Abstract
Although human immunodeficiency virus (HIV)-infected subjects without acquired immunodeficiency syndrome have a high frequency of HIV-specific CD8 T lymphocytes, freshly isolated lymphocytes frequently lack detectable HIV-specific cytotoxicity. However, this effector function becomes readily apparent after overnight culture. To investigate reasons for T-cell dysfunction, we analyzed T-cell expression of the cytolytic protease granzyme A and of CD3ζ, the signaling component of the T-cell receptor complex. An increased proportion of CD4 and CD8 T cells from HIV-infected donors contain granzyme A, consistent with the known increased frequency of activated T cells. In 28 HIV-infected donors with mild to advanced immunodeficiency, a substantial fraction of circulating T cells downmodulated CD3ζ (fraction of T cells expressing CD3ζ, 0.74 ± 0.16 v 1.01 ± 0.07 in healthy donors; P < .0000005). CD3ζ expression is downregulated more severely in CD8 than CD4 T cells, decreases early in infection, and correlates with declining CD4 counts and disease stage. CD3ζ expression increases over 6 to 16 hours of culture in an interleukin-2–dependent manner, coincident with restoration of viral-specific cytotoxicity. Impaired T-cell receptor signaling may help explain why HIV-specific cytotoxic T lymphocytes fail to control HIV replication.
IN HUMAN immunodeficiency virus (HIV)-infected individuals who have not developed acquired immunodeficiency syndrome (AIDS)-opportunistic infections, there is a high frequency of circulating CD8+ T cells bearing T-cell receptors (TcRs) that recognize HIV-infected cells.1 As many as 1 in 100 circulating T cells may be capable of responding to a particular HIV peptide.2,3 Despite the vigorous expansion of antiviral CD8+ T cells, which can develop into effector cells that lyse HIV-infected targets and secrete soluble factors that suppress HIV replication, the cellular immune response is unable to control viral production. It has been estimated that as many as 10 billion virions are produced each day, even in asymptomatic infected individuals.4 5 The reasons for the failure of T-cell function could be because of many factors, including the known paucity of viral-specific CD4 helper cells that are important regulators of CD8 T-cell function, the state of chronic inflammation characteristic of HIV infection, or immune dysfunction in the setting of antigen excess.
When freshly isolated peripheral blood mononuclear cells (PBMCs) from HIV-infected donors are assayed for HIV-specific cytolytic activity, laboratories have differed widely in their detection of viral-specific cytotoxicity. Several early reports suggested that 80% to 90% of HIV-infected donor PBMCs had HIV-specific cytotoxic T lymphocyte (CTL) activity readily demonstrable above background.6-8 In these studies the cytotoxicity assay time ranged from 6 to 16 hours and the cut-off for positive activity was less than what has become the standard of 10% above lysis of wild-type vaccinia-infected cells. We found that in shorter cytotoxicity assays (4-hour Cr release), high levels of specific cytotoxicity by fresh PBMCs were uncommon but developed after in vitro culture in the presence of mitogen and interleukin-2 (IL-2).9
Recently we found that viral-specific cytolytic activity develops after overnight culture. This rapid increase in specific cytotoxicity, typically from undetectable above background to 10% to 30% HIV-specific cytotoxicity, as measured in a 4-hour 51Cr release assay at an effector:target ratio of 25 to 50:1, could not be explained by clonal expansion of viral-specific T cells in less than one day. This suggested that the circulating viral-specific CD8+ T cells were either precursor CTLs, which had not differentiated into mature effector CTLs containing cytotoxic granules, or were functionally defective. Because of the high frequency of activated circulating CD8 T cells10-12 and the high likelihood of a prior encounter with HIV antigen, the first hypothesis was unlikely and indeed proved not to be the case. Circulating CD8 and CD4 T cells in HIV-infected subjects compared with healthy donors have an unusually high percentage of cells containing cytolytic granules as measured by intracellular staining for the most abundant granule serine protease, granzyme A (grnA).
Cancer patients and tumor-bearing mice develop progressive immunodeficiency, which resembles in some ways that of HIV-infected patients, including decreased delayed type hypersensitivity, decreased T-cell cytolytic activity, and impaired lymphokine and proliferative responses. Lymphocytes infiltrating solid tumors (TILs) of various types and circulating lymphocytes in Hodgkin's disease and B-cell lymphoma patients have a defect in signaling by the TcR linked to abnormally low expression of CD3ζ.13-16 This suggested that a defect in CD3ζ expression might also occur in HIV-infected subjects.
The first step in T-cell activation by antigen-presenting cells is engagement of the TcR complex composed of a clonotypic dimeric TcR, required for antigen recognition, in noncovalent association with CD3, a multicomponent signal transduction complex. CD3 consists of CD3δ, CD3ε, and CD3γ chains and a ζ-chain–containing dimer, which may be a homodimer or heterodimer with η or Fcε receptor γ chain (reviewed in Weiss and Littman17). The ζ chain is also an important signaling molecule in natural killer (NK) cells, which do not express the TcR and other CD3 components. All components of the CD3 complex are required to transport the TcR to the T-cell surface,18 but the ζ chain differs from the other components in rapid cycling from the cell surface.19 CD3ζ is central to transmission of the TcR activation signal. Failure of signal transduction should lead to failure of effector T-cell function, including cytokine secretion and cytolysis.
In 28 HIV-infected donors with varying degrees of immunodeficiency, we found significant downmodulation of CD3ζ, especially in CD8 T cells. About a quarter of circulating T cells lacked expression of CD3ζ above background, and in cells that had some ζ expression, it was significantly downmodulated. However, upregulation of CD3ζ expression occurred over 6 to 16 hours in culture and coincided with restoration of viral-specific cytotoxicity.
MATERIALS AND METHODS
Subjects.
Subjects were healthy volunteers or HIV-1 seropositive patients of varied disease stages (Table 1). Nineteen subjects had had no clinical symptoms of HIV infection, and 6 subjects (601, 602, 604, 605, 6155, and 6159) had CD4 counts above 600/mm3. Six patients (216, 221, 228, 235, 354, and 355) met the Centers for Disease Control (CDC) criteria for AIDS.20 Subjects no. 601 and 6225 were recent seroconverters (3 and 8 months earlier, respectively) and subjects no. 602, 6155, and 6159 could be considered slow progressors. Informed consent was obtained from each subject. This study was approved by the Center for Blood Research Institutional Review Board. Samples were either freshly obtained or cryopreserved using a programmed cell freezer (Gordinier Model 9000; Gordinier, Roseville, MI). Flow cytometry results obtained from thawed cells were comparable with those from freshly isolated cells in two samples studied.
Flow cytometry.
For CD3ε staining, PBMCs (2 to 10 × 105/tube), isolated by Ficoll-Hypaque density centrifugation from heparinized blood, were suspended in 50 μL fluorescence-activated cell sorting (FACS) buffer (2% fetal calf serum [FCS], 0.2 mg/mL NaN3in phosphate-buffered saline [PBS]) to which 4 μL phycoerythrin (PE)-conjugated CD3ε SK7 monoclonal antibodies (MoAbs; Becton Dickinson, Mountain View, CA) or isotype-matched control was added. After incubation for 20 minutes at 4°C, cells were washed with 1 mL FACS buffer and resuspended in FACS buffer with 1% formaldehyde before analysis. For CD3ζ and grnA staining, cells were resuspended in 50 μL Hanks' balanced salt solution (HBSS) and permeabilized using the Caltag Laboratories (Burlingame, CA) Fix and Perm Kit according to the manufacturer's protocol. Fixed cells were incubated for 15 minutes at room temperature with either 2 μL CD3ζ MoAb 6B10.2 (Santa Cruz, Santa Cruz, CA), a 1:5 dilution of grnA CB9 MoAb culture supernatant,21 or 2.5 μL MsIgG1 isotype-matched control antibody (Coulter, Hialeah, FL). After washing with 5 mL HBSS, cells were stained with 2 μL PE-conjugated F(ab′)2 goat antimouse Ig (DAKO, Carpenteria, CA). For some samples, direct CD3ζ staining was performed with fluorescein isothiocyanate (FITC)-conjugated 6B10.2 (Santa Cruz) and elimination of the second step. After two further washes, cells were resuspended in PBS with 1% formaldehyde for analysis. Flow cytometry analysis was performed on a tightly gated lymphocyte population using FACScalibur (Becton Dickinson). Cellquest (Becton Dickinson) software was used to overlay and subtract the histograms of the control antibody-stained cells from the CD3-stained cells. For each sample the ratio of the number of cells staining for CD3ζ to those staining for CD3ε was calculated. CD3ζ three-color staining was done as previously described for CD3ζ single staining before the addition of 2 μL CD4-Cy5 (Pharmingen, San Diego, CA) and 2 μL CD8-FITC (Immunotech, Westbrook, ME), 4 μL CD20-FITC (Becton Dickinson), and 2 μL CD3-Cy5 (Immunotech), or IgG-FITC and -Cy5 conjugated controls. The samples were incubated for 15 minutes at 4°C, washed with 5 mL HBSS, and resuspended in PBS with 2% FCS and 1% formaldehyde for analysis.
Cell lines.
T-cell lines were generated by culture of PBMCs at 1 × 106/mL in T-cell medium (RPMI 1640 supplemented with 2 mmol/L glutamine, 2 mmol/L HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μmol/L β-mercaptoethanol) to which was added 10% FCS. In some cases, varying concentrations of recombinant human IL-2 (Cetus, Emeryville, CA) or antihuman IL-2–neutralizing antibody 5334.21 (R&D Systems, Minneapolis, MN) was added at the initiation of culture. Autologous B lymphoblastoid cell lines (B-LCLs) were generated for each subject using standard methods.
Cytotoxicity assay.
HIV-specific cytotoxicity of PBMCs, freshly isolated or after overnight culture, was analyzed by 4-hour 51Cr release assay against autologous B-LCL targets infected with vaccinia recombinant vectors encoding HIV genes as previously described.9 Target cells were infected with vaccinia vectors encoding lacZ (vSC8),env of the BH8 isolate of HIV-1IIIB (vPE16),gag of the HXB.2 subclone (vDK1), and all but the last 22 residues of HXB.2 RT (vCF21).6,9 22
RESULTS
HIV-specific cytotoxicity is greatly enhanced by overnight in vitro culture and the increase is IL-2 dependent.
Although antiviral HIV-specific cytotoxicity is detectable in freshly isolated PBMCs from some HIV-infected donors, more commonly, antiviral cytotoxicity only reaches significant levels after in vitro culture. In previous studies we measured large increases in viral-specific cytotoxicity after phytohemagglutinin stimulation and in vitro culture for 2 to 3 weeks in the presence of IL-2. To study the kinetics and IL-2 dependence of the emergence of viral-specific cytotoxic activity in vitro, we measured cytotoxicity against HIV-expressing autologous targets after overnight and 3-day culture in IL-2–containing media by PBMCs from five subjects whose freshly isolated PBMCs lacked significant activity. Representative data from four HIV-infected subjects and a representative healthy donor are shown in Figs 1 and 2. Antiviral cytotoxic activity emerged in the cultures from HIV-infected donors in an IL-2–dependent manner within 16 hours to levels comparable with those detected after 2 to 3 weeks of culture. At intermediate concentrations of IL-2 (30 and 150 IU/mL), HIV-specific cytotoxicity was somewhat increased after 3 days compared with overnight, but at that time nonspecific lymphokine-activated killer (LAK)-like activity also began to be detected as lysis of control vaccinia-infected B-LCLs. Because one could expect, at most, one cell division in overnight cultures, the development of antiviral cytotoxicity could not depend on the selective proliferation of HIV-specific precursor CTLs.
HIV-specific cytotoxicity by PBMCs from a representative HIV-infected donor (no. 234; A) is below background (10%) for freshly isolated samples but rapidly develops after culture overnight or for 3 days in IL-2. Healthy donor PBMCs (B) have no HIV-specific cytotoxicity before or after culture but develop LAK-like activity in an IL-2–dependent manner (as evidenced by the lysis of control vaccinia infected autologous B-LCLs). Cytotoxicity is measured by 4-hour51Cr release assay at an effector:target ratio of 50:1 against autologous targets infected with vaccinia recombinant virus expressing HIV gp160 (vPE16), RT (vCF21), and gag (vDK1). The control vaccinia (vSC8) expresses the lacZ gene.
HIV-specific cytotoxicity by PBMCs from a representative HIV-infected donor (no. 234; A) is below background (10%) for freshly isolated samples but rapidly develops after culture overnight or for 3 days in IL-2. Healthy donor PBMCs (B) have no HIV-specific cytotoxicity before or after culture but develop LAK-like activity in an IL-2–dependent manner (as evidenced by the lysis of control vaccinia infected autologous B-LCLs). Cytotoxicity is measured by 4-hour51Cr release assay at an effector:target ratio of 50:1 against autologous targets infected with vaccinia recombinant virus expressing HIV gp160 (vPE16), RT (vCF21), and gag (vDK1). The control vaccinia (vSC8) expresses the lacZ gene.
HIV-specific cytotoxicity increases after overnight culture in an IL-2–dependent manner from PBMCs of HIV-infected donors 228 (A), 237 (B), and 307 (C). Cytotoxicity is measured as in Fig 1against targets expressing lacZ control (vSC8), gp160 (vPE16), RT (vCF21), and gag (vDK1). The percent of CD8 T cells that express CD3ζ increases concomitantly.
HIV-specific cytotoxicity increases after overnight culture in an IL-2–dependent manner from PBMCs of HIV-infected donors 228 (A), 237 (B), and 307 (C). Cytotoxicity is measured as in Fig 1against targets expressing lacZ control (vSC8), gp160 (vPE16), RT (vCF21), and gag (vDK1). The percent of CD8 T cells that express CD3ζ increases concomitantly.
Freshly isolated CD8 T lymphocytes from HIV-infected donors have a high frequency of expression of the CTL granule protein, grnA.
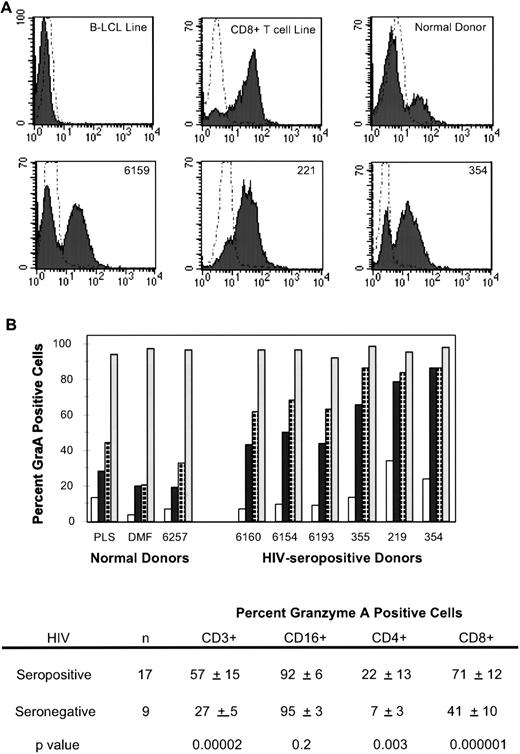
One possible reason for the lack of specific cytotoxicity of circulating peripheral blood lymphocytes (PBLs) could have been lack of the cytolytic machinery, which consists of a pore-forming protein, perforin, and several CTL-specific serine proteases, termed granzymes, which are released when a CTL encounters a target cell.23-25 Precursor CTLs, which have not encountered specific antigens, lack cytotoxic proteins, which are expressed within 1 day of antigenic stimulus. We used three-color flow cytometry analysis of permeabilized PBMCs indirectly stained, with a mouse MoAb CB921 for the most abundant human CTL granzyme grnA26 and directly stained for CD3 and CD16 or CD4 and CD8 expression (Fig 3). In healthy donors, less than 10% of circulating CD4 cells, up to 50% of CD8 cells, and greater than 95% of CD16+ cells (mostly NK cells) stained for grnA. In 17 HIV-infected donors covering a range of disease states, the mean percent of CD4 and CD8 cells that expressed grnA was significantly higher, although the increases in this small sample did not correlate with clinical status. The percent of grnA-staining CD4 cells was 22% ± 13% in HIV-infected subjects versus 7% ± 3% in healthy controls (P = .003, 2-sidedt-test). The difference was even greater for CD8 T cells: 71% ± 12% in HIV-infected subjects versus 41% ± 10% in healthy subjects (P = .000001). This is consistent with the known high frequency of activated circulating CD8 CTLs, expressing human leukocyte antigen (HLA)-DR and CD38 in HIV-infected subjects.11 Virtually all NK cells in both groups stained for grnA. Therefore, lack of antiviral cytotoxicity by freshly isolated PBMCs was not because of lack of cytolytic protein expression.
HIV-infected subjects have an increased frequency of T cells that stain for the cytolytic granule protease grnA. (A) CB9 MoAb stains about 25% of the circulating lymphocytes of a healthy donor but stains the majority of circulating lymphocytes in HIV-infected subjects. (B) An increased number of circulating CD4 (□), CD3 (▪), and CD8 (⊡) T cells in HIV-infected subjects (graphed for representative subjects in order of increasing immunodeficiency) contain the cytolytic effector molecule, grnA. CD16+(▧) NK cells from healthy and HIV-infected donors uniformly contain grnA.
HIV-infected subjects have an increased frequency of T cells that stain for the cytolytic granule protease grnA. (A) CB9 MoAb stains about 25% of the circulating lymphocytes of a healthy donor but stains the majority of circulating lymphocytes in HIV-infected subjects. (B) An increased number of circulating CD4 (□), CD3 (▪), and CD8 (⊡) T cells in HIV-infected subjects (graphed for representative subjects in order of increasing immunodeficiency) contain the cytolytic effector molecule, grnA. CD16+(▧) NK cells from healthy and HIV-infected donors uniformly contain grnA.
Circulating T cells from HIV-infected donors downmodulate CD3ζ expression.
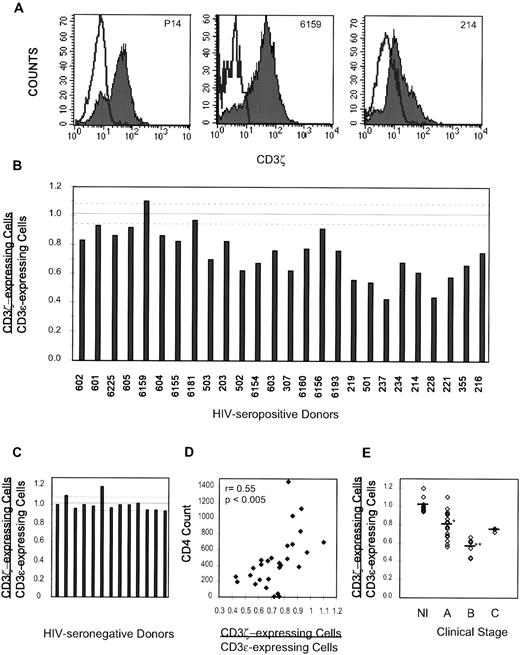
Lymphocytes isolated from tumor-bearing mice and from patients with a variety of tumors have defective T-cell function because of downregulation of CD3ζ, the signaling component of the TcR. We tested whether or not a similar defect might occur in HIV-infected donors. Because CD3ζ is mostly intracytoplasmic and antibodies to CD3ζ recognize determinants not accessible to the cell surface, cells were permeabilized before immunofluorescent staining. PBMCs were directly stained with immunofluorescent antibody to cell surface CD3ε or were permeabilized and stained with antibody to CD3ζ or control. The histograms of gated lymphocytes stained for ζ and control antibody were subtracted to obtain the number of cells expressing ζ (Fig 4A). In PBMCs from healthy donors, the number of cells expressing CD3ζ corresponded to the number of CD3ε-expressing cells (Fig 4C). The ratio of CD3ζ-expressing cells to CD3ε-expressing cells in 14 healthy donor samples was 1.01 ± 0.07, not significantly different from the expected value of 1, if the number of NK cells can be ignored.
CD3ζ is downmodulated in circulating lymphocytes from most HIV-infected donors. (A) The isotype-matched control antibody histogram is subtracted from the CD3ζ histogram (shaded) to obtain the number of ζ+ cells. In normal donor P14 and asymptomatic healthy HIV-infected subject 6159, the number of ζ-staining cells is comparable with the number of cells staining for CD3. However, in donor no. 214, who has oral hairy leukoplakia and thrush, CD3ζ expression is downmodulated. (B) CD3ζ staining for PBLs from 28 HIV-seropositive donors, graphed in order of declining CD4 counts. The dashed lines represent the normal range (C). (D) The percent of T cells that do not stain for ζ increases as the CD4 count falls. (E) CD3ζ downmodulation correlates with CDC disease stage. The ratio for stage A donors, 0.80 ± 0.14, is significantly lower than that of HIV-seronegative healthy donors (*P < .00001) but significantly higher than that of symptomatic stage B donors 0.55 ± 0.09 (**P < .001).
CD3ζ is downmodulated in circulating lymphocytes from most HIV-infected donors. (A) The isotype-matched control antibody histogram is subtracted from the CD3ζ histogram (shaded) to obtain the number of ζ+ cells. In normal donor P14 and asymptomatic healthy HIV-infected subject 6159, the number of ζ-staining cells is comparable with the number of cells staining for CD3. However, in donor no. 214, who has oral hairy leukoplakia and thrush, CD3ζ expression is downmodulated. (B) CD3ζ staining for PBLs from 28 HIV-seropositive donors, graphed in order of declining CD4 counts. The dashed lines represent the normal range (C). (D) The percent of T cells that do not stain for ζ increases as the CD4 count falls. (E) CD3ζ downmodulation correlates with CDC disease stage. The ratio for stage A donors, 0.80 ± 0.14, is significantly lower than that of HIV-seronegative healthy donors (*P < .00001) but significantly higher than that of symptomatic stage B donors 0.55 ± 0.09 (**P < .001).
PBMCs from 28 HIV-infected donors of varying disease stages (Table 1) were analyzed. All but 8 of these subjects had evidence of immunodeficiency by a CD4 count <500 cells/mm3 or clinical symptoms. The ratio of CD3ζ-expressing cells to CD3ε-expressing cells was significantly reduced to 0.74 ± 0.16 (Fig 4B). This was highly significant compared with healthy donors by 2-sided t-test (P < .0000005). CD3ζ expression also correlated with CD4 count, with a higher fraction of cells lacking ζ as the CD4 count dropped (r = 0.55, P < .005, Fig 4D), but did not correlate with plasma viremia in this sample.
The ratio of CD3ζ-expressing cells/CD3ε-expressing cells also correlated with the HIV disease stage as defined by the CDC20 (Fig 4E). The ratio for 19 asymptomatic HIV-infected donors (stage A) was 0.80 ± 0.14, which was significantly different from healthy donors (P = .00001). Only 2 subjects (subjects no. 6159 and 6181) had values in the normal range, and they had CD4 counts above 500/mm3. The ratio greater than 1 for subject 6159 could be attributed to a high percent of circulating NK cells (25% of PBL CD16+), which expressed ζ but not CD3ε. Six donors with minor symptoms of HIV infection (stage B) had significantly lower ratios (0.55 ± 0.09, P < .001 compared with stage A). The three donors with a history of major opportunistic infections, had a somewhat restored ratio (0.74 ± 0.02), but the number of subjects was too few to draw any conclusions.
CD8 T cells preferentially downmodulate CD3ζ.
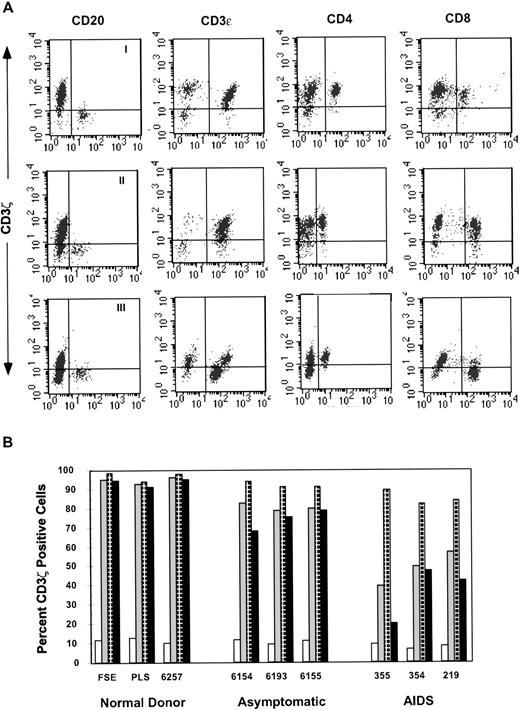
To determine whether all T cells, or a particular subset, had downregulated CD3ζ, we analyzed PBMCs from 20 HIV-infected and 7 healthy donors by three-color staining for CD4 and CD8 or CD20 and CD3ε together with CD3ζ. Each subject was analyzed using CD20 B cells as an internal negative control to set quadrant parameters (Fig 5A). Simultaneous staining with CD3ε and CD3ζ gave comparable results with the method described previously. In most HIV-infected subjects, CD8, but not CD4 T cells had downregulated ζ to the extent that a sizable fraction of CD8 T cells did not stain for ζ above background. Three AIDS patients also showed a slight decrease in the CD4 T-cell subset (Fig 5B). The percentage of CD8 T cells staining for ζ in 20 HIV-infected donor PBMCs was 63% ± 22% compared with 91% ± 4% in healthy donors (P = .004), whereas the percentage of CD4 cells expressing ζ was only slightly reduced: 91% ± 6% for HIV-infected versus 96% ± 2% for controls (P = .05; Table 2).
CD3ζ is downmodulated to a greater extent in CD8 T cells than CD4 T cells. Gated lymphocytes were analyzed for triple staining with antibodies to CD3ζ with CD3 and CD20 or CD4 and CD8. (A) Profile of a healthy donor (I), asymptomatic, slow-progressor subject no. 6156 (II), and AIDS patient no. 355 (III). (B) Representative results from healthy donors, asymptomatic HIV-infected donors and AIDS patients show progressive downmodulation of ζ in CD8 more than CD4 T cells. The percent of CD20 B cells (□), CD3 T cells (▧), CD4 T cells (⊡) and CD8 T cells (▪) that costain with ζ is shown.
CD3ζ is downmodulated to a greater extent in CD8 T cells than CD4 T cells. Gated lymphocytes were analyzed for triple staining with antibodies to CD3ζ with CD3 and CD20 or CD4 and CD8. (A) Profile of a healthy donor (I), asymptomatic, slow-progressor subject no. 6156 (II), and AIDS patient no. 355 (III). (B) Representative results from healthy donors, asymptomatic HIV-infected donors and AIDS patients show progressive downmodulation of ζ in CD8 more than CD4 T cells. The percent of CD20 B cells (□), CD3 T cells (▧), CD4 T cells (⊡) and CD8 T cells (▪) that costain with ζ is shown.
CD4 and CD8 T cells have reduced levels of CD3ζ.
Although most CD8+ and CD4+ T cells stained above background for CD3ζ, the levels of expression for both subsets were reduced (Table 2). The mean ζ fluorescence intensity for CD8 T cells was reduced to 39% of normal (24 ± 11 v 61 ± 19;P < .002) and for CD4 cells to 58% of normal (53 ± 25v 92 ± 33; P = .06). The CD3ζ mean fluorescence of CD8 and CD4 subsets also correlated significantly with CD4 counts with correlation coefficients of 0.70 (P = .01) and 0.64 (P < .05), respectively. Interestingly, CD4 T cells from healthy donors have higher CD3 ε and ζ mean fluorescence intensity than CD8 T cells. This may partly explain why the number of CD4 T cells in HIV infection that did not stain above background was low. CD3 ζ expression must be reduced more in CD4 than in CD8 T cells to measure below the detection threshold.
CD3ζ expression increases after overnight culture in an IL-2–dependent manner.
Because development of antiviral cytotoxicity during overnight culture required the addition of exogenous IL-2, we measured CD3ζ expression after overnight culture in the presence of increasing concentrations of IL-2. We found that CD3ζ expression in PBLs from three donors (no. 228, 237, and 307) also increased when calculated as a percent of T cells staining for ζ above threshold or as mean ζ fluorescence intensity of T cells (data not shown) in an IL-2–dependent manner (Fig2). The change in ζ expression parallels the in vitro increase in cytotoxicity. The increase in CD3ζ expression was detectable beginning at 6 to 10 hours after in vitro culture in three HIV-infected subjects (data not shown).
In some less advanced subjects, CD3ζ expression increased partially in vitro even in the absence of adding exogenous IL-2. Data from four asymptomatic subjects (CD4 counts 493 to 840 cells/mm3) are shown in Fig 6. The percent of CD8 T cells that stained above threshold for CD3ζ increased in culture for the two patients with higher CD4 counts (604 and 605) but decreased in the two subjects with CD4 counts below 500/mm3 (502 and 503). The mean percent of ζ-staining CD8 T cells was not changed after overnight culture (67% ± 5% v 69% ± 10%; P= .76). Because the subjects whose percentage increased in vitro had higher numbers of CD4 T cells capable of producing endogenous IL-2, we determined whether ζ reexpression could be blocked in the presence of an IL-2 blocking antibody. The blocking antibody reduced CD3ζ expression after culture in all four subjects to 59% ± 9%, a significant change compared with the cultures without antibody (P = .01). Similarly, when we added exogenous IL-2 (300 IU/mL), the percent of CD8 T cells staining for CD3ζ increased significantly to 81% ± 3% (P = .02 compared with fresh T cells). This increase was blocked when the IL-2 blocking antibody was added at the beginning of culture; the percent of staining CD8 T cells decreased to 65% ± 6% (P = .04 compared with cultures without antibody), a level comparable with that of fresh cells. These results confirmed a role for IL-2 in the parallel defects of ζ expression and antiviral cytotoxic function.
CD3ζ reexpression in vitro is inhibited by an IL-2–blocking antibody. T cells from four asymptomatic HIV-infected donors (502, ◊; 503, X; 604, ▵; 605, □) cultured overnight with or without exogenous IL-2 (300 IU/mL) and/or anti–IL-2 blocking antibody 5334.21 (30 μg/mL). The percent of CD8 T cells staining above background for CD3ζ is shown. Statistically significant changes are indicated.
CD3ζ reexpression in vitro is inhibited by an IL-2–blocking antibody. T cells from four asymptomatic HIV-infected donors (502, ◊; 503, X; 604, ▵; 605, □) cultured overnight with or without exogenous IL-2 (300 IU/mL) and/or anti–IL-2 blocking antibody 5334.21 (30 μg/mL). The percent of CD8 T cells staining above background for CD3ζ is shown. Statistically significant changes are indicated.
DISCUSSION
We have found that ζ expression is downregulated in CD8, and to a lesser extent in CD4 T cells from most HIV-infected subjects and that it upregulates after overnight culture in serum-containing media in an IL-2–dependent manner. This appears to occur simultaneously with detection of HIV-specific cytotoxicity, which is also IL-2 dependent. The kinetics of ζ reappearance in vitro beginning at about 6 hours explains why laboratories that assayed PBMCs for antiviral cytotoxicity in 6- to 16-hour assays detected a much greater frequency of responders than laboratories that used 4-hour assays. Impaired HIV-specific cytotoxicity in vivo is likely due at least in part to a defect in T helper IL-2 secretion.
Our results are consistent with a recent study that found a signaling defect in the circulating T cells of HIV-infected subjects.27 Using immunoprecipitation and Western blotting, kinase activities of lck, fyn, and ZAP70 were found to be decreased in HIV-infected patients at varying disease stages but not in a group of five long-term nonprogressors. This decrease in kinase activity was related to a conformational change because some but not all antibodies showed decreased reactivity to these proteins from PBLs from HIV-infected donors, and antibody reactivity was restored after protein treatment with dithiothreitol. These abnormalities were found in both CD4 and CD8 cells from two AIDS patients with less than 100 CD4 cells/mm3. ζ chain downmodulation was also found by Western blotting in T cells from several acutely infected patients, recently infected patients, and AIDS patients, although CD4 and CD8 subsets were not separately examined. Downmodulation of ζ presumably increased the threshold for T-cell activation.
Although high concentrations of exogenous IL-2 can overcome CD3ζ downmodulation in vitro, the causes of its downregulation in vivo may be multifactorial. The defect in T-helper cell function that is the hallmark of HIV infection is likely to play a significant role. Functional defects in the proliferative response to recall antigens and to HIV occur early in the disease before the onset of symptoms at the time when we have found defects in CD3ζ expression.28
T-cell dysfunction in HIV infection has been extensively studied with most studies focused on the effects of HIV infection or of HIV gene products on CD4 T cells. Cell surface CD4 is a key regulator of CD4 T-cell activation. When CD4 is ligated together with the TcR, it enhances T-cell activation; however, when it is ligated independently it inhibits subsequent TcR activation or can initiate CD4 T-cell death. Cross-linking of cell surface CD4 with HIV gp120 and antibody to gp120 has been shown to inhibit TcR activation events including phosphorylation of CD3ζ, lck and fyn, and Ca++influx.29-33 Expression of HIV nef within CD4 T cells has also been shown to inhibit T-cell activation, probably by several mechanisms including downmodulation of cell surface CD4 and by interaction of a nef central SH3 binding surface to lck and possibly other TcR-signaling molecules.34-38 These HIV gene products have been implicated in T-cell dysfunction of CD4 T cells but are unlikely to be directly related to the phenomenon we are studying, which disproportionally affects CD8 T cells. However, coculture of CD4 T cells with HIV-infected T-cell hybridomas and monocytes has been shown to reduce IL-2 production.39 This direct effect on IL-2 secretion, together with the reduced CD4 T-cell number and selective deficit in antigen-specific CD4 T cells characteristic of HIV infection, may be important contributing factors to the downmodulation of CD3ζ. Another HIV gene product, tat, has been shown to bind to the cell surface ectoenzyme dipeptidyl aminopeptidase type IV (DP IV or CD26) and to inhibit antigen-specific and anti-CD3 T-cell proliferation. Interestingly the antigen-specific inhibition could be overcome by exogenous IL-2.40 41 Because tat protein has been shown to be secreted from HIV-infected cells, a possible role for tat in CD3ζ downmodulation of CD4 and CD8 T cells is worth exploring.
Other T-cell defects during HIV infection may be related to CD3ζ downmodulation. These include the increased apoptosis of CD4 and CD8 T cells in the blood of HIV-infected donors (with rescue with exogenous IL-2)42 and the increased number of memory CD4 and CD8 T cells and activated CD28− HLA-DR+CD8 T cells.10-12 CD8 T cells from healthy and HIV-infected donors that are CD28− have reduced proliferation to anti-CD3, which increases in the presence of IL-2.43 44
The reasons for CD3ζ downregulation in TILs and PBLs from some cancer patients have not been elucidated, although several reports may provide clues for fruitful avenues of research. In one report, coculture of PBMCs from healthy donors with a human lung cancer cell line induced downmodulation of ζ chain expression, as well as the p56lck and ZAP70 tyrosine kinases. This effect required direct cell-to-cell contact, so it is probably not mediated solely by cytokines. However, coculture with the same tumor cells transfected with IL-2, but not IL-7, granulocyte-macrophage colony-stimulating factor, or tumor necrosis factor-α, was able to reverse the ζ downregulation.45In another report, macrophages that accumulated in the spleens of tumor-bearing mice were able to induce ζ downregulation, also in a contact-dependent manner, in T cells from healthy mice. Moreover, normal macrophages activated by zymosan A and lipopolysaccharide were also able to induce ζ downregulation from healthy T cells.46 Further work by this group showed that treatment of macrophages from tumor-bearing mice with the antioxidant N-acetyl cysteine blocked their ability to induce ζ downregulation, and treatment with oxidants such as H2O2 induced ζ downmodulation.47 Because chronic macrophage activation and oxidant stress are characteristic features of HIV infection,48 a possible role for either of these in the phenomenon we have described in HIV infection merits further inquiry. In another report, costimulation with cross-linked CD3 and CD28 of ζ-downmodulated T cells from Hodgkin's disease patients in vitro induced the reexpression of ζ chain.16 Because a high fraction of circulating CD8, but not CD4, T cells in HIV infection lacked expression of CD28,12 it is possible that this result might also be relevant to the CD3ζ downmodulation that we have found predominantly in CD8 T cells in HIV-infected donors.
Downregulation of CD3ζ in CD8 T cells and consequent loss of their normal functional activity is likely to have significant adverse consequences on control of viral replication in HIV-infected individuals. This result may help explain why the high frequency of HIV-specific precursor CTLs fails to control HIV replication. We have presented preliminary evidence that suggests that ζ downregulation is an early manifestation of HIV disease. It remains to be determined when CD3ζ downregulation occurs in the course of the infection, whether it occurs in long-term nonprogressors and whether it is reversible by treatment with highly effective combination antiretroviral drugs. CD3ζ expression was normal in two subjects we tested with no clinical signs of immunodeficiency. Preliminary study of two patient cohorts treated early with intensive, effective antiretroviral therapy that reduced plasma viremia to undetectable levels for sustained periods, suggested that the defect in ζ expression can be reversed. If so, the prospects for immune control of residual viral reservoirs and their possible eventual elimination will be significantly improved. A flow cytometry-based assay of relative expression of CD3ζ and CD3ε might be a useful tool to monitor immune recovery during drug therapy.
These findings have implications for the design of effective immune-based therapies, especially cellular infusion therapy with CD8 T cells.49-51 For one, it provides a rationale for removing T cells and culturing them in IL-2 briefly to upregulate ζ expression and antiviral function. However, it is unlikely that the infused cells will have a sustained effect if they subsequently downregulate ζ expression and do not continue to function in vivo. Our results also provide a possible additional rationale for IL-2 therapy52,53; an added benefit to IL-2 infusion, in addition to increasing CD4 counts, may be improved CD8 T-cell function. Whether this occurs in vivo, as we observed in our short-term in vitro assays, needs to be explored. The possible salutary effects of related cytokines, such as IL-12 or IL-15, to in vitro ζ reexpression also remains to be studied.
CD3ζ downmodulation in HIV infection and cancer may be examples of a more common mechanism for controlling an exuberant immune response in the setting of chronic inflammation or antigen excess. Although mechanisms for regulation of the CD4 T-cell response in the setting of antigenic excess have been well studied, little is known about how the CD8 T-cell response might be modulated. It is possible that induction of CD4 T-cell anergy induces CD8 T-cell ζ downmodulation. The consequent inhibition of TcR signaling may be an important mechanism for controlling CD8 T-cell cytolysis.
ACKNOWLEDGMENT
We thank M. Perales, H. Jessen, F. Lori, and J. Lisziewicz for patient samples; H. Sprang and F. Emspak for expert technical assistance; and P. Shankar for helpful discussions.
Supported by a Pew Scholar Award in the Biomedical Sciences and National Institutes of Health Grant No. U19-AI36611 (J.L.).
Address reprint requests to Judy Lieberman, MD, PhD, The Center for Blood Research, 800 Huntington Avenue, Boston, MA 02115.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.