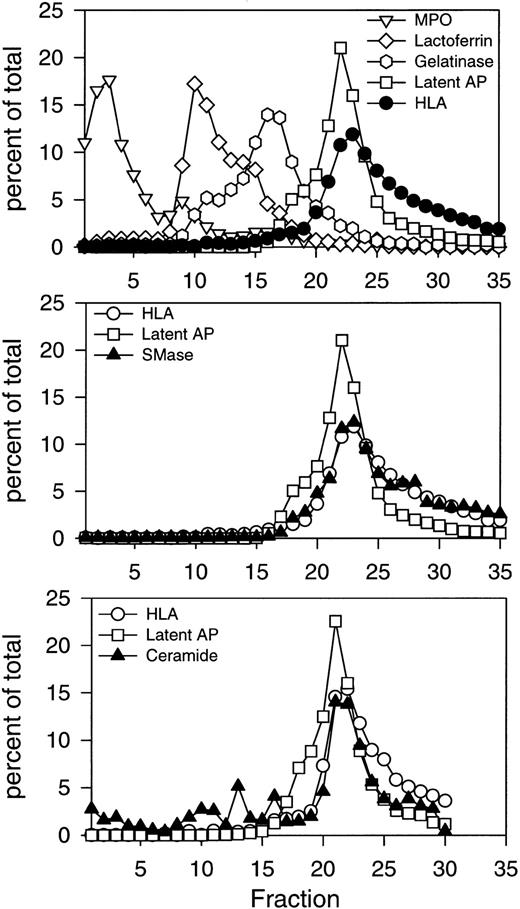
Initial subcellular fractionation experiments of resting PMNs were performed on three-layer Percoll density gradients, which resolve all known mobilizable subsets of granules and secretory vesicles in human PMNs.32 These include azurophil granules, identified by myeloperoxidase; specific granules, identified by lactoferrin; gelatinase granules, identified by gelatinase; and secretory vesicles, identified by latent alkaline phosphatase (Fig 1, top panel). Secretory vesicles were present in the light membrane region together with plasma membranes, recognized by HLA class I. The profiles of the two markers differed, with secretory vesicles being slightly denser and extending into the gradient, while plasma membranes were slightly lighter and remained in the upper part of the gradient where very little latent alkaline phosphatase was detected (Fig 1, top panel). The cytosol was present above the light membrane fractions, beginning with fraction number 25 and extending to fraction number 35. Subcellular fractions were assayed for acid and neutral sphingomyelinase activity as described in Experimental Procedures. The major activity detected was the neutral, Mg2+-dependent sphingomyelinase, which colocalized with the HLA plasma membrane marker (Fig 1, middle panel). The two profiles were virtually superimposable, indicating that the neutral sphingomyelinase was confined to the plasma membrane. No activity was detected in any of the granule subsets.
Subcellular localization of neutral sphingomyelinase and ceramide in resting human PMNs. Isolated, DFP-treated PMNs at 1.5 to 5 × 107 cells/mL, were disrupted by nitrogen cavitation. Ten milliliters of postnuclear supernatant was layered on a three-layer Percoll density gradient and centrifuged as described in Experimental Procedures. The gradient was fractionated into 35 fractions by aspiration from the bottom of the tube. Fractions were assayed for neutral sphingomyelinase, myeloperoxidase (MPO), lactoferrin, gelatinase, HLA class I, and latent alkaline phosphatase (latent AP). Numbers are average of four experiments, normalized to a cell number of 3 × 108 cells, and expressed in percent of the total amount measured in the fractions 1 through 35. The subcellular distribution of ceramide is shown in the bottom panel. Ceramide was measured as described in Experimental Procedures after removal of Percoll by ultracentrifugation. The absolute value for neutral sphingomyelinase activity in the peak fraction was 65.5 pmol sphingomyelin hydrolyzed/min/mL. The peak value for ceramide was 0.44 nmol/mL. Results of one representative experiment are shown.
Subcellular localization of neutral sphingomyelinase and ceramide in resting human PMNs. Isolated, DFP-treated PMNs at 1.5 to 5 × 107 cells/mL, were disrupted by nitrogen cavitation. Ten milliliters of postnuclear supernatant was layered on a three-layer Percoll density gradient and centrifuged as described in Experimental Procedures. The gradient was fractionated into 35 fractions by aspiration from the bottom of the tube. Fractions were assayed for neutral sphingomyelinase, myeloperoxidase (MPO), lactoferrin, gelatinase, HLA class I, and latent alkaline phosphatase (latent AP). Numbers are average of four experiments, normalized to a cell number of 3 × 108 cells, and expressed in percent of the total amount measured in the fractions 1 through 35. The subcellular distribution of ceramide is shown in the bottom panel. Ceramide was measured as described in Experimental Procedures after removal of Percoll by ultracentrifugation. The absolute value for neutral sphingomyelinase activity in the peak fraction was 65.5 pmol sphingomyelin hydrolyzed/min/mL. The peak value for ceramide was 0.44 nmol/mL. Results of one representative experiment are shown.
In Fig 1, no detergent was included in the sphingomyelinase assays. To rule out the possibility that there was latent sphingomyelinase activity in either secretory vesicle or granule fractions, we repeated the sphingomyelinase assays in the presence of 0.1% Triton X-100. No sphingomyelinase activity was detected in the granule fractions in the presence of Triton X-100. In the light membrane fractions, there was a 50% increase in sphingomyelinase activity across all fractions, with no change in the overall distribution of sphingomyelinase activity (data not shown).
When the sphingomyelinase assay was performed in the absence of Mg2+ and at pH 5.0, a small amount of activity was detected in the light membrane fractions (data not shown). This activity most likely represented residual neutral sphingomyelinase activity because it was completely inhibited by ionic mercury and enhanced by dithiothreitol (Table 1), features of the neutral sphingomyelinase.18 20 A small amount of sphingomyelinase activity was also detected in the azurophil granule fractions at pH 5.0. This activity was enhanced in the presence of ionic mercury and inhibited by dithiothreitol (Table 1), characteristic of a “true” acid sphingomyelinase. However, this acid sphingomyelinase activity constituted less than 1% of the total PMN sphingomyelinase activity. Recently, a cytosolic, Mg2+-independent, neutral sphingomyelinase was isolated from HL60 cells. We found that cytosolic activity constituted less than 1% of the total sphingomyelinase activity, indicating that in human PMNs the predominant sphingomyelinase activity is a plasma membrane-associated, Mg2+-dependent, neutral sphingomyelinase.
The involvement of ceramide in intracellular signaling raises an important question as to its cellular localization. Subcellular fractions of resting PMNs were also analyzed for ceramide content (Fig1, bottom panel). Ceramide was detectable in all granule subsets, but the majority was present in the light membrane fractions colocalizing with HLA. These results indicated that ceramide was primarily localized to the plasma membrane fractions of resting PMNs. Interestingly, sphingomyelin, the substrate of sphingomyelinases, was equally distributed in all the granule subsets and light membrane fractions (Table 2).
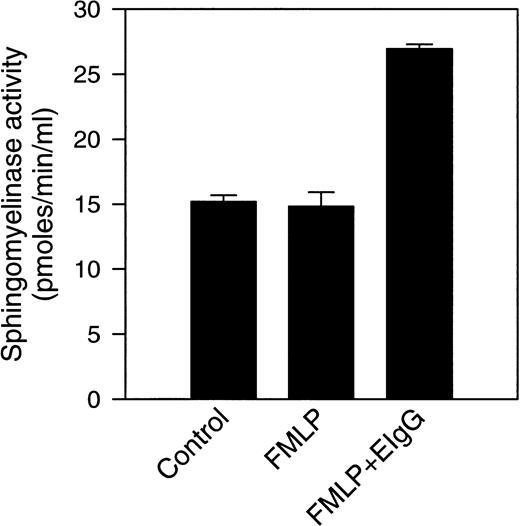
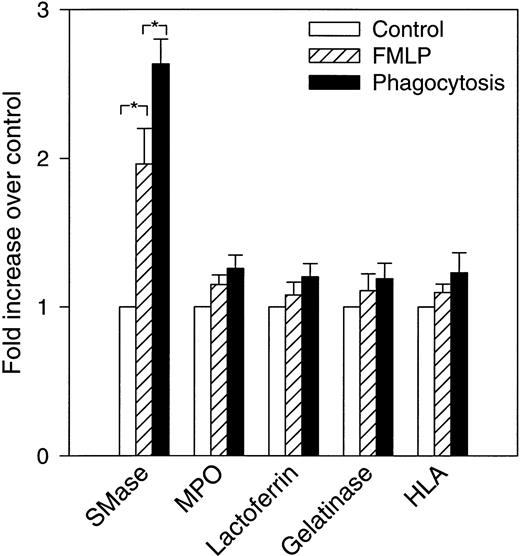
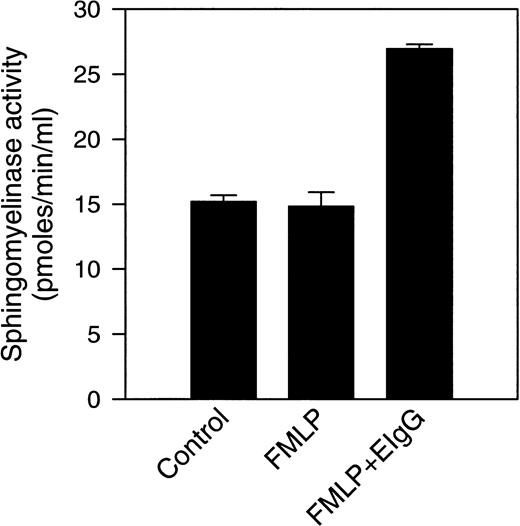
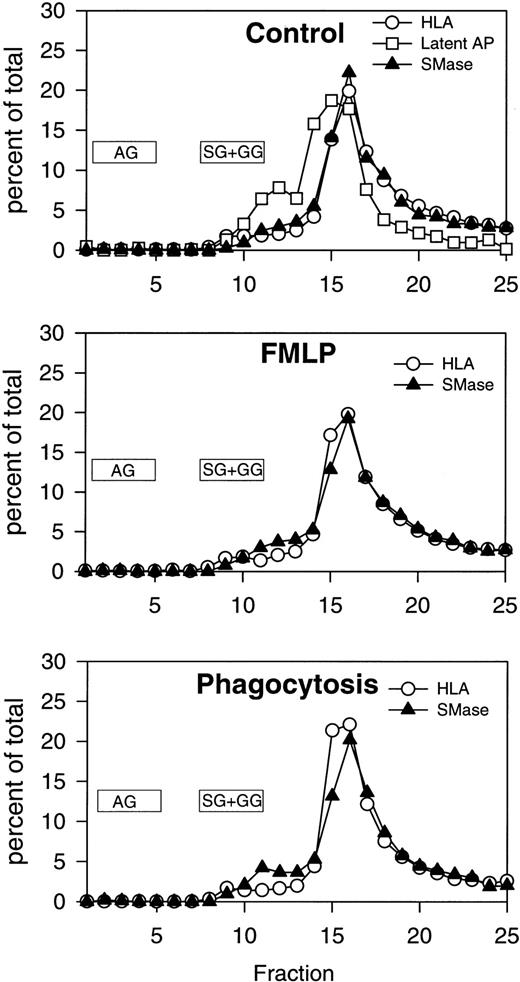
Previous experiments from our laboratory showed that exogenously added cell-permeable, short-chain ceramides inhibit phagocytosis of EIgG by FMLP-activated PMNs.28 In addition, there is activation of an Mg2+-dependent, neutral sphingomyelinase and ceramide formation that occur during phagocytosis. To investigate the subcellular localization of these events, control (resting), FMLP-activated, and FMLP-activated phagocytosing PMNs were subjected to subcellular fractionation. The gradient fractions were assayed for neutral sphingomyelinase activity and ceramide. Because the majority of sphingomyelinase activity was present in the light membrane fractions in unactivated cells, subcellular fractionation was performed on two-layer Percoll gradients. The two-layer gradient, when compared to the three-layer gradient, improves the resolution between secretory vesicles and plasma membranes but fails to separate specific granules from gelatinase granules. When measurements were performed in intact cells in this and previous studies,28 the increase in neutral sphingomyelinase activity was only observed during phagocytosis; ie, we did not see an increase in sphingomyelinase activity with FMLP-treatment alone (Fig 2). The addition of protease inhibitors to these assays did not affect sphingomyelinase activity, suggesting that granule-associated proteases were not inhibiting activity in whole cell assays (data not shown). This is in contrast to our findings in subcellular fractions, where an increase in sphingomyelinase activity was observed in the plasma membrane fractions after stimulation with FMLP (1.9-fold above control, Figs 3 and 4). Upon phagocytosis of EIgG by FMLP-primed PMNs, a further increase in sphingomyelinase activity to 2.7-fold above control was observed (Figs3 and 4). Sphingomyelinase activity increased similarly during EIgG-mediated phagocytosis whether PMNs were pretreated with FMLP or not pretreated with FMLP (Fig 3). These increases in sphingomyelinase activity could not be explained by differences in the disruption of PMNs or differences in the total number of disrupted PMNs loaded onto the gradient, because no significant difference could be shown between control, FMLP-activated, and FMLP-activated phagocytosing cells in total content of the plasma membrane marker HLA, or the granule markers myeloperoxidase, lactoferrin, and gelatinase (Fig 4). As seen in Fig 4, there was a significant increase in sphingomyelinase activity from control to FMLP-activated cells, and also from FMLP-activated cells to FMLP-activated cells engaged in phagocytosis. The activation of the neutral sphingomyelinase occurred in the plasma membrane fractions since the distribution profile of sphingomyelinase colocalized with the HLA profile in control (as also shown above on the three-layer gradient), FMLP-stimulated, and FMLP-stimulated phagocytosing cells (Fig 5).
Sphingomyelinase activity was measured in intact PMNs after FMLP activation, and after phagocytosis of EIgG. Sphingomyelinase activity was measured in control cells, FMLP-activated cells (100 nmol/L), and in cells phagocytosing EIgG (for 30 minutes) after preincubation with FMLP. Values represent the mean ± SD for three experiments.
Sphingomyelinase activity was measured in intact PMNs after FMLP activation, and after phagocytosis of EIgG. Sphingomyelinase activity was measured in control cells, FMLP-activated cells (100 nmol/L), and in cells phagocytosing EIgG (for 30 minutes) after preincubation with FMLP. Values represent the mean ± SD for three experiments.
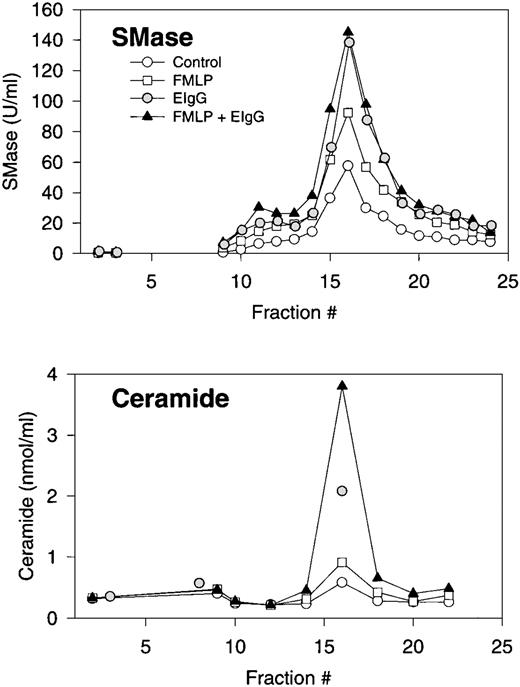
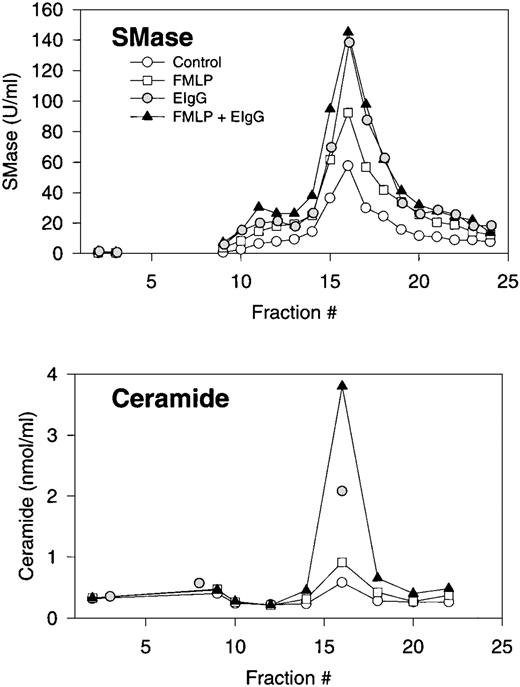
Subcellular fractionation of resting, FMLP-activated, and phagocytosing human PMNs. Distribution profiles of neutral sphingomyelinase and ceramide. Isolated and DFP-treated PMNs were resuspended at 2 × 106/mL in PBS with CaCl2(1 mmol/L) and MgCl2 (1 mmol/L), and warmed to room temperature for approximately 1 hour. Cells were left untreated (control), stimulated with FMLP (100 nmol/L) for 10 minutes (FMLP), or stimulated with FMLP followed by addition of EIgG and incubation for 30 minutes (phagocytosis). Cells were resupended in disruption buffer, disrupted by nitrogen cavitation, and the postnuclear supernatant centrifuged on a two-layer Percoll density gradient. An equal number (ranging from 1.8 to 3.3 × 108 cells) of control cells, FMLP-activated cells, and phagocytosing, FMLP-activated cells were fractionated in each experiment. The gradients were fractionated into 25 fractions by aspiration from the bottom of the tubes, and fractions assayed for neutral sphingomyelinase (SMase) activity, and every second fraction assayed for ceramide after removal of Percoll by ultracentrifugation. Results are the average of three experiments, normalized to a cell number of 3 × 108.
Subcellular fractionation of resting, FMLP-activated, and phagocytosing human PMNs. Distribution profiles of neutral sphingomyelinase and ceramide. Isolated and DFP-treated PMNs were resuspended at 2 × 106/mL in PBS with CaCl2(1 mmol/L) and MgCl2 (1 mmol/L), and warmed to room temperature for approximately 1 hour. Cells were left untreated (control), stimulated with FMLP (100 nmol/L) for 10 minutes (FMLP), or stimulated with FMLP followed by addition of EIgG and incubation for 30 minutes (phagocytosis). Cells were resupended in disruption buffer, disrupted by nitrogen cavitation, and the postnuclear supernatant centrifuged on a two-layer Percoll density gradient. An equal number (ranging from 1.8 to 3.3 × 108 cells) of control cells, FMLP-activated cells, and phagocytosing, FMLP-activated cells were fractionated in each experiment. The gradients were fractionated into 25 fractions by aspiration from the bottom of the tubes, and fractions assayed for neutral sphingomyelinase (SMase) activity, and every second fraction assayed for ceramide after removal of Percoll by ultracentrifugation. Results are the average of three experiments, normalized to a cell number of 3 × 108.
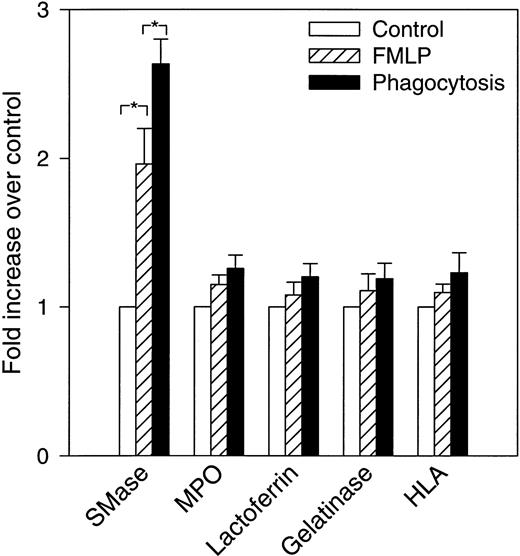
Increase in neutral sphingomyelinase activity in FMLP-activated and phagocytosing PMNs. Control, FMLP-activated, and phagocytosing FMLP-activated cells were fractionated as described in Experimental Procedures and in the legend to Fig 3. The bars show fold increase (+SEM) above control (set to 1) of total measured activity of neutral sphingomyelinase (SMase) and the various marker proteins after FMLP activation and phagocytosis. The total amount is calculated as the sum of measured amount in fractions 1 through 25, in nuclei and unbroken cells, and in exocytosed material (cell supernatant after activation). Data are the average of five experiments. *P < .05 compared with the control by paired t-test. None of the marker proteins differed significantly between control, FMLP, or phagocytosing cells.
Increase in neutral sphingomyelinase activity in FMLP-activated and phagocytosing PMNs. Control, FMLP-activated, and phagocytosing FMLP-activated cells were fractionated as described in Experimental Procedures and in the legend to Fig 3. The bars show fold increase (+SEM) above control (set to 1) of total measured activity of neutral sphingomyelinase (SMase) and the various marker proteins after FMLP activation and phagocytosis. The total amount is calculated as the sum of measured amount in fractions 1 through 25, in nuclei and unbroken cells, and in exocytosed material (cell supernatant after activation). Data are the average of five experiments. *P < .05 compared with the control by paired t-test. None of the marker proteins differed significantly between control, FMLP, or phagocytosing cells.
Subcellular fractionation of resting, FMLP-activated, and phagocytosing human PMNs. Distribution profiles of neutral sphingomyelinase, HLA, and latent alkaline phosphatase. Cells were processed for fractionation as described in Experimental Procedures and in the legend to Fig 3. Fractions were assayed for neutral sphingomyelinase activity, myeloperoxidase, lactoferrin, gelatinase, HLA class I, and latent alkaline phosphatase. Numbers are the average of three experiments (same data as shown in Fig 3), normalized to a cell number of 3 × 108 cells, and expressed in percent of the total amount measured in fractions 1 through 25. Latent alkaline phosphatase is only shown in control cells, because secretory vesicles are almost completely mobilized (and latent AP thus disappearing) after FMLP stimulation. The localization of the majority of azurophil granules (AG) and specific/gelatinase granules (SG+GG) is marked.
Subcellular fractionation of resting, FMLP-activated, and phagocytosing human PMNs. Distribution profiles of neutral sphingomyelinase, HLA, and latent alkaline phosphatase. Cells were processed for fractionation as described in Experimental Procedures and in the legend to Fig 3. Fractions were assayed for neutral sphingomyelinase activity, myeloperoxidase, lactoferrin, gelatinase, HLA class I, and latent alkaline phosphatase. Numbers are the average of three experiments (same data as shown in Fig 3), normalized to a cell number of 3 × 108 cells, and expressed in percent of the total amount measured in fractions 1 through 25. Latent alkaline phosphatase is only shown in control cells, because secretory vesicles are almost completely mobilized (and latent AP thus disappearing) after FMLP stimulation. The localization of the majority of azurophil granules (AG) and specific/gelatinase granules (SG+GG) is marked.
The sphingomyelinase data presented in Fig 5 were obtained in the absence of detergent. To rule out the possibility that the increase in sphingomyelinase activity seen with FMLP activation and phagocytosis was the result of the translocation of sphingomyelinase from an intracellular store of secretory vesicles, the sphingomyelinase measurements were also repeated in the presence of 0.1% Triton X-100. Similar to unactivated PMNs, there was an equal increase in sphingomyelinase activity (≈50%) in every fraction from gradients of control, FMLP-activated, and FMLP-activated phagocytosing PMNs (data not shown). On the other hand, there was only an increase in alkaline phosphatase activity with Triton X-100 treatment in control cells, in agreement with all secretory vesicles being mobilized to the plasma membrane (ie, no latent alkaline phosphatase) in FMLP-activated and FMLP-activated phagocytosing cells. This supports our observation that sphingomyelinase was present in the plasma membrane and not in secretory vesicles, and that the effect of Triton X-100 appeared to be a direct effect on the enzyme.
The activation of sphingomyelinase was accompanied by an accumulation of ceramide, which was also confined to the plasma membrane (Fig 3). No changes in ceramide content were observed in granule fractions (Fig 3). Interestingly, the relative increase in ceramide levels during phagocytosis was greater than the relative increase in sphingomyelinase activity. In addition, ceramide levels increased substantially during phagocytosis in both the presence and absence of FMLP. Interestingly, the increase in ceramide levels in FMLP-primed cells was more than additive to those seen with FMLP and EIgG alone.
PLD activation is a principal signal in PMN activation and has been associated with the uptake of both complement- and IgG-opsonized particles.39-41 Furthermore, PLD is one of the intracellular targets of ceramide action during phagocytosis.28 Thus, we thought it was likely that PLD activity would increase in the plasma membrane fractions in parallel with ceramide formation during PMN activation. A unique property of PLD which provides a specific assay for this enzyme is the transphosphatidylation reaction in which an alcohol is transferred to the phosphatidyl group of a phospholipid substrate to form a phosphatidylalcohol.42 Cells prelabeled with 1-O-[3H]-octadecyl-sn-glycero-3-phosphocholine and incubated in the presence of 200 mmol/L ethanol will synthesize radioactive PEt if PLD is active. Figure 6shows the cellular localization of PA and PEt formation in PMN fractions. Both PA and PEt were found in azurophil, specific, and plasma membrane fractions in control (resting) cells. In FMLP-activated and FMLP-activated phagocytosing cells, PLD activity, as indicated by PEt formation, was markedly greater in the plasma membrane fractions in comparison with the granule fractions (Fig 6). These data show increased PLD activity in plasma membranes during PMN activation, indicating colocalization of ceramide with its cellular target, PLD.
Subcellular fractionation of resting, FMLP-activated, and phagocytosing human PMNs. Distribution profiles of the PLD products, PA and PEt. PMNs were labeled with 1-O-[3H] octadecyl-sn-glycero-3 phosphocholine, activated and fractionated as described in Experimental Procedures. PA and PEt were detected as previously described.38
Subcellular fractionation of resting, FMLP-activated, and phagocytosing human PMNs. Distribution profiles of the PLD products, PA and PEt. PMNs were labeled with 1-O-[3H] octadecyl-sn-glycero-3 phosphocholine, activated and fractionated as described in Experimental Procedures. PA and PEt were detected as previously described.38
DISCUSSION
In this report we describe the subcellular localization of sphingomyelinases in human PMNs. Our findings clearly indicate that a Mg2+-dependent, neutral sphingomyelinase is the predominant form in PMNs, with localization confined to the plasma membrane. This confirms findings in several other cell types, where crude fractionation localized the neutral sphingomyelinase to membranes enriched in plasma membrane markers and possibly microsomes.18-21 23 Our fractionation scheme allowed us to compare the distribution profiles of various well-established markers for the PMN subsets with the profile of sphingomyelinase activity. This showed a strict colocalization with the plasma membrane marker HLA, rather than with the secretory vesicle marker latent alkaline phosphatase (Figs 1 and 5).
In the presence of ionic mercury, we detected an acidic sphingomyelinase activity in azurophil granules, but this activity was less than 1% of the neutral activity found in plasma membranes. The low amount of acidic sphingomyelinase activity in subcellular fractions confirms our findings in intact cells.28 Although azurophil granules are rich in various acid hydrolases, this is unlikely to explain the low level of acidic sphingomyelinase obtained because this enzyme is believed to be insensitive to the action of phosphatases and proteases.11 This is supported by the inability of protease inhibitors to increase acid sphingomyelinase activity in these fractions. The localization of the acidic activity in azurophil granules, regarded as the lysosomal compartment of the PMN, is in agreement with the lysosomal localization of acidic sphingomyelinases in other cell types.16 It appears that PMNs are among the relatively few cell types investigated thus far that largely contain neutral sphingomyelinase rather than acidic sphingomyelinase.43 In comparison, acidic sphingomyelinase activity has been found to be several-fold higher than the neutral activity in rat liver and in the monocytic cell line, U937.20 44
The subcellular localization of ceramide, the product of sphingomyelinase action, has not previously been addressed, although it was shown to increase concomitantly with sphingomyelinase activation in isolated, TNF-α-activated membranes from HL60 cells.45Others have described compartmentalization of ceramide to sphingomyelin-rich membrane domains with the characteristics of caveolae in interleukin-1β (IL-1β)-activated fibroblasts.46 However, it is unclear if caveolae are present in human PMNs. The majority of the phosphatidylinositol-linked proteins including alkaline phosphatase, known to be concentrated in caveolae in other cells, are primarily present in an intracellular compartment in resting PMNs, namely in secretory vesicles1. Our fractionation data showed that neutral sphingomyelinase and ceramide were localized in the plasma membrane fractions of resting as well as activated PMNs, but did not allow any conclusions to be drawn regarding compartmentalization within subdomains of the membranes. Although sphingomyelin, the substrate for sphingomyelinase, was present in equal amounts in the three granule subsets and light membrane fractions (Table 2), it appears that only the plasma membrane pool contributes to the sphingomyelin cycle.
A number of activators of sphingomyelinase have been identified, including growth factors like TNF-α, interferon-γ, and vitamin D3 in HL60 cells, TNF-α in U937 cells, and IL-1β in fibroblasts.6-8,11,45 We have now shown that Fcγ receptor-mediated phagocytosis induces a plasma membrane-associated neutral sphingomyelinase in FMLP-activated human PMNs. In contrast to our measurements in intact cells, we were able to demonstrate that FMLP activation alone resulted in an increase in sphingomyelinase activity that was detected when cells were fractionated. The discrepancy between intact and fractionated PMNs with regard to FMLP activation of sphingomyelinase may be explained by segregation of the plasma membrane-associated sphingomyelinase from an unidentified inhibitor of sphingomyelinase activity during subcellular fractionation. For example, Liu and Hannun43 reported that neutral sphingomyelinase is inactive in the presence of physiological concentrations of glutathione. However, cytosolic glutathione is unlikely to be the source of this inhibition because combining cytosol with plasma membrane fractions did not diminish sphingomyelinase activity in our assays (data not shown). Similar experiments with granule and plasma membrane fractions also failed to implicate granules as the source of this “inhibitory” activity. The activation of neutral sphingomyelinase by FMLP in suspended, fractionated PMNs is in agreement with the observation that FMLP induces ceramide accumulation in PMNs adherent to fibrinogen.26 Future studies will determine whether other inflammatory mediators that activate PMNs through serpentine, G-protein coupled receptors such as IL-8, C5a, leukotriene B4, and platelet-activating factor47 also activate the neutral sphingomyelinase.
Surprisingly, the ceramide content of plasma membranes of phagocytosing cells was twofold to fourfold greater than that of FMLP-stimulated cells (Fig 3). This is considerably more than predicted from the observed 50% difference in sphingomyelinase activity between FMLP-stimulated and phagocytosing cells. This may be a consequence of the assay conditions, because the sphingomyelinase measurements reflect the enzymatic activity present at a single timepoint, whereas ceramide was detected as an endpoint measurement and measures ceramide accumulation. Alternatively, the observed difference may be explained by discrepancies between FMLP-activated and phagocytosing PMNs in activation of other enzymes regulating the ceramide content of membranes, such as ceramidase, ceramide kinase, and ceramide synthase.
The sphingomyelin cycle has hitherto been implicated in responses such as growth, differentiation, apoptosis, gene regulation, and intracellular vesicle transport in a variety of cells and cell lines.3-13 Gene transcription and protein synthesis are unlikely to be of importance for the very rapid responses of PMNs to soluble and particulate stimuli. The lack of significant lysosomal, acidic sphingomyelinase activity in PMNs may support this notion, because ceramide formed in endosomes/lysosomes as a result of TNF-α-induced acid sphingomyelinase activity was reported to be responsible for activation of the NF-κB pathway11 and, thus, for gene regulation in monocytic U937 cells. In PMNs, exogenously added short-chain ceramides inhibit important PMN responses including FMLP-induced oxidant release in adherent and suspended cells, and Fcγ-receptor-mediated phagocytosis.26-28 Therefore, it seems that the sphingomyelin cycle regulates membrane trafficking in PMNs, in accordance with the findings of Pagano et al,12 13who showed that short-chain ceramides inhibited fluid phase and receptor-mediated endocytosis and membrane traffic in Chinese hamster ovary cells.
The mechanisms underlying the effects of ceramide in PMNs are not completely delineated, but the localization of ceramide in the plasma membrane of activated cells indicates that the targets are likely to be associated with or in proximity to the plasma membrane. One of the targets of ceramide action during IgG-mediated phagocytosis is PLD.28 This would suggest that a plasma membrane-associated PLD is activated during FMLP treatment and phagocytosis. Consistent with this hypothesis, increased levels of PEt, indicating increased PLD activity, were found in the plasma membrane fractions of activated PMNs. These findings do not exclude the possibility that PLD and ceramide are in different, but adjacent, membrane compartments or microdomains that interact during phagocytosis. Overall, our present findings indicate that the sphingomyelin cycle in PMNs is restricted to the plasma membrane during both FMLP activation and phagocytosis, and that the intracellular targets of ceramide action, such as PLD, are likely to be proximal to the sites of ceramide formation. This localization is consistent with our hypothesis that ceramide, by accumulating at strategic sites during activation, plays a negative feedback role in modulating PMN phagocytosis.
V.H.-G. and L.K. contributed equally to this work.
Supported by National Institutes of Health Grants No. HL53074 (to S.J.S.), AI20065 (to L.A.B.), DK41487 and DK39255 (to J.A.S.); a grant from the American Heart Association of Michigan (S.J.S.); and by The Danish Medical Research Council (L.K.). J.A.S. is an Established Investigator of the American Heart Association.
Address reprint requests to Suzanne J. Suchard, PhD, Zeneca Pharmaceuticals, 1800 Concord Pike, PO Box 15437, Wilmington, DE 19850-5437.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 6. Subcellular fractionation of resting, FMLP-activated, and phagocytosing human PMNs. Distribution profiles of the PLD products, PA and PEt. PMNs were labeled with 1-O-[3H] octadecyl-sn-glycero-3 phosphocholine, activated and fractionated as described in Experimental Procedures. PA and PEt were detected as previously described.38](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4761/4/m_blod41224006y.jpeg?Expires=1771000719&Signature=Tmk3v9~1CcWXrx4hHNDCwvfNKLI612m~CENITboYIAlPjz94QaZYhRREEGw~qSfhRBoQopJfr5jJ7rwmrOjQUKQEnoZYImd2A7X1T~bv6PRpbBdM03nElueCUCqyxFCFLUM4LIyONW7gbv-hvBHayogndzN43Qy9jDPtyqqZ2gYMjEuvnvG-RnYmmPlD1WC4b4rVRW6xYF77mgipQsd09sH92v0INlK2-31OtzIDqvkwYzLN195WbFUfrUNmdqu-u-14hxHnaoQA-pdOv8gEJvQ77UtXF33lD~P-A7Lxs0J26Yc4rYYifEpWw6L3mlwMs~cPKBG5opswXiccCdQxfw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 6. Subcellular fractionation of resting, FMLP-activated, and phagocytosing human PMNs. Distribution profiles of the PLD products, PA and PEt. PMNs were labeled with 1-O-[3H] octadecyl-sn-glycero-3 phosphocholine, activated and fractionated as described in Experimental Procedures. PA and PEt were detected as previously described.38](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/12/10.1182_blood.v91.12.4761/4/m_blod41224006y.jpeg?Expires=1771235821&Signature=kW24rwsX4uqXKi50QV2qiAGHhWV2r0ojLWWctgnzS~da6aDO00HCJIYbzEZYftGU~plvA1hOXjDIuc0s5QFbhfNQl-F8YB~Qeqo7OmQn47QHzA~A98mxcij7L5sS4DBLQ5v8JK1JU5~FUiFiGGcyWfFWDy69F61yraiSxXQxykiCbFtvwxS-gc7UdlXamlvHkaT70u6alQXVPiruLHiCvheAdOTU2R9NZ4MqhDpOMHZj~VYIuvpgKEF8lsS0I8fOgqD5F~A-4sc5H8hdmCePIhEaRlkaJDq1kknF9Mn8sjDDhDY7In4q6Ku1IqtqjR7RK4G~FHpvhXmJq9YOZw4rEQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)