Abstract
In this report, we show that the Src family nonreceptor protein tyrosine kinase (PTK) Lyn associates with aggregated IgA Fc receptor (FcαR) in the monocytic cell line THP-1. Receptor aggregation and subsequent immunoprecipitation of receptor complexes with huIgA adsorbed to nitrocellulose particles shows that Lyn associates with FcαR by a mechanism sensitive to short treatment with the Src family-selective inhibitor PP1. However, interaction of Lyn with IgG Fc receptor (FcγR) in THP-1 cells was unaffected by short treatment with the PTK inhibitor. Cross-linking of FcαR induced tyrosine phosphorylation of several cellular proteins, including p72Syk, which appears to be a major target of early PTK activity. Unexpectedly, in vitro kinase assays showed that FcαR aggregation-induced tyrosine phosphorylation of Syk did not result in upregulation of Syk activity. Despite the lack of enhanced Syk kinase activity, downstream signaling after FcαR cross-linking was functional and induced the release of significant amounts of interleukin-1 receptor antagonist and interleukin-8. The induction of cytokine release was completely blocked by PP1, thus confirming the biological significance of the association of Lyn with aggregated FcαR. Our data show that early signal transduction after FcαR cross-linking as well as FcαR-mediated activation of cellular effector functions depends on Src family kinase activity. The Src-family PTK involved in FcαR-mediated tyrosine phosphorylation appears to be Lyn, which coprecipitated with aggregated FcαR complexes.
IgS OF THE IgA ISOTYPE prevail over other Ig isotypes in the mucosal compartment, where they play a critical role in protection against environmental challenges. In addition to functions dependent on specific antibody activity, such as neutralization of bacterial toxins and inhibition of attachment of pathogenic microorganisms to the mucosal epithelium, IgA appears to be a regulatory molecule capable of modulating immune and inflammatory responses. The immunoregulatory functions of IgA seem to be confined to interaction of IgA with receptors for the Fc portion of IgA expressed on the surface of cells of the immune system, such as monocytes, macrophages, neutrophils, or T cells, leading to upregulation or downregulation of inflammation or immunologic reactivity under certain conditions.1-3 CD89, the FcαR of phagocytic cells, has been defined as a 55-to 75-kD glycoprotein on monocytes/macrophages and neutrophils, whereas a more heavily glycosylated form is expressed on eosinophils.4,5 The cDNA sequence of FcαR predicts a transmembrane protein with two Ig-like extracellular domains, a 19-amino acid membrane spanning region, and a 41-amino acid cytoplasmic tail.6 Genetic characterization of the human gene encoding CD89 indicates that FcαR represents a distantly related member of the Ig receptor gene family.7
Transmembrane signal transduction induced by aggregation of receptors specific for the Fc portion of IgG or IgE (FcεRI) has been extensively studied over the past several years. Like multisubunit antigen receptors, such as B-cell and T-cell receptor, FcεRI and FcγR lack intrinsic kinase activity and, therefore, depend on the association with nonreceptor protein tyrosine kinases (PTKs). Two families of PTKs have been shown to interact with these receptors, and a sequential activation pathway model was proposed recently.8,9 Current evidence suggests that Src family kinases, eg, Lyn, are activated in the initial events after FcγR or FcεR cross-linking. Lyn has been described to be associated with FcεRI in mast cells and FcγRs I and II on the surface of monocytic cell lines, and cross-linking of these receptors upregulates, more or less, the activity of the kinase.9-14 Interestingly, binding of Lyn to some receptors seems to occur constitutively in the absence of a stimulatory signal, underlining its potential as first-step kinase.9,13,15 Activation of Lyn is followed by tyrosine phosphorylation of conserved sequences present on the signal transducing subunits of the Fc receptors.16 Phosphorylation of these immunoreceptor tyrosine-based activation motifs (ITAMs) is neccessary for binding of the PTK Syk, a member of the second family of tyrosine kinases, which leads to further downstream signaling events, and ultimately results in cellular effector functions.17 18
The importance of Syk in FcεRI- and FcγR-mediated signal transduction is reflected by its presence in aggregated FcR complexes on various myloid cell lineages.9,19-21 Association of Syk with receptor subunits occurs via its two Src homology 2 domains (SH2), which contribute cooperatively to the high-affinity binding, and depends on tyrosine phosphorylation of ITAM sequences.10,22Direct interaction of Syk with further downstream molecules involved in the moblization of intracellular calcium and the activation of Ras has been described, suggesting a role in at least two signal transduction cascades.17,23-25 Additionally, cellular effector functions, such as FcεRI-mediated degranulation of mast cells and FcγR-mediated phagocytosis, depend on the presence of functional Syk kinase.26 27
As compared with the Fc receptors for IgG or IgE, much less is known about the events involved in FcαR-mediated signal transduction. Cross-linking of FcαR results in immediate tyrosine phosphorylation of celluar proteins, although known signaling motifs, such as the ITAM domain, are missing from the cytoplasmic tail of its ligand-binding α-chain.6,7,28 Recently, association with a specialized signaling subunit, FcR γ-chain, has been described in U937 monocytic cells and this interaction was necessary for functional transmembrane signal transduction in a transfected B-cell line.28,29However, little is known about the PTKs responsible for FcαR-mediated tyrosine phosphorylation of cellular proteins. A preliminary report described interaction of Syk with this receptor in U937 monocytic cells, but the Src family PTKs involved in FcαR signaling have not been identified so far.30
We describe here PTKs engaged in transmembrane signaling of FcαR in the monocytic cell line THP-1. To investigate receptor-associated molecules, we cross-linked and subsequently immunoprecipitated FcαR complexes with the natural ligand, huIgA, adsorbed to nitrocellulose (NC) particles. Furthermore, these particles were used to study the effect of FcαR cross-linking on downstream cellular functions, such as the release of cytokines. Our results show that, comparable to other FcRs, aggregation of FcαR recruits PTK Lyn. However, the mechanism of kinase/receptor interaction appears to be different for FcαR and FcγR.
MATERIALS AND METHODS
Cells.
The THP-1 monocytic cell line was obtained from the American Type Culture Collection (ATCC; Rockville, MD) and cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FCS; Hyclone Lab, Logan, UK), 2 mmol/L L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin (GIBCO BRL, Paisley, UK; complete medium). Cell density was maintained between 5 × 105 and 2 × 106/mL.
Reagents and antibodies.
Highly purified human serum IgA (huIgA) and IgG (huIgG) preparations were kindly provided by Dr Y. Linnau (Immuno AG, Vienna, Austria).31 The 4-hydroxy-3-nitrophenacetyl–specific chimaeric huIgA antibody, clone JW393A, was purchased from Serotec (Kidlington, UK). Anti-FcαR (anti-CD89) monoclonal antibody (MoAb), clone My43 (IgM isotype), was obtained from Medarex, Inc (Annandale, NJ), and anti-CD14 MoAb, clone Mo2 (IgM isotype), was obtained from Coulter-Immunotech (Instrumentation Laboratory, Vienna, Austria). Polyclonal rabbit antibodies specific for Lyn, Syk, and Hck were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Antiphosphotyrosine MoAb, clone 4G10, was obtained from Upstate Biotechnology Inc (Lake Placid, NY), and rabbit anti-huIgA F(ab′)2 fragments (F′2RahuIgA) was obtained from Chemicon International, Inc (Temecula, CA). Rabbit polyclonal IgG was purchased from Sera-Lab Limited (Crawley Down, UK). Horseradish peroxidase-labeled sheep antimouse and donkey antirabbit antibodies, [γ-32P]ATP (specific activity, 1.11 TBq/mmol), and the enhanced chemoluminescence assay (ECL) were from Amersham (Little Chalfont, UK). Dimethylsulfoxide was obtained from Sigma-Aldrich Handels Ges.m.b.H. (Vienna, Austria), and Staph aureus(Pansorbin cells) were from Calbiochem-Novabiochem Corp (La Jolla, CA). Bovine serum albumine fraction V (BSA) was from Serva (Heidelberg, Germany).
Preparation of Ig-adsorbed nitrocellulose particles.
NC particles were prepared and coated with human Ig preparations as described.14 Briefly, NC sheets (Schleicher and Schuell, Dassel, Germany) were dissolved in dimethylsulfoxide and particles were precipitated by dropwise addition of 1.5 vol of double-destilled H2O with constant mixing on a shaker. The particles were washed three times with double-destilled H2O and the resuspended pellet was transferred to Petridishes and dried at 50°C on an Eppendorf Thermomixer (Engelsdorf, Germany). Prewetted NC particles (2.5 mg; by weight, 5 mg correspond to 1 cm2 of unprocessed NC sheets) were incubated for 2 hours at 4°C with 2 mg/mL of huIgA or huIgG or with 0.2 mg/mL chimaeric huIgA in phosphate-buffered saline (PBS) with constant shaking at 1.4 × 1,000 rpm. The NC was pelleted at 14,000g for 10 seconds and free protein binding sites were blocked for 30 minutes at 37°C with PBS containing 5% BSA and 0.05% sodium azide on a Thermomixer. NC particles were then washed three times with RPMI 1640 medium without FCS and directly used for cell activation and subsequent immunoprecipitation of aggregated FcRs.
Cell stimulation and isolation of whole lysate proteins.
THP-1 cells were washed and resuspended in RPMI 1640 medium without FCS to a density of 1 × 107/mL. Aliquots of 5 × 105 cells were transferred into 1.5-mL reaction tubes, incubated for 30 minutes at 37°C, and stimulated with antibodies for 5 minutes at 37°C. NC particles adsorbed with human Ig preparations were used at a concentration of 1 mg of coated particles per tube (dry weight). FcαR-specific MoAb My43 (cell culture supernatant) was added 1:2 to the cell suspensions, and the anti-CD14 MoAb Mo2 was used at a concentration of 5 μg/mL. The activation reaction was stopped by addition of 500 μL of ice-cold PBS containing 1 mmol/L sodium orthovanadate (Na3VO4), followed by brief centrifugation and immediate lysis of cells for 20 minutes on ice in Nonidet P-40 lysis buffer (20 mmol/L Tris/HCl, pH 7.5, 150 mmol/L NaCl, 1% Nonidet P-40, 0.1% sodium azide, 1 mmol/L Na3VO4, 1 mmol/L sodium molybdate, Na2MoO4, 2 mmol/L phenylmethanesulfonyl fluoride [PMSF], 5 mmol/L EDTA, and 10 μg/mL of proteinase inhibitors aprotinin and leupeptin). Nuclei and cellular debris were removed by centrifugation for 10 minutes at 14,000g and soluble proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 8% polyacrylamide gels under reducing conditions.
Immunoprecipitation and in vitro kinase assays.
THP-1 cells were washed and resuspended in RPMI 1640 medium to a density of 1.5 × 108/mL. Aliquots of 7.5 × 106 or 1 × 107 cells were transferred into 1.5-mL reaction tubes and cells were activated for 5 minutes with human Ig-adsorbed NC particles (concentrations of NC particles are given in the respective figure legends) as described above. Cell activation was stopped by addition of 0.9 mL of Brij 96 (polyoxyethylene 10 oleyl ether) lysis buffer (20 mmol/L Tris/HCl, pH 7.5, 150 mmol/L NaCl, 1% Brij 96, 0.1% sodium azide, 1 mmol/L Na3VO4, 1 mmol/L Na2MoO4, 2 mmol/L PMSF, 1 mmol/L MgCl2, and 10 μg/mL of proteinase inhibitors aprotinin and leupeptin) and 20 U/mL of Dnase I (Boehringer Mannheim GmbH, Mannheim, Germany). The lysed cells were incubated for 20 minutes on ice to precipitate the NC particles. Supernatants were discarded, and the particles were afterwards washed five times with Brij 96 lysis buffer and precipitated on ice as described. Subsequently, immunocomplexes were eluted with sample buffer and separated by SDS-PAGE on 8% to 12% polyacrylamide gradient gels under nonreducing conditions.
Syk kinase was precipitated from cell lysates of 1.5 × 107 THP-1 cells, which have been stimulated for 5 minutes with My43 or Mo2 as described and lysed in Nonidet P-40 lysis buffer without EDTA. To reduce unspecific binding of proteins to Syk-specific antibody, lysates were incubated on ice for 1 hour with 5 μg/mL of rabbit polyclonal IgG followed by two successive treatments for 30 minutes at 4°C with 50 μL of a 10% Staph aureus slurry. Lysates were then incubated on ice for 1 hour with 5 μg/mL anti-Syk rabbit polyclonal antibody or the same amount of control rabbit IgG. Antibody-bound molecules were precipitated by addition of Staph aureus cells, and immunocomplexes were washed three times with lysis buffer and once with 25 mmol/L HEPES, pH 7.2, containing 150 mmol/L NaCl, 0.1% Nonidet P-40, and 5 mmol/L MnCl2. Pelleted Staph aureus cells were resuspended in 90 μL kinase buffer (25 mmol/L HEPES, pH 7.2, 5 mmol/L MnCl2) and one third of the slurry was used for in vitro kinase assay. The rest of each sample was pelleted and bound proteins were eluted with reducing sample buffer followed by separation on 8% to 12% polyacrylamide gradient gels. To determine the PTK activity of Syk, immunocomplexes were incubated for 15 minutes at 30°C in the presence of 10 μCi [γ-32P] ATP. Bound proteins were then eluted with reducing sample buffer and resolved on 8% to 12% gradient gels. Syk kinase activity was visualized by autoradiography of dried gels.
Immunoblot analysis.
Electrophoretically separated proteins were transferred onto NC sheets and the transfer efficiency was examined by staining with 0.5% Ponceau S. Free protein binding sites were blocked by incubation of immunoblots in Tris-buffered saline, pH 7.5, containing 0.1% Tween-20 and 1% nonfat dry milk (Bio-Rad Lab, Hercules, CA). Tyrosine phosphorylation of cellular proteins was probed with MoAb 4G10 followed by HRP-labeled sheep antimouse antibody. After removal of excess antibody by washing with TBST, specific binding was visualized by ECL. For reprobing of immunoblots, phosphotyrosine-specific antibody was removed by treatment with 100 mmol/L glycine/HCl, pH 2.5 (3 times for 20 minutes), on a shaker. Reprobing with rabbit polyclonal antibodies specific for Syk, Lyn, or Hck was performed after blocking of glycine-treated immunoblots with TBST containing 1% nonfat dry milk. Specific binding was detected by ECL.
Induction of cytokine release.
THP-1 cells were cultured for 40 hours in complete medium in 6-well tissue culture plates (Macro Tray 635 TC; Greiner und Söhne, Kremsmünster, Austria; 3 × 106 cells in a total volume of 2 mL per well) in the presence of calcitriol (1,25-dihydroxyvitamin D3; BIOMOL Research Lab, Plymouth Meeting, PA; final concentration, 80 nmol/L) and recombinant human interferon-γ (IFN-γ; Genzyme, Cambridge, MA; final concentration, 100 U/mL). The cells were then washed and further incubated (3 × 106cells/mL/well) in 24-well tissue culture plates (Becton Dickinson Labware, Lincoln Park, NJ) for 24 hours in the presence of complete medium alone or medium containing soluble monomeric huIgA (at a concentration of 100 μg/mL, if not indicated otherwise) or huIgA adsorbed onto nitrocellulose particles (IgA-NC, where the amount of huIgA-particles added to the cultures corresponded to an huIgA concentration of 100 μg/mL, if not indicated otherwise). As a control, BSA-adsorbed NC-particles were added in the same dilution to parallel cultures. Cells were cultured in complete medium alone or in complete medium containing polymyxin B (PMB; Sigma-Aldrich Handels Ges.m.b.H., Vienna, Austria; final concentration, 10 μg/mL) or PP1 (Calbiochem-Novabiochem Corp, La Jolla, CA); if not indicated otherwise, PP1 was added at a concentration of 100 μmol/L. After 24 hours of incubation in a CO2 incubator at 37°C in humidified air, cell culture supernatants were aspirated and centrifuged for 3 minutes at 9,000g, and cytokine release was examined using commercially available enzyme-linked immunosorbent assay (ELISA) kits for tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-8 (IL-1β-EASIA, TNF-alpha-EASIA, and IL-8-EASIA; Medgenix Diagnostics, Fleurus, Belgium) or IL-1ra (Quantikine Human Interleukin 1 Receptor Antagonist Immunoassay; R&D Systems, Minneapolis, MN). Results are expressed as nanograms per milliliter or as a percentage of control relative to the cytokine release observed in THP-1 cells stimulated in the absence of PTK inhibitor.
Statistical analysis.
Results of the determination of cytokine release are expressed as the mean ± SEM of repeated experiments performed on different days. Statistical evaluation of the observed huIgA-mediated induction of cytokine release by calculating the differences between more then two study groups was performed with the nonparametric Kruskal-Wallis one-way ANOVA by ranks or the Newman-Keuls multiple comparisons test. For statistical evaluation of the difference between two study groups, the nonparametric Mann-Whitney U-test or the Student's t-test for paired samples were used, as appropriate. P < .05 was considered a statistically significant difference.
RESULTS
Cross-linking of FcαR induces tyrosine phosphorylation of cellular proteins in THP-1 cells.
To study transmembrane signaling after FcαR triggering, we cross-linked the receptor with the MoAb My43, or huIgA adsorbed to NC particles, on the surface of the human monocytic cell line THP-1. Both stimuli induced tyrosine phosphorylation of an identical set of cellular proteins, with the exception of an unidentified 115-kD protein, which was phosphorylated exclusively by cross-linking of FcαR with NC particles adsorbed with the natural ligand, huIgA (Fig 1A, lanes 2 and 6 through 10). In contrast, treatment of cells with the CD14-specific MoAb Mo2, with monomeric huIgA, or with NC particles adsorbed with BSA failed to induce tyrosine phosphorylation, indicating that induction of signaling was specific for FcαR ligation and that signal transduction required FcαR cross-linking (Fig 1A, lanes 1, 3, and 11). Unexpectedly, cross-linking of monomeric huIgA with F(ab′)2fragments of a specific antibody failed to induce tyrosine phosphorylation of cellular proteins, suggesting the need for extensive FcαR aggregation by multivalent ligand (Fig 1A, lane 4).
Activation of THP-1 cells with MoAb My43 and huIgA induces tyrosine phosphorylation of cellular proteins. THP-1 cells (5 × 105/sample) were activated at 37°C for 5 minutes by the addition of 5 μg/mL Mo2 (anti-CD14, lane 1), My43 cell culture supernatant 1:2 (anti-CD89, lane 2), 100 μg/mL huIgA (lane 3), 100 μg/mL huIgA + 100 μg/mL F′2RAhuIgA (lane 4), 100 μg/mL F′2RAhuIgA (lane 5), or NC particles (1 mg/sample) incubated in PBS containing different concentrations of huIgA, 3 mg/mL (lane 6), 1 mg/mL (lane 7), 0.33 mg/mL (lane 8), 0.11 mg/mL (lane 9), and 0.037 mg/mL (lane 10), and NC particles incubated in PBS containing 5% BSA only (lanes 11 and 12). Cellular proteins were isolated, separated by SDS-PAGE, and transferred to NC sheets as described in the experimental section. Immunoblotting of tyrosine-phosphorylated proteins was performed with the MoAb 4G10 followed by development with ECL as described (A). The immunoblot was then stripped and reprobed with a rabbit polyclonal anti-Syk antibody (lanes 1 through 11 in [B]) or control rabbit polyclonal IgG (lane 12 in [B]).
Activation of THP-1 cells with MoAb My43 and huIgA induces tyrosine phosphorylation of cellular proteins. THP-1 cells (5 × 105/sample) were activated at 37°C for 5 minutes by the addition of 5 μg/mL Mo2 (anti-CD14, lane 1), My43 cell culture supernatant 1:2 (anti-CD89, lane 2), 100 μg/mL huIgA (lane 3), 100 μg/mL huIgA + 100 μg/mL F′2RAhuIgA (lane 4), 100 μg/mL F′2RAhuIgA (lane 5), or NC particles (1 mg/sample) incubated in PBS containing different concentrations of huIgA, 3 mg/mL (lane 6), 1 mg/mL (lane 7), 0.33 mg/mL (lane 8), 0.11 mg/mL (lane 9), and 0.037 mg/mL (lane 10), and NC particles incubated in PBS containing 5% BSA only (lanes 11 and 12). Cellular proteins were isolated, separated by SDS-PAGE, and transferred to NC sheets as described in the experimental section. Immunoblotting of tyrosine-phosphorylated proteins was performed with the MoAb 4G10 followed by development with ECL as described (A). The immunoblot was then stripped and reprobed with a rabbit polyclonal anti-Syk antibody (lanes 1 through 11 in [B]) or control rabbit polyclonal IgG (lane 12 in [B]).
The PTK Syk is a major target in FcαR-mediated tyrosine phosphorylation.
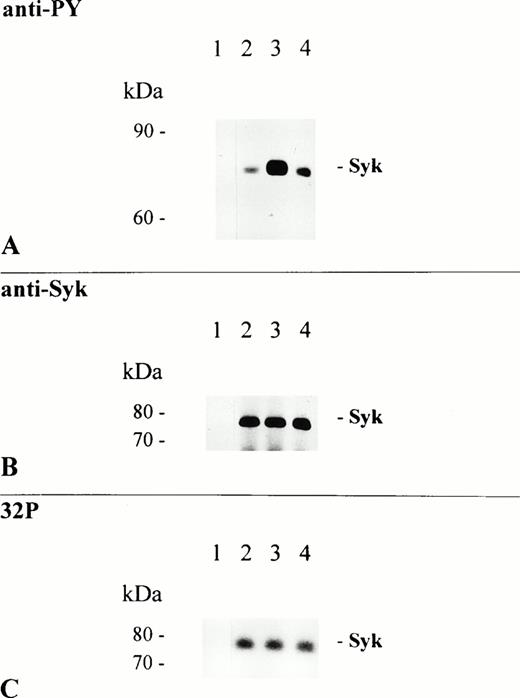
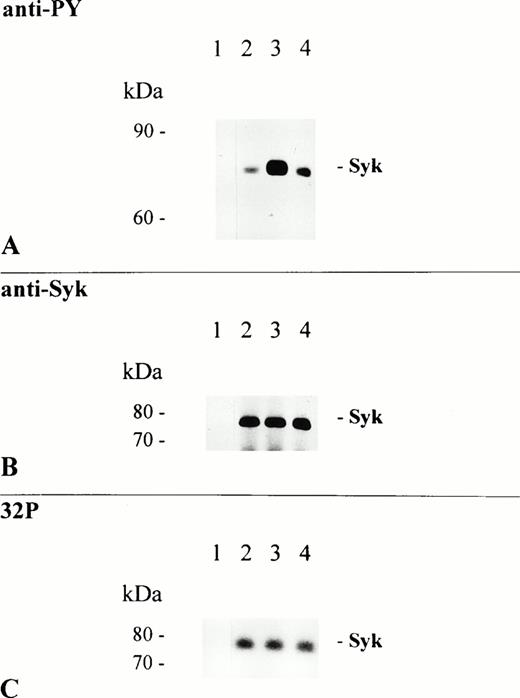
Among the targets that became phosphorylated after cross-linking of FcαR was a predominant protein band of 75 kD. Because involvement of Syk, a PTK of similar molecular mass, has been shown for various FcRs, we wanted to clarify whether the 75-kD band corresponded to p72syk. As shown in Fig 1B, rabbit polyclonal IgG specific for human Syk recognized a single protein band of identical molecular mass. To further characterize the effect of FcαR-mediated signaling on this PTK, we immunoprecipitated Syk kinase from resting and stimulated THP-1 cells and performed anti-PY immunoblot analysis and in vitro kinase assay. FcαR cross-linking with MoAb My43 induced extensive tyrosine phosphorylation of Syk protein (6-fold increase in phosphotyrosine; Fig 2A, lane 3), whereas treatment of cells with the MoAb Mo2 (also of the IgM isotype) had no effect (Fig 2A, lane 2). Although FcαR aggregation after stimulation with My43 induced Syk tyrosine phosphorylation, the enzymatic activity of precipitated Syk was unchanged (Fig 2C). Anti-Syk immunoblot analysis shows that an equal amount of Syk was immunoprecipitated from resting and FcαR-activated cells (Fig 2B).
Syk is a major target of FcαR-induced PTK activity. THP-1 cells were activated at 37°C for 5 minutes by the addition of 5 μg/mL Mo2 (lanes 1 and 2), My43 cell culture supernatant 1:2 (lane 3), and RPMI 1640 cell culture medium only (lane 4). Lysates from 1.5 × 107 cells were immunoprecipitated with a rabbit polyclonal anti-Syk antibody (lanes 2 through 4) or control rabbit polyclonal IgG (lane 1). Two thirds of each immunoprecipitate were separated by 8% to 12% SDS-PAGE, transferred to NC sheets, and immunoblotted with an antiphosphotyrosine antibody (A). The immunoblot was afterwards stripped and reprobed with Syk-specific antibody (B). One third of each immunoprecipitate was used for in vitro kinase assay, as described in the Materials and Methods; separated by 8% to 12% SDS-PAGE; and autophosphorylated Syk kinase was detected on dried gels using autoradiography (C).
Syk is a major target of FcαR-induced PTK activity. THP-1 cells were activated at 37°C for 5 minutes by the addition of 5 μg/mL Mo2 (lanes 1 and 2), My43 cell culture supernatant 1:2 (lane 3), and RPMI 1640 cell culture medium only (lane 4). Lysates from 1.5 × 107 cells were immunoprecipitated with a rabbit polyclonal anti-Syk antibody (lanes 2 through 4) or control rabbit polyclonal IgG (lane 1). Two thirds of each immunoprecipitate were separated by 8% to 12% SDS-PAGE, transferred to NC sheets, and immunoblotted with an antiphosphotyrosine antibody (A). The immunoblot was afterwards stripped and reprobed with Syk-specific antibody (B). One third of each immunoprecipitate was used for in vitro kinase assay, as described in the Materials and Methods; separated by 8% to 12% SDS-PAGE; and autophosphorylated Syk kinase was detected on dried gels using autoradiography (C).
The Src family kinase Lyn coprecipitates with FcαR.
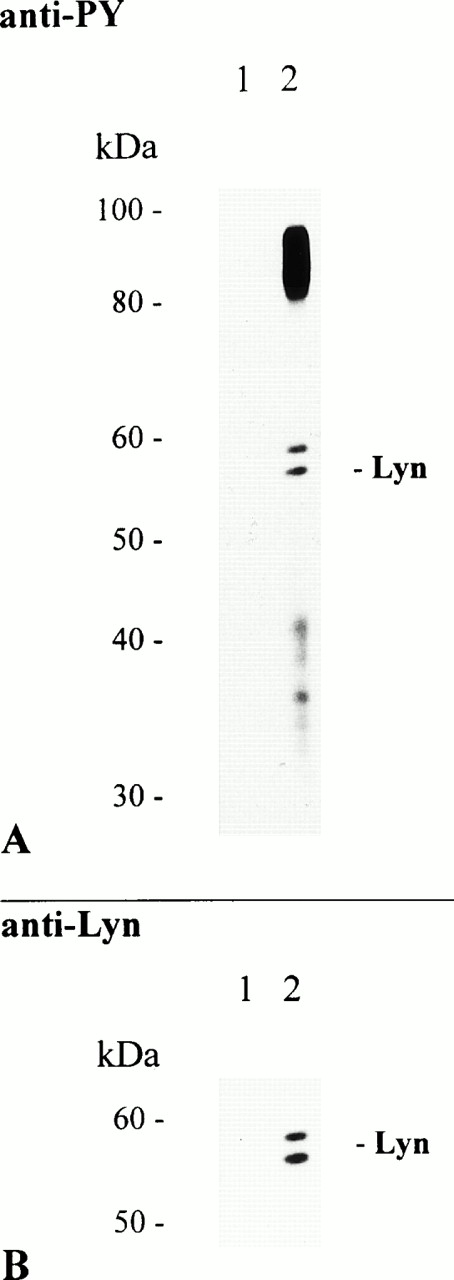
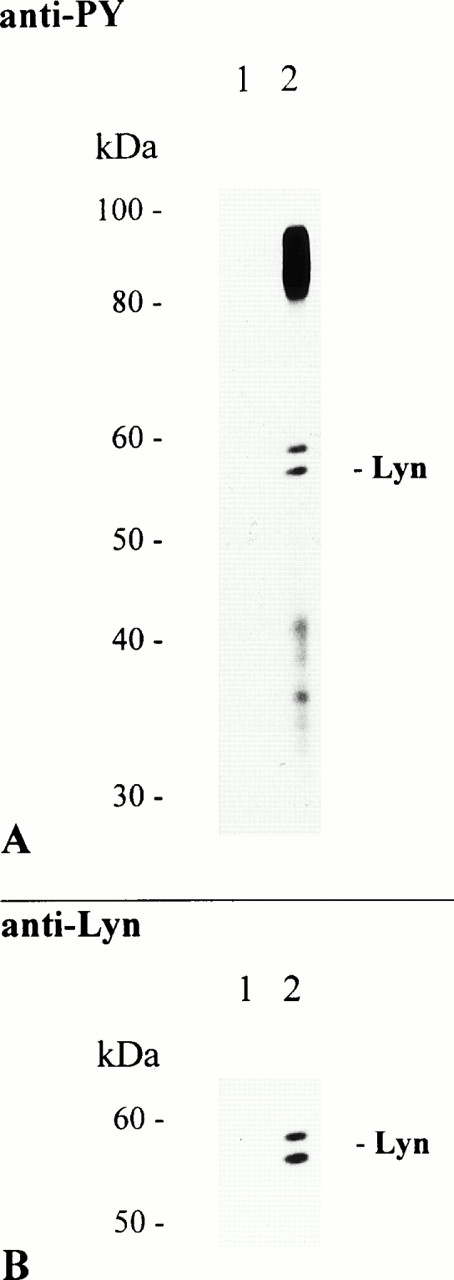
The results shown above prompted us to investigate whether PTKs associate with FcαR. Therefore, we adsorbed huIgA to NC particles and used these complexes for cross-linking and subsequent immunoprecipitation of aggregated FcαR. Several tyrosine-phosphorylated proteins were present in the FcαR-specific immunoprecipitate, whereas no proteins were found with NC particles coated with BSA or two different preparations of human myeloma IgM (Fig 3A).14 Among the proteins that coprecipitated with the receptor was a notable double band of 56 and 58 kD. Similar migration patterns have been described for the Src family kinases Hck and Lyn.32-34 To further characterize the double band, we stripped the immunoblot in low pH buffer and reprobed it with antibodies specific for human Hck or Lyn. Our results show that the anti-Lyn antibody reacted with protein bands of identical molecular mass, whereas no proteins were detected with the Hck-specific antibody (Fig 3B, and data not shown). Additionally, Lyn kinase was also present in receptor complexes immunoprecipitated with NC particles adsorbed with the MoAb My43, but not with MoAb Mo2 (data not shown).
Lyn coimmunoprecipitates with aggregated FcαR. THP-1 cells (7.5 × 106/sample) were activated at 37°C for 5 minutes with 2 mg of NC particles adsorbed with BSA only (lane 1) or huIgA (lane 2). Immunoprecipitation, separation of precipitated proteins by 8% to 12% SDS-PAGE, and antiphosphotyrosine immunoblot analysis of separated proteins were performed as described before (A). The immunoblot was then stripped and reprobed with a rabbit polyclonal anti-Lyn antibody (B).
Lyn coimmunoprecipitates with aggregated FcαR. THP-1 cells (7.5 × 106/sample) were activated at 37°C for 5 minutes with 2 mg of NC particles adsorbed with BSA only (lane 1) or huIgA (lane 2). Immunoprecipitation, separation of precipitated proteins by 8% to 12% SDS-PAGE, and antiphosphotyrosine immunoblot analysis of separated proteins were performed as described before (A). The immunoblot was then stripped and reprobed with a rabbit polyclonal anti-Lyn antibody (B).
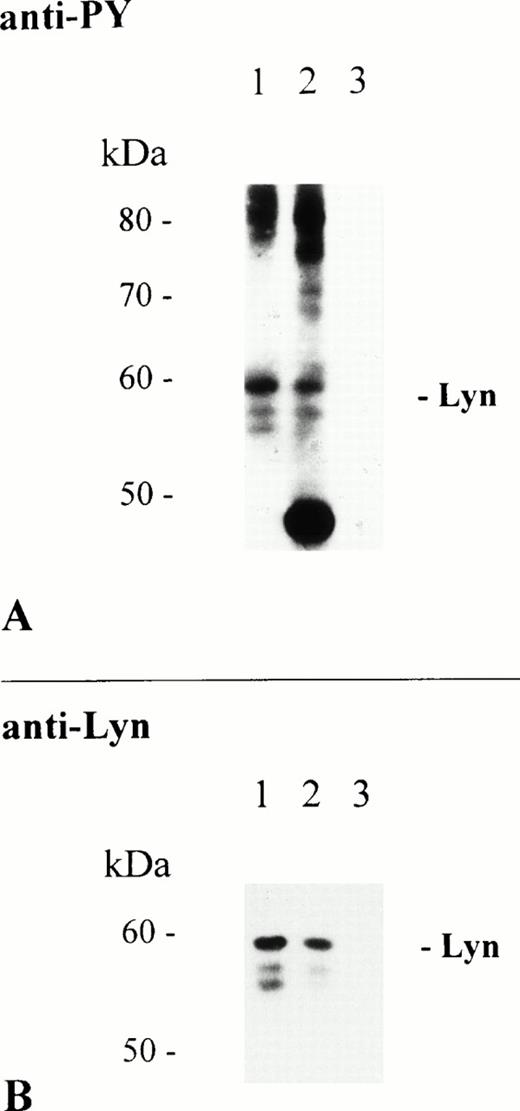
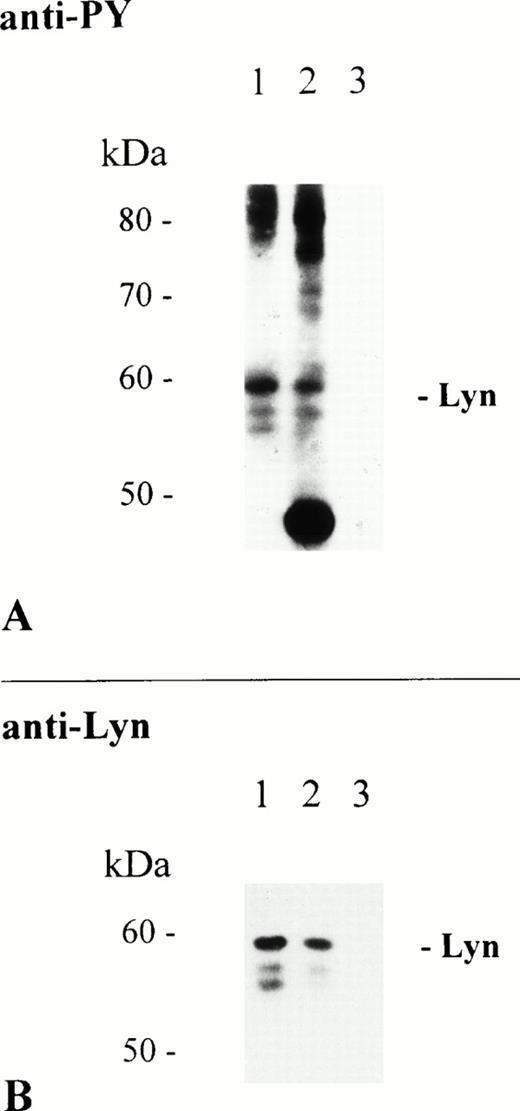
To exclude that precipitation of Lyn kinase was due to specific antibody activities present in the huIgA preparation, a chimaeric huIgA antibody specific for the hapten NP (4-hydroxy-3-nitrophenacetyl) was used to immunoprecipitate FcαR. Interaction of this hapten-specific antibody with THP-1 cells depends entirely on its huIgAFc domain and is, therefore, restricted to ligation of FcαR. Anti-PY analysis shows that proteins of 55, 56, and 58 kD were present in the receptor-specific immunoprecipitates using either huIgA or chimaeric huIgA adsorbed to NC particles (Fig 4A, lanes 1 and 2, respectively). Again, protein bands of identical molecular mass were recognized in both immunoprecipitates by reprobing of the immunoblot with the Lyn-specific antibody (Fig 4B).
Immunoprecipitation of FcαR depends on the Fc domain of huIgA. THP-1 cells (1 × 107/sample) were activated at 37°C for 5 minutes with 2.5 mg of NC particles adsorbed with huIgA (lane 1), chimeric huIgA (lane 2), or BSA only (lane 3). Immunoprecipitation, separation of precipitated proteins by 8% to 12% SDS-PAGE, and antiphosphotyrosine immunoblot analysis of isolated proteins were performed as described. Immunoblot analysis of tyrosine phosphorylated proteins (A). Reprobing of the same immunoblot with Lyn-specific antibody (B).
Immunoprecipitation of FcαR depends on the Fc domain of huIgA. THP-1 cells (1 × 107/sample) were activated at 37°C for 5 minutes with 2.5 mg of NC particles adsorbed with huIgA (lane 1), chimeric huIgA (lane 2), or BSA only (lane 3). Immunoprecipitation, separation of precipitated proteins by 8% to 12% SDS-PAGE, and antiphosphotyrosine immunoblot analysis of isolated proteins were performed as described. Immunoblot analysis of tyrosine phosphorylated proteins (A). Reprobing of the same immunoblot with Lyn-specific antibody (B).
Tyrosine kinase inhibitor PP1 differentially affects association of Lyn with FcαR and FcγR.
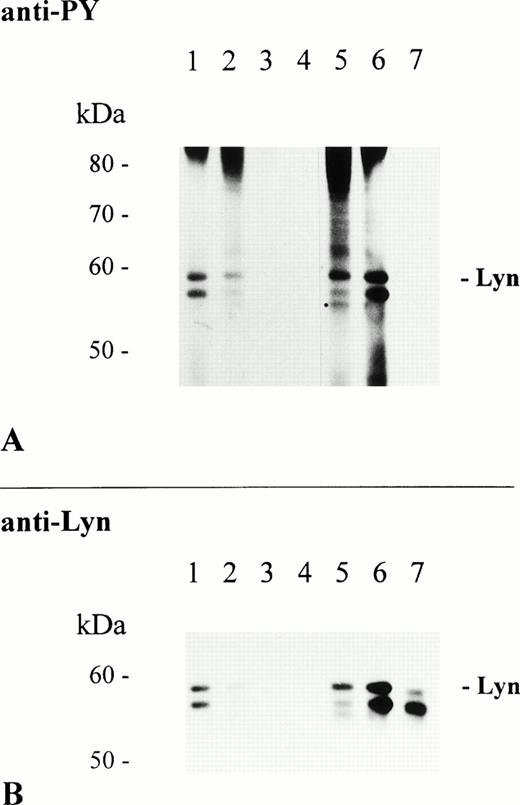
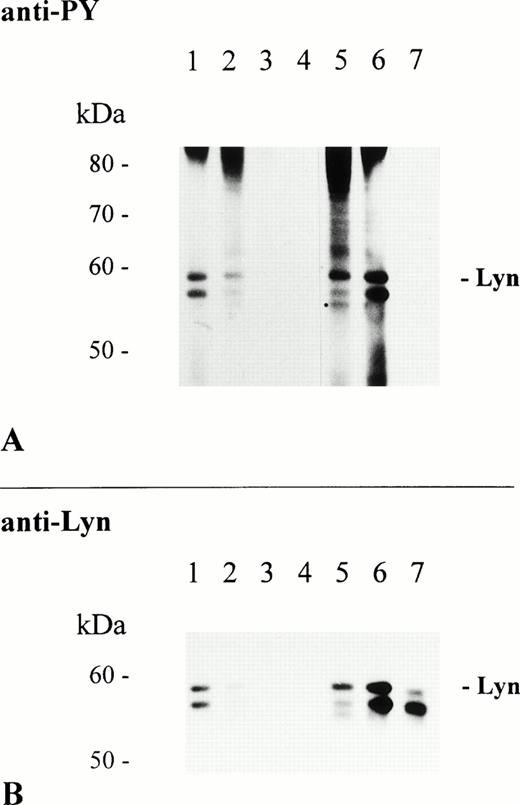
Treatment of RBL-2H3 cells with the Syk-specific inhibitor piceatannol has been shown to partially inhibit FcεR-mediated protein tyrosine phosphorylation, but to completely block downstream cellular responses.35 The molecule responsible for tyrosine phosphorylation of the remaining phosphorylated proteins appeared to be the Src family kinase Lyn, which is constitutively associated with the FcεR β-chain and, hence, may function upstream of Syk.9We used the Src family-selective inhibitor PP1 to study the role of Src kinases in FcαR-mediated protein tyrosine phosphorylation.36 Activation of THP-1 cells in the presence of 100 μmol/L PP1 abolished FcαR-mediated protein tyrosine phosphorylation of whole lysate proteins, including Lyn and Syk (data not shown). PP1 blocked FcαR- and FcγR-mediated tyrosine phosphorylation of Lyn kinase in a dose-dependent way and phosphorylation of Lyn was completely abolished at a concentration of 100 μmol/L (Fig 5A, lanes 3 and 7). Interestingly, association of Lyn with FcαR was sensitive to short-term treatment with PP1 and completely blocked at 100 μmol/L. However, interaction of Lyn with FcγR was not affected by PP1 (Fig 5B, lanes 1 through 3 and 5 through 7, respectively). These results support the hypothesis that association of Lyn with FcαR or FcγR differs in the need of Src kinase activity before and/or after receptor aggregation.
Association of Lyn with FcαR is sensitive to treatment with PP1. THP-1 cells (1 × 107/sample) were incubated at 37°C for 30 minutes in RPMI 1640 cell culture medium only (lanes 1, 4, and 5) and cell culture medium containing 10 μmol/L of PP1 (lanes 2 and 6) or 100 μmol/L of PP1 (lanes 3 and 7). Subsequently, cells were activated at 37°C for 5 minutes with 2.5 mg of NC particles adsorbed with huIgA (lanes 1 through 3), huIgG (lanes 5 through 7), or BSA only (lane 4). Immunoprecipitation, separation of precipitated proteins by 8% to 12% SDS-PAGE, and antiphosphotyrosine immunoblot analysis of isolated proteins were performed as described in Materials and Methods (A). Reprobing of the same immunoblot with Lyn-specific antibody (B).
Association of Lyn with FcαR is sensitive to treatment with PP1. THP-1 cells (1 × 107/sample) were incubated at 37°C for 30 minutes in RPMI 1640 cell culture medium only (lanes 1, 4, and 5) and cell culture medium containing 10 μmol/L of PP1 (lanes 2 and 6) or 100 μmol/L of PP1 (lanes 3 and 7). Subsequently, cells were activated at 37°C for 5 minutes with 2.5 mg of NC particles adsorbed with huIgA (lanes 1 through 3), huIgG (lanes 5 through 7), or BSA only (lane 4). Immunoprecipitation, separation of precipitated proteins by 8% to 12% SDS-PAGE, and antiphosphotyrosine immunoblot analysis of isolated proteins were performed as described in Materials and Methods (A). Reprobing of the same immunoblot with Lyn-specific antibody (B).
Cross-linking of FcαR with huIgA adsorbed to NC particles induces IL-1ra and IL-8 release in THP-1 cells.
In a previous study, we showed that stimulation of human mononuclear cells or isolated human monocytes with huIgA induces the production of IL-1ra, a naturally occurring inhibitor of IL-1 activity previously shown to be produced by monocytes/macrophages in response to FcγR stimulation or LPS.37,38 The present results confirm and extend these previous findings by showing that cross-linking of FcαR by huIgA adsorbed onto nitrocellulose particles induces IL-1ra release in THP-1 cells (Fig 6A). In addition, stimulation of THP-1 cells by FcαR cross-linking induces the production of IL-8 (Fig 6B), an 8-kD chemokine and angiogenic factor previously shown to be produced by cells of the monocyte/macrophage lineage, epithelial cells, or endothelial cells in response to lipopolysaccharide (LPS), FcγR cross-linking, or cytokines such as TNF-α.39 40
Src family PTK activity is required for induction of IL-1ra and IL-8 in THP-1 cells after cross-linking of FcαR by huIgA-adsorbed NC particles. THP-1 cells (3 × 106cells/mL/well) were stimulated for 24 hours with huIgA adsorbed to nitrocellulose particles (IgA-NC); parallel cultures were incubated in the presence of corresponding concentrations of soluble huIgA or BSA-adsorbed nitrocellulose particles (NC). In (A) and (B), cells were cultured in complete medium (Med) or in complete medium containing PMB (final concentration, 10 μg/mL) or PP1 (final concentration, 100 μmol/L). In (C), THP-1 cells were stimulated for 24 hours with huIgA adsorbed to NC particles (final concentration of huIgA, 100 μg/mL) in the presence of PP1 at the indicated concentrations. Release of IL-1ra and IL-8 was examined in cell-free supernatants as described in Materials and Methods. Results are expressed as nanograms per milliliter (mean ± SEM of 3 experiments [A] or 6 experiments [B]) or (C) as a percentage of control (mean ± SEM of 3 experiments) relative to the cytokine release observed in cells stimulated with huIgA-particles in the absence of PP1 (cytokine release, in nanograms per milliliter, mean ± SEM: IL-1ra, 7.99 ± 0.84; IL-8, 1.41 ± 0.04). (*) Statistically significant difference between cultures stimulated with huIgA-NC and cells cultured in the presence of huIgA or NC alone (both in the presence or absence of polymyxin B, 10 μg/mL;P<0.05, Newman-Keuls multiple comparisons test; P = .004, analysis of variance). (#) Statistically significant difference as compared with cultures containing soluble huIgA or NC particles alone (P < .01, Kruskal-Wallis test for comparison of more then two samples). (x) Statistically significant difference as compared with cells stimulated with huIgA adsorbed to NC particles in the absence of PP1 (P = .01, Mann-Whitney U test). (+) Statistically significant inhibition of cytokine release after stimulation with huIgA adsorbed to NC particles (P < .025, Student's t-test for paired samples).
Src family PTK activity is required for induction of IL-1ra and IL-8 in THP-1 cells after cross-linking of FcαR by huIgA-adsorbed NC particles. THP-1 cells (3 × 106cells/mL/well) were stimulated for 24 hours with huIgA adsorbed to nitrocellulose particles (IgA-NC); parallel cultures were incubated in the presence of corresponding concentrations of soluble huIgA or BSA-adsorbed nitrocellulose particles (NC). In (A) and (B), cells were cultured in complete medium (Med) or in complete medium containing PMB (final concentration, 10 μg/mL) or PP1 (final concentration, 100 μmol/L). In (C), THP-1 cells were stimulated for 24 hours with huIgA adsorbed to NC particles (final concentration of huIgA, 100 μg/mL) in the presence of PP1 at the indicated concentrations. Release of IL-1ra and IL-8 was examined in cell-free supernatants as described in Materials and Methods. Results are expressed as nanograms per milliliter (mean ± SEM of 3 experiments [A] or 6 experiments [B]) or (C) as a percentage of control (mean ± SEM of 3 experiments) relative to the cytokine release observed in cells stimulated with huIgA-particles in the absence of PP1 (cytokine release, in nanograms per milliliter, mean ± SEM: IL-1ra, 7.99 ± 0.84; IL-8, 1.41 ± 0.04). (*) Statistically significant difference between cultures stimulated with huIgA-NC and cells cultured in the presence of huIgA or NC alone (both in the presence or absence of polymyxin B, 10 μg/mL;P<0.05, Newman-Keuls multiple comparisons test; P = .004, analysis of variance). (#) Statistically significant difference as compared with cultures containing soluble huIgA or NC particles alone (P < .01, Kruskal-Wallis test for comparison of more then two samples). (x) Statistically significant difference as compared with cells stimulated with huIgA adsorbed to NC particles in the absence of PP1 (P = .01, Mann-Whitney U test). (+) Statistically significant inhibition of cytokine release after stimulation with huIgA adsorbed to NC particles (P < .025, Student's t-test for paired samples).
Induction of cytokine release by particle-bound huIgA was dose-dependent and became statistically significant even at a relatively low concentration of particle-adsorbed huIgA (ie, 100 μg/mL). In contrast, levels of IL-1ra and IL-8 produced by cells cultured in the presence of the same amount of BSA-adsorbed nitrocellulose particles were only slightly elevated as compared with background cytokine release, indicating that induction of cytokine release by particle-bound huIgA was specific and mediated through triggering of FcαR. Cross-linking of FcαR by particle-bound ligand was required for induction of IL-1ra and IL-8 release, because stimulation of THP-1 cells with soluble monomeric huIgA at the same concentration had no effect on cytokine production, although flow cytometric analysis confirmed strong binding of soluble huIgA to the cells under these conditions (data not shown). In contrast to phorbol 12-myristate 13-acetate (PMA), which stimulates production of IL-8, IL-1ra, TNF-α, and IL-1β by directly activating protein kinase C independent of surface receptor triggering, particle-adsorbed huIgA specifically induced IL-1ra and IL-8 release accompanied by only very low levels of TNF-α or IL-1β release (TNF-α release, in nanograms per milliliter, mean ± SEM [n]: BSA-adsorbed particles, 0.29 ± 0.04 [9]; huIgA-coated particles, 0.76 ± 0.14 [9]; PMA [100 ng/mL], 3.98 ± 1.07 [5]; IL-1β, in nanograms per milliliter, mean ± SEM [n]: BSA-adsorbed particles, 0.12 ± 0.02 [9]; huIgA [100 μg/mL]-coated particles, 0.82 ± 0.22 [9]; PMA [100 ng/mL], 8.45 ± 1.66 [5]; ratio IL-1ra/IL-1β: huIgA coated onto NC particles, 28 ± 7; PMA: 5 ± 1;P = .00135, Mann-Whitney U-test). Furthermore, particle-adsorbed huIgA triggered cytokine release even under conditions in which endotoxin-induced cytokine release was blocked by the addition of polymyxin B (data not shown), thus indicating that FcαR cross-linking and LPS stimulate cytokine release in THP-1 cells through different mechanisms (Fig 6A and B).
Induction of IL-1ra and IL-8 release after cross-linking of FcαR by multivalent ligand requires Src family protein tyrosine kinase activity.
In the present study, we show that signaling events after FcαR cross-linking involve association of the PTK Lyn with FcαR and tyrosine phosphorylation of proteins involved in signal transduction such as Syk. The results presented in Fig 6B and C show that Src family PTK activity is essential for FcαR-mediated activation of cellular functions, because the Src family-specific inhibitor PP1 abolished the huIgA-mediated induction of IL-1ra and IL-8 release in THP-1 cells. PP1-induced inhibition of cytokine production was dose-dependent over a 3-log range, with almost complete inhibition of cytokine release at a concentration of 100 μmol/L of PP1 (Fig 6C).
DISCUSSION
In the present report, we show that, in the monocytic cell line THP-1, the Src family kinase Lyn physically associates with FcαR after receptor aggregation by multivalent ligand. The association of Lyn with FcαR was found in immunoprecipitates of the native ligand of the receptor, huIgA, or an MoAb of the IgM isotype directed against the ligand-binding site of FcαR, thus excluding precipitation of FcγR-bound Lyn. Furthermore, precipitation of Lyn kinase due to specific antibody activities was excluded by the use of a chimeric huIgA consisting of a hapten-specific murine F(ab′)2component and the Fc part of human IgA.
Our approach, to cross-link and immunoprecipitate FcαR with huIgA-adsorbed NC particles, does not allow us to study whether Lyn is constitutively associated with FcαR in unstimulated cells. However, the sensitivity of this interaction to short treatment with the PTK inhibitor PP1 argues in favor of an aggregation-induced association dependent on immediate kinase activity. PP1 has been shown to selectively inhibit Src family PTKs, including Lck, Fyn, Src, and Hck, at nanomolar concentrations, whereas no effect on the kinase activity of ZAP-70, a member of the Syk-family of PTKs, was observed even in the presence of 100 μmol/L of the inhibitor.36 Furthermore, Amoui et al41 recently showed that Lyn in vitro kinase activity was highly sensitive to PP1, whereas Syk activity was not influenced by the inhibitor. The selectivity of PP1 provides, therefore, strong evidence that the PTK regulating FcαR/Lyn association is a Src family kinase, probably Lyn itself.
Presently, we do not know how Lyn interacts with FcαR at the molecular level, whereas various mechanisms have been described for recruitment of Lyn to other FcRs expressed on myeloid cells, such as FcεRI, FcγRI, and FcγRII.9,12,13 However, the dependence on Src kinase activity suggests that the target region on FcαR for Lyn binding may be a tyrosine-containing sequence motif. In this case, association of Lyn should occur to molecules other than the Ig-binding α chain, because of the lack of tyrosine residues in the cytoplasmic tail of the receptor.6 Additionally, binding must be of considerable strength, because FcαR/Lyn complexes did not readily dissociate in 1% Nonidet P-40 lysis buffer (data not shown). Recently, FcαR has been described to associate with the ITAM-containing FcR γ chain in U937 monocytic cells, and association of Lyn kinase and γ chain has been shown in the same cell line.13,28 However, association of Lyn with ITAM-containing receptor subunits such as the FcR γ chain or the FcεRI β chain has been described to occur constitutively and does not depend on activation-induced phosphorylation of tyrosine residues.9,13 In contrast to FcγR, interaction of Lyn with FcαR was sensitive to treatment with PP1 (Fig 5), suggesting that other mechanisms than binding to the FcαR-associated γ chain account for the observed association of Lyn kinase with FcαR. Coimmunoprecipitation of Lyn with the Ig-binding α chain of FcγRI has been shown in monocytic cells lines under conditions in which γ chain dissociates from the receptor complex.11,13 Like FcαR, the cytoplasmic tail of the ligand-binding α chain of FcγRI is devoid of tyrosine-containing signaling motifs and functional signal transduction depends on ITAM-bearing receptor subunits.42 Direct binding of Lyn to the α chain of FcαR implies a more active role of the Ig-binding subunit in signal transduction processes induced by receptor aggregation than generally assumed. Recently, Hou et al43showed that the membrane-proximal amino acids in the cytoplasmic tail of the FcγRIII α chain are necessary for cell activation. In their report, tyrosine phosphorylation of cellular proteins was considerably impaired in cell lines transfected with a mutant α chain that lacked the respective amino acids, although association of FcγRIII with ζ chain was not affected. These results suggest, therefore, a role for the membrane-proximal amino acids of the α chain in recruiting nonreceptor PTKs to aggregated FcγRIII. The purpose of the membrane-proximal amino acids of the FcαR α chain is presently unkown. However, they could also play a role in signal transduction, for instance by recruiting Lyn to the aggregated receptor or by stabilizing Lyn/FcαR complexes.
Sequential activation of different PTKs appears to be a general feature of multisubunit antigen receptors and FcRs of hematopoietic cells.8 In myeloid cells, involvement of members of the Src and Syk families of PTKs in transmembrane signaling after FcεR or FcγR aggregation has been described.11,13,15,19,20,44 In our study, tyrosine phosphorylation of Syk kinase increased sixfold after FcαR cross-linking, whereas baseline in vitro kinase activity of Syk was unchanged (Fig 2). The observed lack of increased tyrosine kinase activity after FcαR cross-linking appears to be in conflict with previous reports showing that tyrosine phosphorylation of Syk, following aggregation of FcγRI in U937 monocytic cells or FcεRI in mast cells, was accompanied by extensive upregulation of in vitro kinase activity of Syk.21,35 Furthermore, Ueland et al30 previously reported that aggregation of FcαR in U937 cells resulted in increased tyrosine phosphorylation of Syk as well as upregulation of its in vitro kinase activity. Discrepancies between these previous findings and the results of the present report with regard to enhancement of Syk in vitro kinase activity could be due to distinct characteristics of the different cell lines studied. In contrast to U937 cells, aggregation of FcγRI or FcγRII in THP-1 cells has been shown to result in a tremendous induction of Syk tyrosine phosphorylation but only weak (2- to 3-fold) upregulation of its in vitro kinase activity.45 In our study, considerable baseline kinase activity of Syk could be found in THP-1 cells and recent evidence indicates that downstream signaling can proceed despite the lack of demonstrable upregulation of Syk kinase activity. Aggregation of Syk/CD16 chimeric proteins on the surface of the CD8+ cytolytic T-cell line WH3 was sufficient to allow induction of specific cytolysis of target cells, although kinase activity of Syk was unchanged after Syk/CD16 cross-linking.46 The finding that cross-linking of FcαR on THP-1 cells by huIgA bound to nitrocellulose particles is capable of inducing the release of IL-1ra and IL-8 indicates that downstream signaling events after FcαR triggering are intact despite the lack of significant FcαR-mediated upregulation of Syk kinase activity. Induction of IL-1ra and IL-8 release could be completely inhibited by the Src family-specific PTK-inhibitor PP1, thus confirming the biological significance of the newly described association of Lyn with FcαR after receptor cross-linking. The lack of detectable Hck kinase, which has been shown to associate with FcγRs in THP-1 cells, additionally emphazises the crucial role of Lyn for FcαR-induced signaling processes.11,12 Although no other Src family kinases have been described to be involved in signal transduction via FcRs in THP-1 cells, involvement of Src kinases apart from Lyn cannot be completely ruled out. Aggregation of FcαR induced the release of considerable amounts of IL-1ra, a cytokine with potent anti-inflammatory properties in vitro and in vivo, as well as IL-8.47 Although IL-8 was initially described as a proinflammatory neutrophil chemotactic factor secreted by lipopolysaccharide-stimulated mononuclear cells, IL-8 has recently been shown to play a more complex role in the regulation of the inflammatory response, eg, by exerting a wide range of modulatory effects on neutrophil-endothelial adhesive interactions.39,40 Our finding that cross-linking of FcαR by particle-bound huIgA induces the release of IL-1ra and IL-8 confirms and extends previous studies suggesting that huIgA, in addition to the protective functions mediated by specific antibody activity, plays an active role in the regulation of inflammatory responses by interacting with the FcαR on cells of the monocyte lineage.31,37 48
ACKNOWLEDGMENT
The authors thank Eleonore Gschaider and Astrid Lehner for expert technical assistance and Paul Breit for excellent photographic assistance.
Address reprint requests to Heinz Gulle, PhD, Institute of Immunology, University of Vienna, Borschkegasse 8A, A-1090 Vienna, Austria.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Activation of THP-1 cells with MoAb My43 and huIgA induces tyrosine phosphorylation of cellular proteins. THP-1 cells (5 × 105/sample) were activated at 37°C for 5 minutes by the addition of 5 μg/mL Mo2 (anti-CD14, lane 1), My43 cell culture supernatant 1:2 (anti-CD89, lane 2), 100 μg/mL huIgA (lane 3), 100 μg/mL huIgA + 100 μg/mL F′2RAhuIgA (lane 4), 100 μg/mL F′2RAhuIgA (lane 5), or NC particles (1 mg/sample) incubated in PBS containing different concentrations of huIgA, 3 mg/mL (lane 6), 1 mg/mL (lane 7), 0.33 mg/mL (lane 8), 0.11 mg/mL (lane 9), and 0.037 mg/mL (lane 10), and NC particles incubated in PBS containing 5% BSA only (lanes 11 and 12). Cellular proteins were isolated, separated by SDS-PAGE, and transferred to NC sheets as described in the experimental section. Immunoblotting of tyrosine-phosphorylated proteins was performed with the MoAb 4G10 followed by development with ECL as described (A). The immunoblot was then stripped and reprobed with a rabbit polyclonal anti-Syk antibody (lanes 1 through 11 in [B]) or control rabbit polyclonal IgG (lane 12 in [B]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.383/3/m_blod4024201.jpeg?Expires=1769821944&Signature=PkVBkeCHrDzsGzQMUO8BpoJlyRivuLXDha30jVKQrMFe6N~GOROBuqwU54o1hp1elcz0PYyBMQWOa5Ug8vbzEd8XjXLdX0LzpPws7t2o4sz2r8nTMd6SYbp0eP6wQ3wfDNtMfrwmxM~4IlLOxGiUi1ThzDqGExyXofcBUPLHceI~D77Xxyu8Wv8S53hS~PtfRLyQxlV68SJX0oiM2njka7pKVVND5Os1gwxJwWMYY9iqlWT84qAo8mqdRoufEJz~5vBI217f59y0kfOs5wgJ7DZtZsTpzV8b7m5reH8gqiY1xNVezMB31YvAye8kRtpYxLRmVrulWHVtNtnlpJE20A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Src family PTK activity is required for induction of IL-1ra and IL-8 in THP-1 cells after cross-linking of FcαR by huIgA-adsorbed NC particles. THP-1 cells (3 × 106cells/mL/well) were stimulated for 24 hours with huIgA adsorbed to nitrocellulose particles (IgA-NC); parallel cultures were incubated in the presence of corresponding concentrations of soluble huIgA or BSA-adsorbed nitrocellulose particles (NC). In (A) and (B), cells were cultured in complete medium (Med) or in complete medium containing PMB (final concentration, 10 μg/mL) or PP1 (final concentration, 100 μmol/L). In (C), THP-1 cells were stimulated for 24 hours with huIgA adsorbed to NC particles (final concentration of huIgA, 100 μg/mL) in the presence of PP1 at the indicated concentrations. Release of IL-1ra and IL-8 was examined in cell-free supernatants as described in Materials and Methods. Results are expressed as nanograms per milliliter (mean ± SEM of 3 experiments [A] or 6 experiments [B]) or (C) as a percentage of control (mean ± SEM of 3 experiments) relative to the cytokine release observed in cells stimulated with huIgA-particles in the absence of PP1 (cytokine release, in nanograms per milliliter, mean ± SEM: IL-1ra, 7.99 ± 0.84; IL-8, 1.41 ± 0.04). (*) Statistically significant difference between cultures stimulated with huIgA-NC and cells cultured in the presence of huIgA or NC alone (both in the presence or absence of polymyxin B, 10 μg/mL;P<0.05, Newman-Keuls multiple comparisons test; P = .004, analysis of variance). (#) Statistically significant difference as compared with cultures containing soluble huIgA or NC particles alone (P < .01, Kruskal-Wallis test for comparison of more then two samples). (x) Statistically significant difference as compared with cells stimulated with huIgA adsorbed to NC particles in the absence of PP1 (P = .01, Mann-Whitney U test). (+) Statistically significant inhibition of cytokine release after stimulation with huIgA adsorbed to NC particles (P < .025, Student's t-test for paired samples).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.383/3/m_blod4024206.jpeg?Expires=1769821944&Signature=piQHkpRe9aIwbuRcMR0O-y32XGEWI3vGK4rrjL12Y0OidZUV1iW8sMafNFbOuKvIIqFVHgijgGjQ75J5tq48IXxIcLuYsAqI2Di4f4WcQ-xljVxiJUaKHKhRAH-IZUJI~WXOAVagAU9d-0Azx48a10cUekFVAn~~HLmLkj-c-uQPGJ3AtHQAdTQXbx7t5OxOiWgw5TyvWy7MuRML9yQW37fIvkGTUWwvSydJt7MYYh2jc3a8yjyo~cWO01PfoqYAwJXfUQgEUY-F7RvvmSFDQnhE9y1mdwW-wICsNMm8bsJe-YOorf3n9~iyLeh2u4NjiGznRwZg2JSLZwciflp~tg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Activation of THP-1 cells with MoAb My43 and huIgA induces tyrosine phosphorylation of cellular proteins. THP-1 cells (5 × 105/sample) were activated at 37°C for 5 minutes by the addition of 5 μg/mL Mo2 (anti-CD14, lane 1), My43 cell culture supernatant 1:2 (anti-CD89, lane 2), 100 μg/mL huIgA (lane 3), 100 μg/mL huIgA + 100 μg/mL F′2RAhuIgA (lane 4), 100 μg/mL F′2RAhuIgA (lane 5), or NC particles (1 mg/sample) incubated in PBS containing different concentrations of huIgA, 3 mg/mL (lane 6), 1 mg/mL (lane 7), 0.33 mg/mL (lane 8), 0.11 mg/mL (lane 9), and 0.037 mg/mL (lane 10), and NC particles incubated in PBS containing 5% BSA only (lanes 11 and 12). Cellular proteins were isolated, separated by SDS-PAGE, and transferred to NC sheets as described in the experimental section. Immunoblotting of tyrosine-phosphorylated proteins was performed with the MoAb 4G10 followed by development with ECL as described (A). The immunoblot was then stripped and reprobed with a rabbit polyclonal anti-Syk antibody (lanes 1 through 11 in [B]) or control rabbit polyclonal IgG (lane 12 in [B]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.383/3/m_blod4024201.jpeg?Expires=1769821945&Signature=HBXyAWFZhpAT2MjWh14gK7BHvy3cbB6pg1161zHZCaCnAndRMJ~AdYTl7VDKNHMEoiaKnT-R4HpS7TkRZSGQMSrucQHmKdjTcxloP19MlBHzTntlmk2GtKtTKiC9VO7ijfl-R6MnA63nK9u7Dyky~8512UK5uykwOQGDqZfzUC1akeIYWWVFoXHP1SoTzvEXJk51x4au3Hf0L7O6Jxfsd-k9~XnLus3W2EAq6SCnmyhDKf6SnVTx9KNx-ts336bZqR-5pH0RlE6L1uWl~Gu380XqkBvMTckchpfJu83Wort0Z5YPEyrxmPFwH-y8zyGne97TzkCM5NWQHdDDFAGUYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Src family PTK activity is required for induction of IL-1ra and IL-8 in THP-1 cells after cross-linking of FcαR by huIgA-adsorbed NC particles. THP-1 cells (3 × 106cells/mL/well) were stimulated for 24 hours with huIgA adsorbed to nitrocellulose particles (IgA-NC); parallel cultures were incubated in the presence of corresponding concentrations of soluble huIgA or BSA-adsorbed nitrocellulose particles (NC). In (A) and (B), cells were cultured in complete medium (Med) or in complete medium containing PMB (final concentration, 10 μg/mL) or PP1 (final concentration, 100 μmol/L). In (C), THP-1 cells were stimulated for 24 hours with huIgA adsorbed to NC particles (final concentration of huIgA, 100 μg/mL) in the presence of PP1 at the indicated concentrations. Release of IL-1ra and IL-8 was examined in cell-free supernatants as described in Materials and Methods. Results are expressed as nanograms per milliliter (mean ± SEM of 3 experiments [A] or 6 experiments [B]) or (C) as a percentage of control (mean ± SEM of 3 experiments) relative to the cytokine release observed in cells stimulated with huIgA-particles in the absence of PP1 (cytokine release, in nanograms per milliliter, mean ± SEM: IL-1ra, 7.99 ± 0.84; IL-8, 1.41 ± 0.04). (*) Statistically significant difference between cultures stimulated with huIgA-NC and cells cultured in the presence of huIgA or NC alone (both in the presence or absence of polymyxin B, 10 μg/mL;P<0.05, Newman-Keuls multiple comparisons test; P = .004, analysis of variance). (#) Statistically significant difference as compared with cultures containing soluble huIgA or NC particles alone (P < .01, Kruskal-Wallis test for comparison of more then two samples). (x) Statistically significant difference as compared with cells stimulated with huIgA adsorbed to NC particles in the absence of PP1 (P = .01, Mann-Whitney U test). (+) Statistically significant inhibition of cytokine release after stimulation with huIgA adsorbed to NC particles (P < .025, Student's t-test for paired samples).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.383/3/m_blod4024206.jpeg?Expires=1769821945&Signature=v7GpcWaRzw02dXU-eo-F8ZT~PoFZ20Hgr17GVEuP0lTqvMmas8KEXQa0Fn9rSJb7jMSIYoxE0UyIbVvci2MzwtMRarsCJbitLv1dvJGd4HyQEA4J6-VhLFhDn32326nvwvzVD~PPTF4ZuRVXt8rOaHyOOlZs6XjErtUtfh8txHRhve6jZcb~NuCdmqEdX1LZmL5YzYiAUHl5dhYSp~Uh8qDS3b3JvdykgFAhLMHwRoY10El2rEMTjJifMICVoKZi2eS7TsX3kQLFuCWCoFty1Scz6TqwbrjwN5XnD~tMRK42AyOqYN1XXehqJ6ModRrmS2io23M2Ym7X807zgRu3QQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)