Abstract
Interleukin-1β (IL-1β) is a pleiotropic proinflammatory cytokine. Mechanisms leading to its secretion include not only release of newly synthesized protein, but also cleavage of a preformed immature precursor protein into an active secretory form by the intracellular protease caspase-1 (formerly termed IL-1–converting enzyme [ICE]). Caspase-1 belongs to a rapidly growing family of cysteine proteases with substrate specificity for aspartate involved in cellular apoptosis. We have used an assay determining the caspase-1 activity based on cleavage of a fluorogenic peptide substrate to elucidate its role in lipopolysaccharide (LPS)-induced secretion of IL-1β. We show that LPS induces moderate caspase-1 activity in the monocytic cell line THP-1, in freshly isolated peripheral blood monocytes, and in human umbilical vein endothelial cells (HUVECs) in a time- and dose-dependent fashion. Caspase-1 activation by LPS was associated with cleavage of the IL-1β precursor protein that was followed by release of the mature IL-1β protein in monocytic cells. In contrast, subsequent release of IL-1β by HUVECs was not significant. LPS-induced caspase-1 activation appeared not to result from modulation of caspase-1 transcript accumulation and inhibition of caspase-1 activity was accomplished by two specific inhibitors, YVAD-CHO and YVAD-CMK, capable of alleviating the release of mature IL-1β. Taken together, these results show that LPS moderately activates caspase-1 and that caspase-1 activation contributes to LPS induction of IL-1β secretion.
THE INTERLEUKIN-1 (IL-1) family of cytokines consists of structurally and functionally related molecules with pleiotropic activities involved in immune and inflammatory responses.1 IL-1β, one member of this family, has been implicated in a wide range of physiologic and pathologic processes, including mitogenic T-cell stimulation, wound healing, cellular adhesion, cytokine and eicosanoid production, inflammation, and sepsis.2-4 Other members of this family include IL-1α and a naturally occuring antagonist, referred to as IL-1 receptor antagonist (IL-1RA), which share sequence homologies and the usage of similar receptors.3 The IL-1 family members are produced by various cell types. Monocytes and macrophage are the major source of IL-1β. IL-1α and IL-1β target the same cellular receptor, which is referred to as the type I IL-1 receptor (IL-1RI). Engagement of IL-1RI leads to signal transduction, whereas the IL-1 receptor type II is thought to act as a decoy receptor.5 IL-1β is intracellularly cleaved and exported in large quantities upon stimulation of its producer cell, whereas IL-1α is synthesized in much lower quantities and does not appear to be actively secreted.6 7
IL-1β is synthesized as an inactive 31- to 33-kD precursor protein (pIL-1β) that is cleaved by a protease to generate the mature secretory protein (mIL-1β).8,9 A cysteine protease responsible for cleavage of IL-1β has been recently identified and termed IL-1β–converting enzyme (ICE).10,11 Several enzymes sharing sequence homology and the ability to cleave proteins at an Asparagine-site have been identified during the past years and were recently named caspases.12Besides ICE, which was assigned to the name caspase-1, nine other caspases are known, with several ones being involved in the regulation of programmed cell death (apoptosis). However, recently, it was shown that caspase-1 does not play a role in Fas-mediated apoptosis and intracellular IL-1β was found to be an inhibitor of apoptosis, leaving the role of caspase-1 during apoptosis unclear.13-15 Caspase-1 cleaves the 31-kD precursor protein (pIL-1β) at Asp116-Ala117, whereby it creates the 17.5-kD mature biologically active cytokine.16 It also cleaves the substrate at Asp27-Gly28 to yield products of 28 kD whose physiologic relevance is, however, presently not understood.11,16-18Although caspase-1 is unable to cleave IL-1α,19 it was, however, recently found to be capable of cleaving an inactive precursor of interferon-γ–inducing factor (IGIF; or IL-18) with high efficiency.20 Caspase-1 thus is also involved in regulation of interferon-γ production and subsequent activation of T cells. It furthermore was found recently that in vascular smooth muscle cells an inhibitor of caspase-1 is present that suppresses the release of mature IL-1β in the presence of ICE.21 The most potent reversible inhibitor of caspase-1 activity is the specific tetrapeptide aldehyde acyl-Tyr-Val-Ala-Asp-CHO or YVAD-CHO,22 which, by forming a thiohemiacetal with the active cysteine site, prevents release of mIL-1β by lipopolysaccharide (LPS)-activated blood monocytes.23YVAD-CHO selectively binds to the substrate-recognition sequence of caspase-1. Another potent and selective irreversible caspase-1 inhibitor is the α-substituted ketone chloromethylketone (YVAD-CMK) with the general structure, acyl-Tyr-Val-Ala-Asp-chloromethylketone. Inhibition of cysteine protease activity of caspase-1 is achieved by expulsion of the carboxylate group to form a thiomethylketone with the active site Cys 285.24
Unlike IL-1β, caspase-1 is constitutively expressed by its producer cells.25 The major form of caspase-1 represents a 45-kD precursor protein (p45) found in the cytoplasm that completely lacks cleavage activity. Active caspase-1 has been purified from THP-1 monocytic cells and has been shown to consist of an equimolar ratio of 10- and 20-kD proteins, termed p10 and p20.26 LPS stimulation of human monocytes or THP-1 cells fails to change the amount of p45 or its activity and does not induce appearance of detectable p20 caspase-1. cDNA cloning showed that both forms (p10 and p20) were found within the p45 precursor protein containing four cleavage sites, leading to formation of the protein subunits that are flanked by Asp-X bonds, so that it is conceivable to assume that the proenzyme is activated autocatalytically.16 Cleavage and activation of the native p45 caspase-1 precursor has been recently characterized by the use of specific inhibitors and antibodies recognizing various regions of caspase-1.27 Low-level expression and instability led to problems not only in isolation and purification of caspase-1, but also in estimating its activity in cells or cell lysates.25
One of the strongest inducers of IL-1 secretion is LPS (endotoxin), a component of cell walls of Gram-negative bacteria.28 LPS, bound by the LPS binding serum protein (LBP), is transported to its cellular receptor, the CD14 molecule, where it induces its specific cellular responses.29,30 Responses of CD14− cells, eg, endothelial cells, to LPS stimulation require the presence of soluble CD14 (sCD14).31,32 To examine LPS-induced caspase-1 activation in different cell types, we have used a caspase-1 bioassay employing a fluorogenic substrate that contains a specific peptide sequence of IL-1β, acyl-Tyr-Val-Ala-Asp-AMC.16 Cleavage activity of caspase-1 is shown by increased concentrations of the fluorogenic moiety of the substrate, aminomethylcoumarin (AMC), which can be visualized by fluorimetry. Using this assay, caspase-1 activity can be precisely monitored. We show that LPS induces biologically active caspase-1 in monocytic and endothelial cells, which spurs the generation and release of mature IL-1β.
MATERIALS AND METHODS
Culture and cell treatment.
Human umbilical vein endothelial cells (HUVECs) were obtained and characterized as described before.33,34 Cells were maintained and stimulated by LPS as indicated. Monocytes were obtained from healthy voluntary donors, separated by density gradient centrifugation, and washed with RPMI 1640 medium. Separated cells were resuspended in RPMI1640 containing 1% penicillin/streptomycin, 1% glutamine, 1% sodium pyruvate, and 10% human AB serum. Aliquots of 5 mL were incubated for 2 hours at 37°C in a humidified atmosphere of 5% CO2 in air. Nonadherent cells were discarded and adherent cells were washed with RPMI 1640 before stimulation with LPS (100 ng/mL) in RPMI. The human monocytic leukemia cell line THP-1 was obtained from the American Type Culture Collection (Rockville, MD). Cells were grown in RPMI 1640 medium containing 1% penicillin/streptomycin, 1% glutamine, 1% sodium pyruvate, and 10% fetal calf serum. To induce differentiation associated with increased surface expression of CD14, cells were exposed to 80 nmol/L DH-VD3 for 3 days at 37°C.35 Cells were stimulated with different concentrations of LPS using a medium containing 10% AB serum. Viability and function of cells were optimized by maintenance of cells in the log phase of growth and monitoring for greater than 95% viability as indicated by trypan blue exclusion.
Inhibition of enzyme activities.
Caspase-1 activity was inhibited in vivo and in vitro by addition of the reversible inhibitor acyl-Tyr-Val-Ala-Asp-CHO or the irreversible inhibitor acyl-Tyr-Val-Ala-Asp-chloromethylketone at concentrations of 0.2 to 20 μmol/L. The inhibitors were applied 2 hours before LPS stimulation (in vivo) and 5 minutes before caspase-1 substrate addition (in vitro), respectively. Viability of cells was monitored by trypan blue exclusion and calculated by the percentage of trypan blue-positive cells divided by protein content.
Lysis of cells.
After washing with ice-cold phosphate-buffered saline (PBS) nonadherent cells were resuspended at 106 cells/100 μL of ice-cold lysis buffer, and adherent cells were scraped off the plate into 100 μL lysis buffer/10 cm2 culture plate surface. Lysis buffer consisted of 20 mmol/L Tris-acetate, pH 7.5, 1% Triton X-100, 0.1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L Na3VO4, 10 mmol/L Na-α-glycerophosphate, 50 mmol/L NaF, 5 mmol/L Na-pyrophosphate, 1 mmol/L benzamidine, 270 mmol/L sucrose, 1 mmol/L phenylmethyl sulfonyl fluoride, and 20 μmol/L leupeptin. Lysates were placed on ice for 20 minutes, followed by centrifugation at 10,000g for 15 minutes at 4°C. The postmitochondrial supernatant fraction was removed, frozen in liquid nitrogen, and stored in aliquots at −80°C. Protein concentration of samples was determined by the Bradford method using bovine serum albumin (BSA) as standard.36
Electrophoresis and immunoblotting.
Protein samples of 50 μg were prepared for electrophoresis as described.34 Samples were separated on 12% or 15%, respectively, sodium dodecyl sulfate (SDS)-polyacrylamide gels using a buffer system as previously described.37 After electrophoresis, the gel was soaked in transfer buffer containing 48 mmol/L Tris, 39 mmol/L glycine, and 0.04% SDS for several minutes and the proteins were transferred to Hybond-C extra membranes by semidry blotting. Membranes were blocked overnight at 4°C with 5% BSA in Tris-buffered saline (TBS) containing 0.1% Tween 20 (TBST). They were washed intensively according to the manufacturer's instructions (Amersham, Braunschweig, Germany), incubated with polyclonal rabbit antihuman IL-1β antiserum, and incubated for 2 hours at room temperature. After washing, membranes were incubated with horseradish peroxidase-linked protein A (protein A-POD) in TBST (1:10,000) containing 1% BSA for 90 minutes at room temperature. After a final washing, blots were developed using the ECL system (Amersham) and immunoreactive proteins were visualized on film.
Quantitation of mIL-1β levels by enzyme immunoassay (EIA) and immunoprecipitation of IL-1β.
The TiterZyme IL-1β EIA kit (Biermann GmbH Diagnostica, Bad Nauheim, Germany) was used for quantitative determination of human IL-1β levels in cell culture supernatants. This assay was performed according to manufacturer's guidelines. A total of 1.5 mL supernatant also was incubated with 15 μg of a polyclonal anti–IL-1β antibody (Genzyme, Cambridge, MA), followed by protein A/G plus agarose (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) addition and centrifugation according to the manufacturer's instructions. The pellet was taken up in sample buffer and run on a 15% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), followed by a IL-1β ECL Western blot as described above.
Measurement of caspase-1 activity.
The assay measuring caspase-1 activity in cell lysates is based on published protocols.16,26 38 Test volumes of 100 μL consisted of 100 μg protein in a final buffer of 50 mmol/L Tris-acetate, pH 7.4, containing 1 mmol/L dithiothreitol, 0.5 mmol/L EDTA, and 20% glycerol. Ten microliters of a stock solution of the acetylated and AMC-conjugated peptide substrate, acetyl-Tyr-Val-Ala-Asp-7-amino-4-methyl-coumarin (1 μg substrate/μL dimethyl sulfoxide), was added to the test tubes. The final concentration was 150 μmol/L. Ten minutes after incubation at 37°C, the reaction was terminated by quick-freezing in liquid nitrogen. Triplets of samples and the substrate control containing no protein were thawed immediately before recording fluorescence intensity. The reaction product AMC was detected at λex = 350 nm and λem = 435 nm. The absorbance was correlated to caspase-1 concentration by using a standard curve of AMC. Specific activity was read as units per milligram of protein. One unit was defined as an activity that releases 1 pmol AMC per minute.
Statistical analysis of the results.
Results shown are representative of at least three independent experiments. Statistical analysis was performed at a level of probability of .01 < P < .05 (caspase-1 measurements) orP < .02 (measurements of IL-1β release), respectively, by using the Student's t-test.
Reagents.
L-glutamine, penicillin, streptomycin, and PBS Dulbecco's solution were obtained from GIBCO BRL, Life Technologies GmbH (Eggenstein, Germany). RPMI1640 medium, sodium pyruvate, and EDTA were purchased from Biochrom KG (Berlin, Germany). The anti-CD14 monoclonal antibody MY4 was from Coulter Electronics GmbH (Krefeld, Germany). Horseradish peroxidase-linked protein A, Hybond-C extra membranes and Rainbow colored protein molecular weight markers were obtained from Amersham-Buchler (Braunschweig, Germany). Salmonella minnesota Re595 LPS, sodium fluoride, sodium-orthovanadate, fetal calf serum, and human AB serum were from Sigma-Aldrich (Deisenhofen, Germany). Acyl-Tyr-Val-Ala-Asp-AMC (YVAD-AMC), acyl-Tyr-Val-Ala-Asp-CHO (YVAD-CHO), and acyl-Tyr-Val-Ala-Asp-chloromethylketone (YVAD-CMK) were purchased from Bachem Biochemica GmbH (Heidelberg, Germany). 7-amino-4-methylcoumarin (AMC) was from Fluka (Neu-Ulm, Germany). Rabbit antihuman IL-1β antibodies were from Biermann GmbH Diagnostica (Bad Nauheim, Germany) or Genzyme Corp (Cambridge, MA). 1,25-dihydroxyvitamin D3 (DH-VD3) was obtained from Biomol (Hamburg, Germany). All other chemicals were of analytical grade and were purchased from Carl Roth GmbH & Co (Karlsruhe, Germany).
RESULTS
LPS-induced caspase-1 activation in DH-VD3-treated THP-1 cells requires the presence of serum.
In the first set of experiments aimed at assaying for caspase-1 activity, the fluorescence intensity of the caspase-1 cleavage product AMC was monitored to obtain a linear standard curve (not shown). The monocytic THP-1 cell line was prestimulated with DH-VD3 to induce differentiation towards a macrophage-like phenotype and to induce surface expression of the cellular LPS receptor CD14. Cells were then stimulated with 100 ng/mL LPS in the absence or presence of 10% human AB-serum. Caspase-1 activity was not detectable in lysates of uninduced THP-1 cells or in lysates of THP-1 cells that were cultured in the absence of serum; however, it was readily detectable after incubation of DH-VD3–pretreated cells in a medium containing serum and 100 ng/mL LPS for 18 hours (not shown)
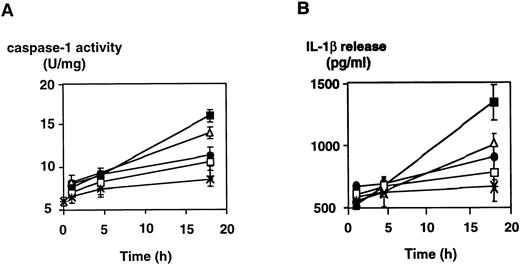
LPS-induced caspase-1 activity is concentration- and time-dependent and is associated with intracellular accumulation and release of mature IL-1β.
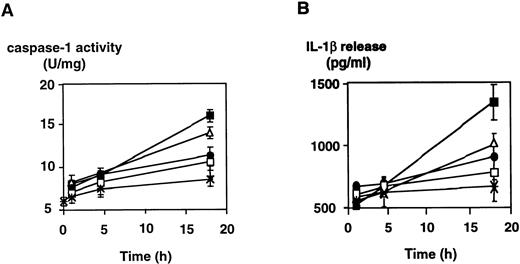
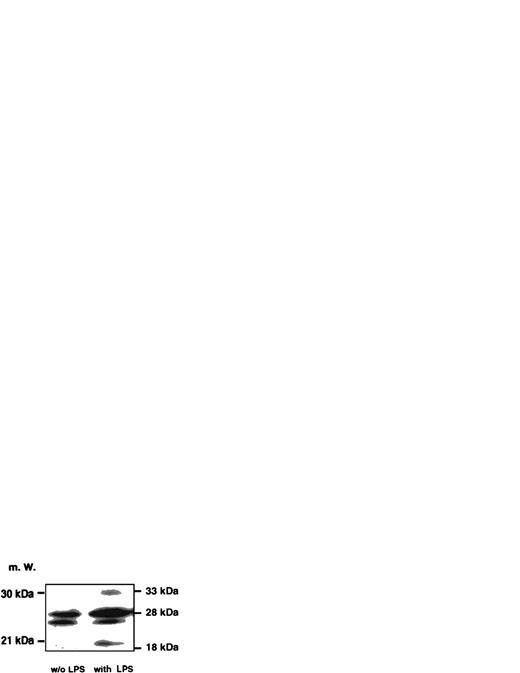
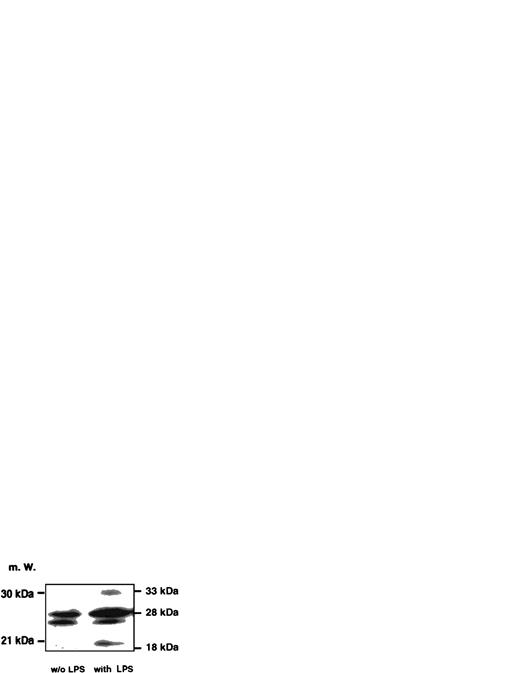
Induction of caspase-1 activity in DH-VD3–pretreated THP-1 cells was time-dependent and dependent on the concentrations of LPS used. As shown in Fig 1, the addition of LPS to THP-1 cell cultures increases caspase-1 activity, with 100 ng LPS/mL being the optimum stimulatory concentration. Further increase of the LPS concentration was not associated with better inducibility of caspase-1. Time- and dose-dependency of caspase-1 induction also correlated with levels of IL-1β detectable in supernatants of THP-1 cells as assessed by enzyme-linked immunosorbent assay (ELISA; Fig 1B). To examine whether LPS-induced activation of caspase-1 was also associated with increases of intracellular levels of mature IL-1β, Western blot analysis was performed using an anti–IL-1β antibody and cell lysates of stimulated and nonstimulated THP-1 cells. As shown in Fig 2, the addition of LPS led to an increase of both cleavage products of caspase-1, the 18-kD mature IL-1β protein, and the 28 kD fragment. An increase of the 31/33-kD precursor protein was also observed, indicating that LPS was inducing both synthesis of the IL-1β precursor protein and activation of caspase-1, leading to enhanced cleavage of the precursor.
Dose- and time-dependent activation of caspase-1 and induction of IL-1β secretion by LPS in the monocytic cell line THP-1. DH-VD3–pretreated THP-1 cells were stimulated with increasing concentrations of LPS (S minnesota Re595) as indicated. (A) Cells were lysed at the time points indicated and caspase-1 activity was assessed as described in the Materials and Methods. (B) In parallel, cell-culture supernatants were collected and assayed for IL-1β by ELISA. Shown are mean values of three independent experiments ± SD. (×) Without LPS; (□) 1 ng/mL LPS; (•) 10 ng/mL LPS; (▵) 100 ng/mL LPS; (▪) 1,000 ng/mL LPS.
Dose- and time-dependent activation of caspase-1 and induction of IL-1β secretion by LPS in the monocytic cell line THP-1. DH-VD3–pretreated THP-1 cells were stimulated with increasing concentrations of LPS (S minnesota Re595) as indicated. (A) Cells were lysed at the time points indicated and caspase-1 activity was assessed as described in the Materials and Methods. (B) In parallel, cell-culture supernatants were collected and assayed for IL-1β by ELISA. Shown are mean values of three independent experiments ± SD. (×) Without LPS; (□) 1 ng/mL LPS; (•) 10 ng/mL LPS; (▵) 100 ng/mL LPS; (▪) 1,000 ng/mL LPS.
Western blot analysis probing IL-1β in cell lysates of unstimulated and LPS-stimulated THP-1 cells. An amount of 50 μg/lane of cell lysates obtained from DH-VD3–pretreated THP-1 cells was blotted and incubated with an anti–IL-1β antibody as described in the Materials and Methods. The left panel represents lysates of unstimulated cells, whereas the right panel depicts lysates of cells stimulated with 100 ng/mL of S minnesota Re 595 LPS. Marked are sizes of the IL-1β precursor protein (33 kD) and the caspase-1 IL-1β cleavage products of 28 kD and 18 kD. The 18-kD fragment represents the mature secretory IL-1β (mIL-1β).
Western blot analysis probing IL-1β in cell lysates of unstimulated and LPS-stimulated THP-1 cells. An amount of 50 μg/lane of cell lysates obtained from DH-VD3–pretreated THP-1 cells was blotted and incubated with an anti–IL-1β antibody as described in the Materials and Methods. The left panel represents lysates of unstimulated cells, whereas the right panel depicts lysates of cells stimulated with 100 ng/mL of S minnesota Re 595 LPS. Marked are sizes of the IL-1β precursor protein (33 kD) and the caspase-1 IL-1β cleavage products of 28 kD and 18 kD. The 18-kD fragment represents the mature secretory IL-1β (mIL-1β).
LPS induces moderate caspase-1 activity in freshly isolated peripheral blood monocytes and HUVECs, correlating with release of mature IL-1β in monocytes only.
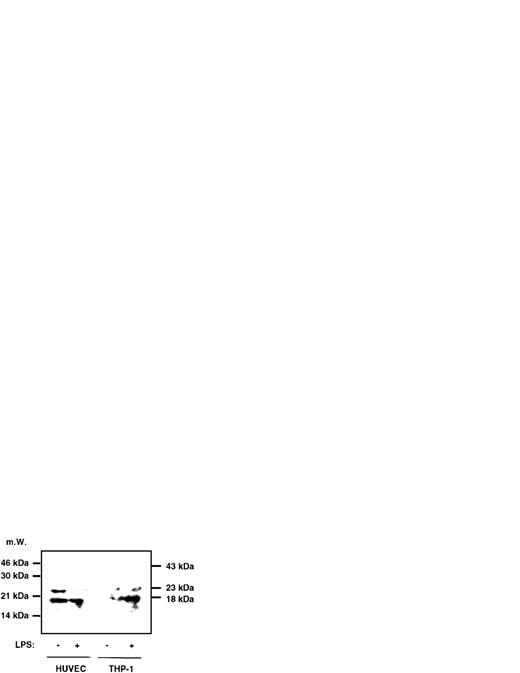
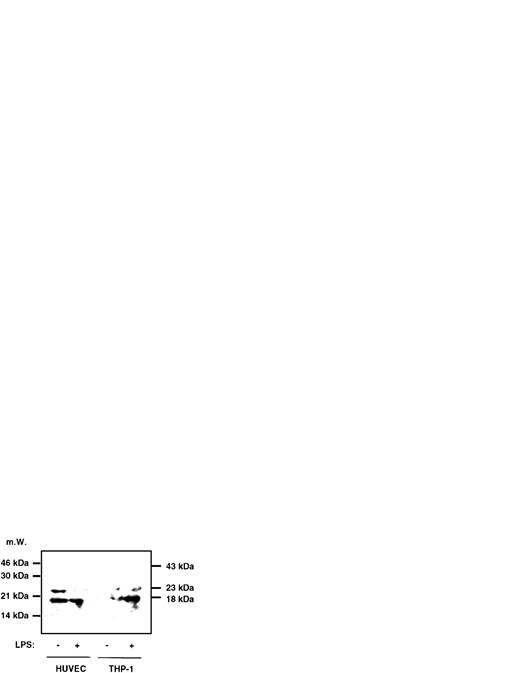
To assess whether LPS induces caspase-1 activity also in freshly isolated monocytes or in LPS-responsive, yet CD14−cells such as endothelial cells, experiments were performed with freshly isolated peripheral blood-derived human monocytes and HUVECs. The results, which are shown in Fig 3, indicate that, in monocytes, an approximately twofold, significant increase of caspase-1 activity can be achieved by stimulating cells with LPS and that this increase is paralleled by a twofold enhanced IL-1β secretion (Fig3A). In HUVECs, constitutive levels of IL-1β release could not be significantly enhanced by LPS. However, caspase-1 activity could be moderately increased by addition of LPS, which also was statistically significant (Fig 3B). To determine whether mature IL-1β or proIL-1β was released into the supernatant of monocytes and HUVECs upon stimulation by LPS, we performed an immunoprecipitation experiment, followed by Western blotting. In both cell types, no precursor IL-1β molecule, which would migrate at 31 kD, could be detected (Fig 4). However, mature IL-1β at 18 kD gave rise to a signal, and the previous result of an inducibility in monocytes but not in HUVECs was confirmed in this assay.
LPS induces (▧) caspase-1 activity and (▪) mIL-1β release in freshly isolated peripheral blood-derived human monocytes and HUVECs. Cells were isolated and cultured in vitro as described in the Materials and Methods and stimulated with 100 ng/mL of LPS (S minnesota Re 595). (A) Freshly isolated human monocytes exhibited a constitutive level of IL-1β release of approximately 950 pg/mL and a constitutive caspase-1 activity of 8 U/mg that both remained constant over time when cells were not stimulated (not shown). Monocytes were assayed for caspase-1 activity of lysates and mIL-1β release into the culture supernatant at the time points indicated. (B) HUVECs were assayed for caspase-1 activity of lysates and mIL-1β release into the culture supernatant after 24 hours in the absence and presence of LPS. Constitutive levels of IL-1 release and caspase-1 activity did not change over time in the absence of LPS (not shown). Shown are the mean values of three independent experiments ± SD. Asterisks indicate a statistically significant difference compared with controls ([A] were considered significant with .01 < P < .02 and [B] with P < .05, respectively).
LPS induces (▧) caspase-1 activity and (▪) mIL-1β release in freshly isolated peripheral blood-derived human monocytes and HUVECs. Cells were isolated and cultured in vitro as described in the Materials and Methods and stimulated with 100 ng/mL of LPS (S minnesota Re 595). (A) Freshly isolated human monocytes exhibited a constitutive level of IL-1β release of approximately 950 pg/mL and a constitutive caspase-1 activity of 8 U/mg that both remained constant over time when cells were not stimulated (not shown). Monocytes were assayed for caspase-1 activity of lysates and mIL-1β release into the culture supernatant at the time points indicated. (B) HUVECs were assayed for caspase-1 activity of lysates and mIL-1β release into the culture supernatant after 24 hours in the absence and presence of LPS. Constitutive levels of IL-1 release and caspase-1 activity did not change over time in the absence of LPS (not shown). Shown are the mean values of three independent experiments ± SD. Asterisks indicate a statistically significant difference compared with controls ([A] were considered significant with .01 < P < .02 and [B] with P < .05, respectively).
Immunoprecipitation followed by Western blotting of supernatants of HUVECs and THP-1 cells. HUVECs and THP-1 cells were stimulated with 0.1 μg/mL LPS for 4 hours and 1.5 mL of the supernatant was immunoprecipitated with a polyclonal anti–IL-1β antiserum, followed by Western blotting as described in the Materials and Methods. No signal in any lane could be observed at 31 kD, the size of the IL-1β precursor molecule, whereas at 18 kD a signal can be seen in HUVECs and stimulated THP-1 cells. At 23 kD, the lower band of Ig can be observed. The position of the Ig heavy chain is 43 kD.
Immunoprecipitation followed by Western blotting of supernatants of HUVECs and THP-1 cells. HUVECs and THP-1 cells were stimulated with 0.1 μg/mL LPS for 4 hours and 1.5 mL of the supernatant was immunoprecipitated with a polyclonal anti–IL-1β antiserum, followed by Western blotting as described in the Materials and Methods. No signal in any lane could be observed at 31 kD, the size of the IL-1β precursor molecule, whereas at 18 kD a signal can be seen in HUVECs and stimulated THP-1 cells. At 23 kD, the lower band of Ig can be observed. The position of the Ig heavy chain is 43 kD.
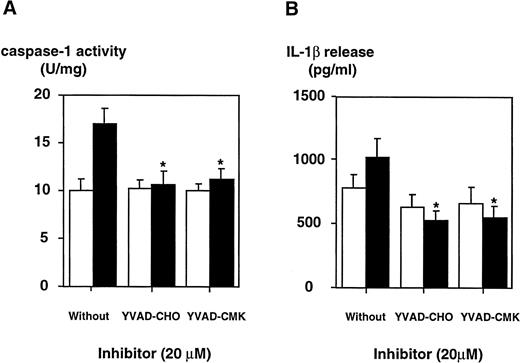
Specific caspase-1 inhibitors block LPS-induced activation of caspase-1 and IL-1β release in THP-1 cells.
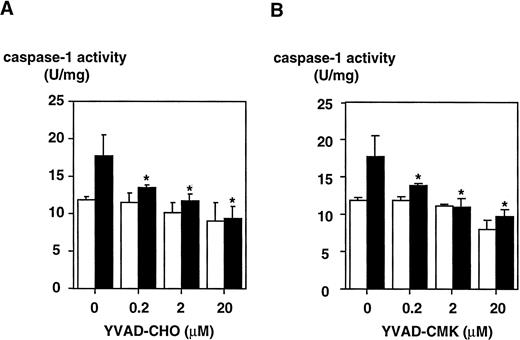
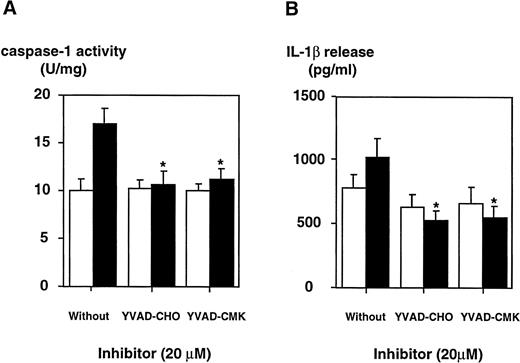
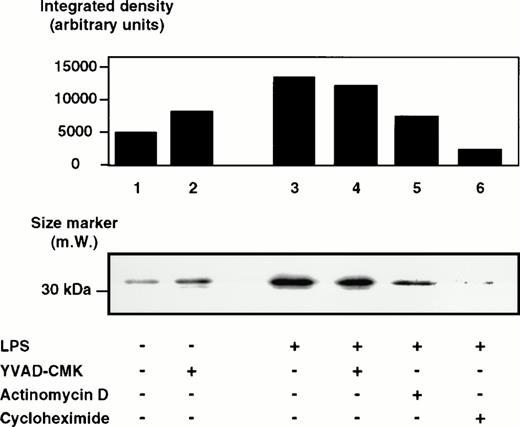
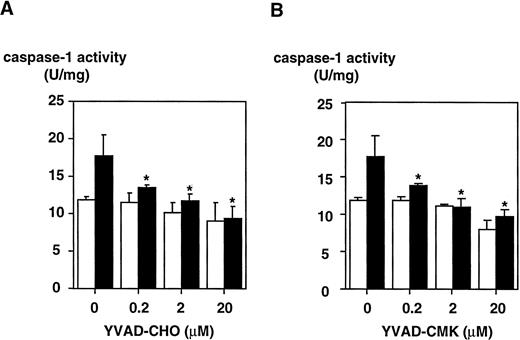
To determine whether LPS effects on the caspase-1 activation are specific, two peptide inhibitors were instrumental, a reversible inhibitor, YVAD-CHO, and an irreversible one, YVAD-CMK. To exclude cytotoxic effects, cell viability was assessed by trypan blue exclusion and no cytotoxicity of both inhibitors could be observed (Table 1). YVAD-CHO and YVAD-CMK were added in vitro to lysates of LPS-induced and control cells, respectively, and caspase-1 activity was assessed (Fig 5). Caspase-1 activity was found to be decreased in lysates of LPS-induced and control cells by both inhibitors in a concentration-dependent fashion. At concentrations of 0.2 μmol/L, LPS-induced increase in caspase-1 activity was blocked significantly by both inhibitors (.01 < P < .02 for YVAD-CHO and .01 < P < .05 for YVAD-CMK, respectively). This condition reflects a molar ratio of 1:750 (inhibitor to substrate). Additionally, caspase-1 inhibitors were added to the culture medium of cells, and caspase-1 activity as well as IL-1β release induced by LPS was measured. Figure 6shows that treatment of THP-1 cells with caspase-1 inhibitors in vivo alleviates both LPS-induced caspase-1 activity and IL-1β release significantly (P < .01). To exclude that caspase-1 inhibitors affected IL-1β precursor synthesis, Western blot analyses were performed in the absence or presence of YVAD-CMK. Figure7 shows that the addition of the caspase-1 inhibitor failed to significantly diminish levels of the IL-1β precursor protein. On the contrary, addition of YVAD-CMK led to a slight increase in constitutive levels of intracellular precursor protein. In the presence of LPS, YVAD-CMK slightly reduced IL-1 precursor levels. As controls, the transcriptional inhibitor of protein synthesis actinomycin D and the translation inhibitor cycloheximide strongly suppressed synthesis of the IL-1β precursor protein. In summary, it was shown that LPS-induced caspase-1 actvity can be blocked specifically by the inhibitors YVAD-CHO and YVAD-CMK.
Inhibition of caspase-1 activity by a reversible (YVAD-CHO) and an irreversible (YVAD-CMK) caspase-1 inhibitor measured in lysates of DH-VD3–pretreated THP-1 cells. DH-VD3–pretreated THP-1 cells were stimulated with 100 ng/mL of S minnesota Re 595 LPS as indicated. Cell lysates were prepared and caspase-1 activity was assessed 18 hours after stimulation as described in the Materials and Methods. The inhibitors were added in vitro to the lysates 5 minutes before measurement of caspase-1 before adding YVAD-AMC. (A) Results obtained with the reversible caspase-1 inhibitor YVAD-CHO. (B) Results obtained with the irreversible caspase-1 inhibitor YVAD-CMK. Shown are mean values of three independent experiments ± SD. Differences in (A) were significant with .01 < P < .02 and in (B) with .01 <P < .05, respectively. (□) Without LPS; (▪) with LPS.
Inhibition of caspase-1 activity by a reversible (YVAD-CHO) and an irreversible (YVAD-CMK) caspase-1 inhibitor measured in lysates of DH-VD3–pretreated THP-1 cells. DH-VD3–pretreated THP-1 cells were stimulated with 100 ng/mL of S minnesota Re 595 LPS as indicated. Cell lysates were prepared and caspase-1 activity was assessed 18 hours after stimulation as described in the Materials and Methods. The inhibitors were added in vitro to the lysates 5 minutes before measurement of caspase-1 before adding YVAD-AMC. (A) Results obtained with the reversible caspase-1 inhibitor YVAD-CHO. (B) Results obtained with the irreversible caspase-1 inhibitor YVAD-CMK. Shown are mean values of three independent experiments ± SD. Differences in (A) were significant with .01 < P < .02 and in (B) with .01 <P < .05, respectively. (□) Without LPS; (▪) with LPS.
The inhibition of caspase-1 activity by specific inhibitors in DH-VD3–pretreated THP-1 cells is paralleled by a decrease in the release of mIL-1β. DH-VD3–pretreated THP-1 cells were stimulated with 100 ng/mL of S minnesota Re 595 LPS in the presence of the caspase-1 inhibitors YVAD-CHO and YVAD-CMK, respectively, as indicated. Cell lysates and supernatants were prepared 18 hours after stimulation. (A) Caspase-1 activity assessed as described in the Materials and Methods. (B) Amount of IL-1β released as assessed by ELISA. Shown are mean values of four independent experiments ± SD. Differences are significantly with P < .01. (□) Without LPS; (▪) with LPS.
The inhibition of caspase-1 activity by specific inhibitors in DH-VD3–pretreated THP-1 cells is paralleled by a decrease in the release of mIL-1β. DH-VD3–pretreated THP-1 cells were stimulated with 100 ng/mL of S minnesota Re 595 LPS in the presence of the caspase-1 inhibitors YVAD-CHO and YVAD-CMK, respectively, as indicated. Cell lysates and supernatants were prepared 18 hours after stimulation. (A) Caspase-1 activity assessed as described in the Materials and Methods. (B) Amount of IL-1β released as assessed by ELISA. Shown are mean values of four independent experiments ± SD. Differences are significantly with P < .01. (□) Without LPS; (▪) with LPS.
Western blot analysis of pIL-1β in lysates of DH-VD3–pretreated THP-1 cells that were treated with various inhibitors before LPS stimulation. DH-VD3–pretreated THP-1 cells were stimulated with 100 ng/mL of S minnesota Re 595 LPS in the absence or presence of various inhibitors, as indicated. Total lysate (30 μg protein/lane) was fractionated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted. The 33-kD IL-1β precursor protein reacts with an anti–IL-1β antibody as described in the Materials and Methods. Concentrations of the inhibitors used were 5 ng/mL actinomycin D, 5 μg/mL cycloheximide, and 20 μmol/L for YVAD-CHO and YVAD-CMK.
Western blot analysis of pIL-1β in lysates of DH-VD3–pretreated THP-1 cells that were treated with various inhibitors before LPS stimulation. DH-VD3–pretreated THP-1 cells were stimulated with 100 ng/mL of S minnesota Re 595 LPS in the absence or presence of various inhibitors, as indicated. Total lysate (30 μg protein/lane) was fractionated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted. The 33-kD IL-1β precursor protein reacts with an anti–IL-1β antibody as described in the Materials and Methods. Concentrations of the inhibitors used were 5 ng/mL actinomycin D, 5 μg/mL cycloheximide, and 20 μmol/L for YVAD-CHO and YVAD-CMK.
DISCUSSION
Induction of cytokine production by LPS has attracted much attention in the past because of the profound stimulatory effects that LPS exerts on cells of the monocyte/macrophage and the endothelial cell lineages. Although this stimulatory effect may have protective effects in the physiologic host response to inflammatory challenges, if unbalanced, it may contribute to acute and chronic pathologic states of the organism. For instance, a dramatic induction of IL-1β occurs during inflammation and sepsis and is caused by LPS. The regulation of this process has been studied by several groups, including our own.3,34,39 Secretion of IL-1β by monocytes is tightly regulated, and the amount of IL-1β secreted by these cells is much higher than that produced by any other cell type in the body.39-41 Processing of the precursor of IL-1β to the fully active and secretory cytokine is regulated by the specific cystein protease caspase-1. However, up to now, regulation of caspase-1 activity has not been studied in greater detail because of technical difficulties in measuring caspase-1 activity.18,25,41,42
It has been shown that LPS enhances both the rate of transcription of the IL-1β gene and accumulation of IL-1β transcripts. However, enhanced gene expression alone cannot account for the rapid and strong induction of IL-1β release by LPS seen in several cell types. We show here that activation of caspase-1 is another mechanism whereby LPS can induce IL-1β release. According to our results, LPS acts most likely on both levels: transcripitonal induction of the IL-1β gene, followed by an intracellular increase of the 30-kD IL-1β precursor, which is paralleled by a moderate activation of caspase-1, leading to cleavage and release of the mature IL-1β protein. Activation of caspase-1 results from a catalytic event, deliberating an inactive form of caspase-1 into an active one. Reverse transcriptase-polymerase chain reaction experiments performed by us suggest that a transcriptional induction of the caspase-1 gene does not play a role during LPS-induced caspase-1 activation (data not shown). Others have recently shown that Langerhans cells and epidermal-derived dendritic cell lines constitutively display high levels of caspase-1 mRNA that are only moderately enhanced by LPS. Our data are also consistent with studies showing that LPS-induced and noninduced THP-1 cells contain comparable levels of caspase-1 protein, indicating that LPS does not effect levels of caspase-1.25
It has been suggested that caspase-1 is largely associated with the cell membrane and enhances directly the export of mature IL-1β after cleavage of the precursor protein.18 This hypothesis is consistent with our observation that mIL-1β is found only in small quantities inside the cell, indicating that processing and secretion are temporarily linked. Our data also show that the kinetics of caspase-1 activation and secretion of IL-1β in monocytes, but not in endothelial cells, are similar, further suggesting that the state of caspase-1 activation in myeloid cells directly relates to secretion of IL-1β. In HUVECs, caspase-1 is also moderately induced; however, this induction does not lead to significant IL-1β release. Moreover, inhibition of caspase-1, which may have potential clinical ramifications as a tool during IL-1β–mediated processes, was shown by us to inhibit IL-1β release. Inhibition by two specific compounds, YVAD-CHO and YVAD-CMK, was almost as effective as the potent but toxic inhibitor of protein synthesis cycloheximide (data not shown). It was recently shown that caspase-1 (ICE) knock-out mice exhibit a striking resistance towards experimental induction of septic shock.43 This finding is supported by recent results showing that caspase-1 not only leads to cleavage and release of IL-1β but also of interferon-γ–inducing factor (IGIF; IL-18).20 Thus, specific caspase-1 inhibitors that act selectively and are less toxic may be valuable tools to suppress caspase-1 activity and subsequent release of a number of cytokines and thus may be useful in interfering with the onset and course of sepsis and septic shock. However, in our experimental approach, we were able to inhibit only approximately 50% of LPS-induced caspase-1 activity, and it has to be shown in animal experiments whether this inhibition is sufficient to block the LPS effects in vivo. Experiments addressing this question are currently underway in our laboratory.
Activation of caspase-1–like proteases has also been linked to the induction of programmed cell death (apoptosis).44-46 During this process, activation of caspases ultimately leads to the cleavage of the poly-(ADP-ribose) polymerase (PARP) and thus to inhibition of DNA repair. Recent findings indicate that FAS-mediated apoptosis is mediated by other caspases, such as CPP32 or FLICE/Mach and not by caspase-1.47-49 On the other hand, it was recently found that apoptosis induced by infection, ie, human immunodeficiency virus infection of cells is strictly caspase-1–mediated.50Several investigators furthermore showed that LPS can induce apoptosis in different cell types.51-54 Whether LPS-induced activation of caspase-1 mediates programmed cell death of monocytes/endothelial cells needs further analysis. Nonetheless, there were no signs of apoptosis induction in the cells obseved over the observation period reported here. Completely elucidating the cellular events induced by LPS will aid in understanding inflammatory processes and may potentially lead to novel therapeutic strategies in sepsis and shock.
ACKNOWLEDGMENT
The authors thank Nicole Siegemund and Ina Krukenberg for their excellent technical assistance.
Supported by Grant No. Schu 828/1-4 from the Deutsche Forschungsgemeinschaft (DFG) and Grants No. 01KI9475 and 01KV9507 from the Bundesministerium für Bildung und Forschung (BMBF).
Address reprint requests to Ralf R. Schumann, MD, Institute of Microbiology and Hygiene, University Medical Center “Charité,” Dorotheen Str 96, D-10117 Berlin, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 3. LPS induces (▧) caspase-1 activity and (▪) mIL-1β release in freshly isolated peripheral blood-derived human monocytes and HUVECs. Cells were isolated and cultured in vitro as described in the Materials and Methods and stimulated with 100 ng/mL of LPS (S minnesota Re 595). (A) Freshly isolated human monocytes exhibited a constitutive level of IL-1β release of approximately 950 pg/mL and a constitutive caspase-1 activity of 8 U/mg that both remained constant over time when cells were not stimulated (not shown). Monocytes were assayed for caspase-1 activity of lysates and mIL-1β release into the culture supernatant at the time points indicated. (B) HUVECs were assayed for caspase-1 activity of lysates and mIL-1β release into the culture supernatant after 24 hours in the absence and presence of LPS. Constitutive levels of IL-1 release and caspase-1 activity did not change over time in the absence of LPS (not shown). Shown are the mean values of three independent experiments ± SD. Asterisks indicate a statistically significant difference compared with controls ([A] were considered significant with .01 < P < .02 and [B] with P < .05, respectively).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.577/3/m_blod4021503.jpeg?Expires=1768039686&Signature=HrZ4Mo~vgdHsNCqZoO5gsJVsUliP3Igni8VrCWeAikEV2haklIMFsOGKZzpjlw37SNLIgVVbSqU~kPYqKpVIrq4Ae5XMOLXjZ4NSQk0lkfsjN7jXEZ4z6FxwVGZCluAaZ8AdxcW768uannn1vSuOMe6iBLtVVsw5RjDFSbnyq4KTPtQz-BJIZBluVRMAEV8HJWjwwEuGW4el6HP6QbDkAtR~qryyodmdVLXakU~EHDa4Jt~NAcna8QuWHOzXisoFw6GDTb5K4yBr7MLcwhe~HHQphrzYg~3ZXqOUo2k2sAR6SNaPw8w3ZkGbtBkbQIYWa9LMarvZQD7tZ-wIviITXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 3. LPS induces (▧) caspase-1 activity and (▪) mIL-1β release in freshly isolated peripheral blood-derived human monocytes and HUVECs. Cells were isolated and cultured in vitro as described in the Materials and Methods and stimulated with 100 ng/mL of LPS (S minnesota Re 595). (A) Freshly isolated human monocytes exhibited a constitutive level of IL-1β release of approximately 950 pg/mL and a constitutive caspase-1 activity of 8 U/mg that both remained constant over time when cells were not stimulated (not shown). Monocytes were assayed for caspase-1 activity of lysates and mIL-1β release into the culture supernatant at the time points indicated. (B) HUVECs were assayed for caspase-1 activity of lysates and mIL-1β release into the culture supernatant after 24 hours in the absence and presence of LPS. Constitutive levels of IL-1 release and caspase-1 activity did not change over time in the absence of LPS (not shown). Shown are the mean values of three independent experiments ± SD. Asterisks indicate a statistically significant difference compared with controls ([A] were considered significant with .01 < P < .02 and [B] with P < .05, respectively).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.577/3/m_blod4021503.jpeg?Expires=1768421082&Signature=z2U2VulE768k6DInrCLJGkYUdi2Ft8~Iu6tmFSzieSITqqaHj0V0VFjoLBfkng~ptj~ImMZm8ehaRxDePQHIM19B8hO7w2UXxw77ocwohUCA~PQDldx6Fgw6ZyUSPY4MCSD3DgwgUegQ9qWePnhnw1-Z1H0OYSuav0HxsqZcvy4u8cdNrpUcNYq45-RiXjnryEhEi6adJ91gub9~To3l0VQjouGnRcQutMovJUHb7lrm3sDVSLZ6WXWvysaumFhCTUafGqR8qBxkdp-2sDo8-ezp2vx7O32wxkEP68DV9Vo5O3R6gtjce9IisuIyTKLf0dVrwCJEBqosshzHjPKxoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)