Abstract
The B-cell defect in X-linked agammaglobulinemia (XLA) is caused by mutations in the gene for Bruton's tyrosine kinase (BTK). Using the anti-BTK monoclonal antibody (48-2H), a flow cytometric analysis of intracytoplasmic BTK protein expressed in monocytes was successfully performed. To examine the possible identification of XLA patients and female carriers by this assay, we studied 41 unrelated XLA families with (35) or without (6) known BTK mutations. A flow cytometric assay showed deficient expression of the BTK protein in 40 of 41 patients, complete BTK deficiency in 35, and partial BTK deficiency in 5. One patient exhibited a normal level of BTK expression. All 6 patients with partial BTK deficiency or normal BTK expression had missense BTK mutations. The cellular mosaicism of BTK expression in monocytes from obligate carriers was clearly shown in 35 of 41 families. The results suggested that most BTK mutations in XLA might result in deficient expression of the BTK protein. We conclude that deficient expression of BTK protein can be evaluated by a flow cytometric assay, and the clinical usefulness and limitations in diagnosis of XLA patients and carriers are discussed.
X-LINKED agammaglobulinemia (XLA) is the prototypical humoral immunodeficiency first described by Bruton in 1952.1 It is characterized by a paucity of circulating B cells and a marked reduction in serum levels of all Ig isotypes, which causes susceptibility to recurrent and severe bacterial infections in affected males.2-4 The defect in XLA is considered to be due to inefficient expansion of pre-B cells into later B-cell stages or incomplete differentiation of B-cell precursors to pre-B cells.4,5 Using linkage analysis, the XLA gene was first mapped to the long arm of the human X-chromosome in the region of Xq21.3-Xq22.6 In early 1993, the gene responsible for XLA was identified as a cytoplasmic tyrosine kinase, named Bruton's tyrosine kinase (BTK).7,8 BTK belongs to a group of related cytoplasmic tyrosine kinases, known as the Btk/Tec family, and consists of five distinct structural domains, which encompass the N-terminus, pleckstrin homology (PH) domain, Tec homology (TH) domain, Src homology 3 (SH3) domain, SH2 domain, and the catalytic kinase (SH1) domain.9-14 The BTK gene analysis has facilitated the identification of various mutations, including point mutations, insertions, or deletions, in XLA cases.15 Mutations have been identified in all five domains of BTK and have been observed to be associated with a reduction in BTK mRNA, BTK protein, and kinase activity.8 16-20
In female XLA carriers, B cells manifest the skewed inactivation of the mutated X-chromosome, reflecting the role of the XLA gene in early B-cell development.21-23 On the other hand, non-B hematopoietic cells in XLA carriers undergo random inactivation of the normal and mutated X-chromosomes. The product of the BTK gene has been detected in B cells and other hematopoietic cells, such as myeloid cells.8,24,25 Thus, it is possible that demonstration of BTK mosaicism in non-B hematopoietic cells could lead to the detection of obligate XLA carriers. In addition, BTK expression in non-B cells must be as clinically informative for evaluation of the BTK deficiency as the results of BTK mutations in XLA patients, because patients lack circulating B cells.2-4 Generally, the direct detection of BTK mutations by gene analysis is time-consuming and labor-intensive in diagnostic studies or the genetic counseling of XLA families. Furthermore, mutations in the coding regions of BTK gene are not identified in some cases, even if they fulfill the criteria for XLA and show no BTK kinase activity.18
Consequently, we attempted to devise a flow cytometric method for evaluation of cellular BTK expression using a monoclonal antibody (MoAb) specific for BTK.18 We show that flow cytometric evaluation of BTK expression in monocytes might constitute a rapid and sensitive approach for detection of XLA patients and female carriers. The clinical usefulness of this approach was then assessed in unrelated 41 XLA families.
MATERIALS AND METHODS
Subjects.
We studied 41 unrelated XLA patients (mean age, 17 years; range, 2 to 34 years) and their mothers in Japan. Thirty-five of these patients had been analyzed for BTK mutations in previous studies.18,26,27 Among them, 31 patients were found to have mutations in their BTK genes, such as point mutations (missense or nonsense), deletions, and insertions. In 4 XLA patients (families 32 through 35), no mutations were identified in the coding region of BTK, although they exhibited markedly reduced levels of the BTKtranscripts.18 Six patients (families 36 through 41) who had not received the BTK genetic analysis were sporadic cases suggestive of XLA, because they exhibited an absence of circulating B cells and reduced serum Ig levels beginning in early childhood. Healthy adult volunteers served as normal controls. Heparinized blood samples of 5 to 10 mL were collected into the syringes containing heparin after informed consent was obtained.
Cell preparation.
Heparinized venous blood was separated into neutrophils and mononuclear cells by dextran sedimentation and Ficoll-Hypaque gradient centrifugation as described.28 Neutrophils were more than 98% pure as assessed by May-Grünwald-Giemsa staining. For immunoblot analysis of the BTK protein, CD3+ T cells, CD20+ B cells, CD16+ natural killer (NK) cells, and CD14+ monocytes were purified from mononuclear cells by an Epics Elite flow cytometer (Coulter Electronics, Inc, Hialeah, FL) using fluorescein isothiocyanate (FITC)-conjugated corresponding MoAbs, all of which were purchased from Dako Japan (Kyoto, Japan). The purified cell populations were more than 95% positive for each marker as determined by a flow cytometric analysis.
Immunoblot analysis of the BTK protein.
Immunoblot analysis was performed as described previously.29 In brief, 1 million cells were lysed with 10 μL of lysis buffer (1% Triton-X 100, 10 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, 5 mmol/L EDTA, 2 mmol/L phenylmethylsulfonyl fluoride, 20 mmol/L ε-amino-n-capronic acid, 20 mmol/L iodoacetamide, 0.01% soybean trypsin inhibitor, and 10 μg/mL aprotinin) for 40 minutes on ice and were centrifuged for 10 minutes at 15,000g. The supernatants were mixed with an equal volume of sodium dodecyl sulfate (SDS) sample buffer and boiled for 3 minutes. The samples were size fractionated on a 10% SDS-polyacrylamide gel and electroblotted on nitrocellulose filters. The blots were blocked in 5% skim milk in phosphate-buffered saline (PBS; pH 7.4) for 1 hour, reacted with anti-BTK (48-2H: IgG1) MoAb18 at 2 μg/mL or anti–β-actin MoAb (Sigma Chemical Co, St Louis, MO) at 1 μg/mL for 1 hour, and then incubated with a 1:2,000 dilution of peroxidase-labeled goat antimouse IgG antibody (Biosource International, Camarillo, CA) for 1 hour. The blots were rinsed at least four times for 10 minutes in PBS with 0.05% Tween 20 between incubations. Blots were developed by using the ECL Western blotting detection system (Amersham International plc, Amersham, UK). Prestained molecular weight markers (Rainbow colored protein; Amersham International plc) were used as the molecular size standard.
Flow cytometric assay.
Intracellular staining with anti-BTK MoAb was performed as described.29 The cells were first fixed in 4% paraformaldehyde in PBS for 20 minutes at room temperature and then permeabilized in 0.1% Triton X-100 in Tris-buffered saline (pH 7.4) with 1 mg/mL bovine serum albumin for 5 minutes. Subsequently, these fixed, permeabilized cells were reacted with 2 μg/mL of anti-BTK (48-2H) or control IgG1 (Dako Japan) MoAbs for 20 minutes on ice, washed, and then incubated with a 1:2,000 dilution FITC-conjugated goat antimouse IgG1 antibody (Zymed Laboratories, San Francisco, CA) for 20 minutes. In some experiments, mononuclear cells were stained with phycoerythrin (PE)-labeled CD20 (IgG2a; Coulter Immunology, Hialeah, FL) or CD14 (IgG2a; Dako Japan) MoAb before cellular permeabilization to discriminate B cells or monocytes, respectively, from other cells. The stained cells were analyzed on a Cytoron Absolute flow cytometer (Ortho-Clinical Diagnostics, Tokyo, Japan).
RESULTS
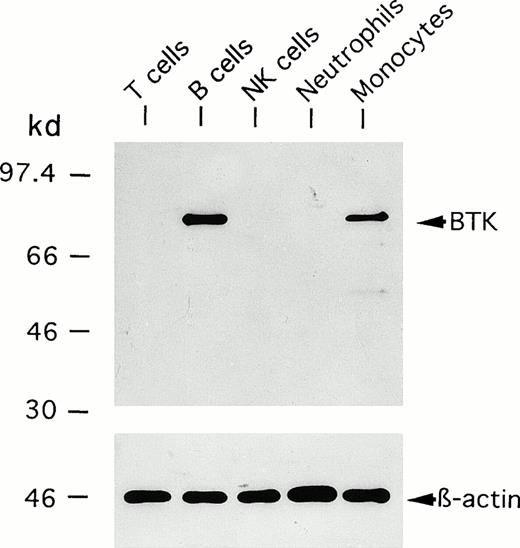
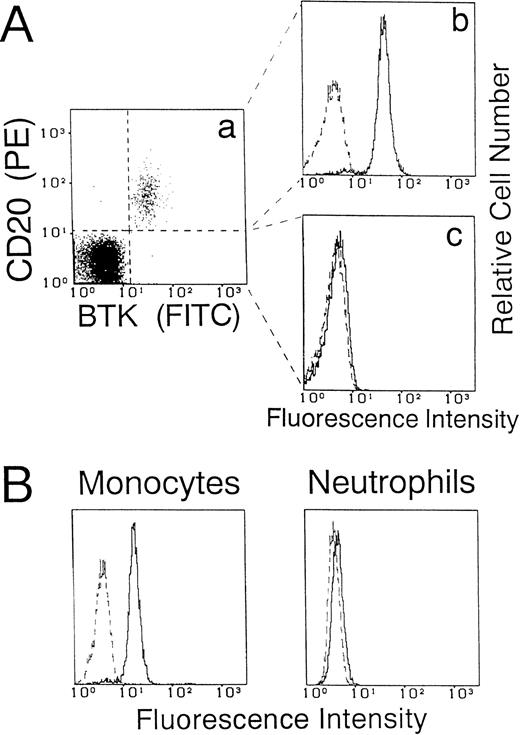
We first performed an immunoblot analysis of T cells, B cells, NK cells, monocytes, and neutrophils using the anti-BTK MoAb (48-2H). Figure 1 shows representative results obtained using cells from a normal adult donor. A 77-kD band identical to the BTK protein was detected in the lysate of B cells, but not of T cells, NK cells, or neutrophils. Importantly, the same band was evident in monocytes. It should be stressed that the anti-BTK MoAb detected very few additional bands in the immunoblot. We further examined whether a flow cytometric assay using this antibody could show different BTK expression among blood leukocyte populations. The cells were fixed and permeabilized to detect intracytoplasmic BTK protein. As shown in Fig 2A, two-color analysis of lymphocytes showed that the BTK protein was expressed in most B cells, but not in non-B cells. Furthermore, monocytes, but not neutrophils, expressed BTK (Fig 2B).
Immunoblot detection of BTK expressed in blood leukocyte populations from normal donors. Neutrophils were isolated from the blood by dextran sedimentation and Ficoll-Hypaque gradient centrifugation. CD3+ T cells, CD20+ B cells, CD16+ NK cells, and CD14+ monocytes were purified from mononuclear cells by electronic sorting. The cells were subjected to immunoblot analysis using anti-BTK or anti–β-actin MoAbs.
Immunoblot detection of BTK expressed in blood leukocyte populations from normal donors. Neutrophils were isolated from the blood by dextran sedimentation and Ficoll-Hypaque gradient centrifugation. CD3+ T cells, CD20+ B cells, CD16+ NK cells, and CD14+ monocytes were purified from mononuclear cells by electronic sorting. The cells were subjected to immunoblot analysis using anti-BTK or anti–β-actin MoAbs.
Flow cytometric analysis of BTK expression in blood leukocytes from normal donors. (A) Two-color immunofluorescence analysis of BTK expression in B cells and non-B cells. Mononuclear cells were stained with PE-labeled anti-CD20 MoAb, fixed, and permeabilized. The cells were then reacted with anti-BTK or irrelevant control MoAbs and further incubated with FITC-labeled secondary antibody. The dot plot map (a) shows the two-color pattern of lymphocytes gated by forward and right angle right scatter. A second gate was set to include CD20+ B cells (b) and non-B cells (c), and the BTK expression in each cell population is presented as a histogram. (B) Analysis of BTK expression in monocytes and neutrophils. Mononuclear cells or neutrophils were stained for BTK as described above. BTK expression in monocytes was evaluated by gating on CD14+ population. The dashed line indicates the control antibody. Five thousand cells were evaluated in each gated population.
Flow cytometric analysis of BTK expression in blood leukocytes from normal donors. (A) Two-color immunofluorescence analysis of BTK expression in B cells and non-B cells. Mononuclear cells were stained with PE-labeled anti-CD20 MoAb, fixed, and permeabilized. The cells were then reacted with anti-BTK or irrelevant control MoAbs and further incubated with FITC-labeled secondary antibody. The dot plot map (a) shows the two-color pattern of lymphocytes gated by forward and right angle right scatter. A second gate was set to include CD20+ B cells (b) and non-B cells (c), and the BTK expression in each cell population is presented as a histogram. (B) Analysis of BTK expression in monocytes and neutrophils. Mononuclear cells or neutrophils were stained for BTK as described above. BTK expression in monocytes was evaluated by gating on CD14+ population. The dashed line indicates the control antibody. Five thousand cells were evaluated in each gated population.
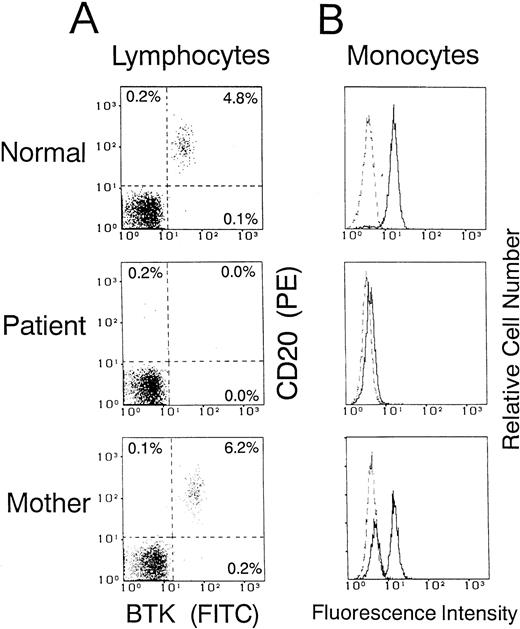
We then used the flow cytometric assay to evaluate BTK expression in XLA patients and to detect cellular mosaicism of BTK expression in their mothers as obligate carriers. Figure3 shows one example from family 19 with deletion in the PH domain. Whereas B cells were markedly decreased in the blood of the patient, the mother possessed a substantial number of circulating B cells expressing BTK (Fig 3A). As shown in Fig 3B, the patient obviously exhibited negligible expression of the BTK protein in monocytes. In addition, the flow cytometric assay demonstrated the clearly bimodal or mosaic pattern of BTK expression in monocytes of the mother, indicating that they consisted of both BTK+ and BTK−cells.
Flow cytometric analysis of cellular BTK expression in a normal donor and in an XLA patient (family 19) and his mother. Dot plot maps of BTK versus CD20 gated for lymphocytes (A) and histograms gated for CD14+ monocytes (B) were obtained as described in the legend of Fig 2. Five thousand cells were evaluated in each gated population. The dashed line indicates the control antibody.
Flow cytometric analysis of cellular BTK expression in a normal donor and in an XLA patient (family 19) and his mother. Dot plot maps of BTK versus CD20 gated for lymphocytes (A) and histograms gated for CD14+ monocytes (B) were obtained as described in the legend of Fig 2. Five thousand cells were evaluated in each gated population. The dashed line indicates the control antibody.
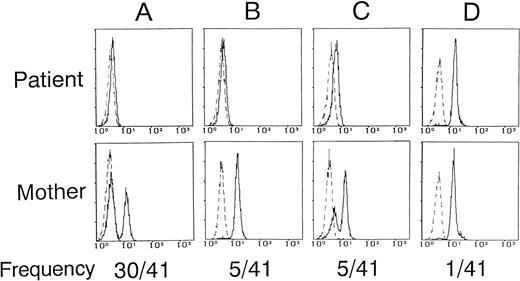
To further validate the flow cytometric assay, we analyzed 41 unrelated XLA patients and their mothers. The results, including known BTK mutations, are summarized in Table 1. Figure 4 shows the relationships between patients and mothers as to monocyte BTK expression profiles. In most of the patients (40 of 41 families) studied, the flow cytometric assay did not detect significant amounts of BTK protein or only detected markedly reduced levels of BTK protein, despite the intense BTK expression in 32 normal controls. This BTK deficiency status was almost complete (<1% anti-BTK stainable or positive cells in monocytes) in 35 patients (Fig4A and B). In 5 (families 1 through 4 and 7) patients showing BTK deficiency, the low but detectable levels of BTK (4% to 32% positive) seemed to be expressed in monocytes (Fig 4C). Arbitrarily, we called the former and the latter complete and partial BTK deficiencies, respectively. The complete BTK deficiency included various BTK mutations such as nonsense, insertion, deletion, and even missense. The complete BTK deficiency was also found in 4 patients (families 32 through 35) who had no mutations in the BTK coding region but exhibited reduced levels of BTK transcripts.18 Six sporadic cases (families 36 through 41) who had not received genetic analysis showed the complete BTK deficiency as well. The partial BTK deficiency was only detected in cases with missense mutations (families 1 through 4 and 7), which might reflect the partially impaired stability of the BTK protein. As shown in Fig 4D, the patient in family 6, who had the missense mutation in the catalytic loop of the SH1 domain,18 exhibited the intense expression of the BTK protein in monocytes (97% positive) comparable to that of normals.
Relationship of monocyte BTK expression profiles between XLA patients and their mothers. Each histogram represents analysis of CD14+ monocytes. The solid and dashed lines indicate the staining with anti-BTK and control antibodies, respectively. Five thousand cells were evaluated in each gated population. (A) The patient had the complete BTK deficiency, and the mother showed the mosaic BTK expression (eg, family 13). (B) The patient had the complete BTK deficiency, but the mother showed the single-positive BTK expression (eg, family 14). (C) The patient had the partial BTK deficiency, and the mother showed the mosaic BTK expression with dimly BTK+ cells and brightly BTK-expressing cells (eg, family 7). (D) Both the patient and the mother expressed the normal BTK expression (eg, family 6). The frequency (no. of families/total no. of families) is depicted below the histogram.
Relationship of monocyte BTK expression profiles between XLA patients and their mothers. Each histogram represents analysis of CD14+ monocytes. The solid and dashed lines indicate the staining with anti-BTK and control antibodies, respectively. Five thousand cells were evaluated in each gated population. (A) The patient had the complete BTK deficiency, and the mother showed the mosaic BTK expression (eg, family 13). (B) The patient had the complete BTK deficiency, but the mother showed the single-positive BTK expression (eg, family 14). (C) The patient had the partial BTK deficiency, and the mother showed the mosaic BTK expression with dimly BTK+ cells and brightly BTK-expressing cells (eg, family 7). (D) Both the patient and the mother expressed the normal BTK expression (eg, family 6). The frequency (no. of families/total no. of families) is depicted below the histogram.
The patterns of BTK expression in mothers' monocytes are summarized in Table 1 and Fig 4. The bimodal pattern of BTK expression in monocytes may indicate cellular mosaicism in mothers as obligate XLA carriers. However, the ratio of brightly staining BTK+ monocytes to weakly staining BTK+ monocytes varied from mother to mother (Table 1). The flow cytometric assay disclosed that normal monocytes frequently had approximately 3% BTK− cells. We classified mothers' monocytes as mosaic when the percentage of brightly anti–BTK-stainable monocytes was less than 90%, which was almost equal to the mean minus 3 SD of positivity of monocyte BTK expression in normals. Based on this criterion, the mosaic BTK patterns in the mothers' monocytes were obtainable in 35 of 41 (∼85%) of families (Table 1 and Fig 4). This mosaicism was observed in the majority of families with complete or partial BTK deficiencies (Fig 4A and C). The mosaicism in the mother's monocytes was also found in all families without any identifiable mutations (families 32 through 35) and 5 sporadic cases (families 36 through 40), supporting the BTK-related agammaglobulinemia in both groups. In the remaining 6 families (6, 14, 15, 17, 23, and 41), the mosaic pattern of BTK expression in the mothers' monocytes was not obtained (Fig 4B and D). The normal pattern of monocyte BTK expression was expected in the mother of family 6, because the patient expressed BTK at the normal levels, which might lead to the nonmosaic BTK expression in the mother's monocytes even in the presence of X-chromosomal mosaicism (Fig 4D). In the other 5 families (14, 15, 17, 23, and 41), all of which are sporadic cases, the patients showed the complete BTK deficiency. The single positive pattern of BTK expression in mothers' monocytes in these cases raised the possibility that BTK deficiency in the patients might arise from the de novo mutation or gonadal mosaicism.30 Nucleotide sequence analysis of the genomicBTK gene in the white blood cells of mothers was performed for families 14 and 15. The analysis showed that the mutated allele was absent in somatic cells of both mothers, supporting the possibility discussed above (data not shown). It is also possible that the excessively skewed inactivation of the mutated X-chromosome may occur in the mothers' monocytes.31 However, these two possibilities were not examined for the mothers in families 17, 23, and 41.
DISCUSSION
We have described a practical flow cytometric assay for identifying affected males and female carriers in XLA families. The immunoblot analysis using the anti-BTK MoAb (48-2H) showed that the BTK protein was selectively expressed in monocytes and B cells among blood leukocytes. Other investigators have also observed BTK expression in monocytes as well as B cells.19 Although several laboratories have generated MoAbs or antisera against the BTK protein,8,25,32 33 these antibodies have been shown to cross-react with proteins other than BTK in the immunoblot analysis or immunoprecipitation. In contrast, our anti-BTK MoAb 48-2H detects the BTK protein with very few additional bands in the immunoblot, implying the suitability of this antibody for flow cytometric use. Consistent with the results of the immunoblot analysis, we showed that a flow cytometric assay could identify preferential expression of the intracytoplasmic BTK protein in both monocytes and B cells.
A flow cytometric assay on monocyte BTK expression was used to evaluate BTK deficiency status and to compare with BTK mutations in XLA patients. This assay showed that, in almost all of the XLA patients (∼98%) studied here, BTK protein was absent or weakly detectable in monocytes, whereas strong BTK expression was found in monocytes from normal donors. Our anti-BTK MoAb was generated against the SH3 domain of BTK.18 It was predictable that the defective BTK protein expression was demonstrated in patients with mutations causing the premature termination at the 3′ terminal region from the SH3 domain (families 8 through 10, 17 through 21, and 31) or the deletion of the SH3 domain-containing region (families 22 and 23). Additionally, it was predicted that 4 patients (families 32 through 35) in whom reduced levels of BTK mRNA had been found despite the absence of mutations in the coding region18 might exhibit defective BTK expression. However, these cases were only 15 (37%) of 41 families examined. In nonsense mutations (families 11 through 16), deletions (families 24 through 28), or insertions (families 29 and 30) not affecting the SH3 domain, the flow cytometric assay would likely detect BTK proteins with various sizes. Nevertheless, the defective BTK expression was observed in all of these cases in our assay. Additionally, in the cases with several missense mutations, the mutated BTK proteins were detected at markedly reduced levels, which is consistent with our previous observation that some of the BTK proteins carrying missense mutations in the SH1 domains were not detected by anti-BTK immunoblotting.18 The normal BTK expression pattern, suggesting the production of normal amounts of altered BTK, was found only in 1 case (family 6) with a missense mutation in the catalytic loop of the SH1 domain.18 Vihinen et al34 have described a similar case with the missense mutation in the same loop.
The majority of the nonsense mutations and deletions may unstabilize the mRNA or the protein, as has been shown in previous studies onBTK8,16-20 and other genes.35,36Furthermore, it has been reported that several missense mutations result in the reduced stability of the BTK protein16,18 and other proteins such as WASP,37 especially in hematopoietic cells. However, it must be noted here that failure to detect mutated BTK proteins does not necessarily imply the absence or instability of proteins. There remains an alternative possibility that the anti-BTK MoAb (48-2H) used in this study might be unable to recognize some of the mutated BTK proteins. Although our unpublished data indicated that this antibody can recognize several artificially mutated BTK proteins (including Arg28 to Pro, Gln41 to Lys, Trp124 to Gly, Tyr223 to Phe, Trp251 to Leu, Lys430 to Arg, Tyr551 to Phe mutations, and, in addition, a truncated protein only having the unique region and SH3 domain), it is impossible to test the latter possibility for all mutated BTK proteins studied here, and further studies using other anti-BTK antibodies will be necessary. Collectively, the present study in 41 unrelated Japanese XLA families showed that most (40 of 41) mutations in XLA, including 5 different missense mutations, resulted in the absence or reduced levels of the BTK protein detectable by a flow cytometric assay using the MoAb 48-2H. A database of BTK mutations has currently compiled 318 unrelated XLA families, which includes 123 families carrying missense mutations.15 To understand the precise molecular mechanism of the pathogenesis of XLA and genotype-phenotype relationships, it may be important to investigate which mutations result in the instability of BTK proteins or which mutations result in the loss of the function of some domain without affecting the stability of BTK proteins. The flow cytometric analysis with combinations of MoAb described here and other antibodies will be useful in this regard.
It is noteworthy that the Arg28 mutation to Pro mutation (family 1), which is located in the amino acid residue in the mutation of XID mice,38 resulted in a greatly reduced level of BTK protein expression. In our previous report,18 we showed a similar level of kinase activity in the Arg28 to Pro-mutated BTK, comparable to that of the wild-type BTK. This discrepancy is apparently due to the difference in experiment methodologies. The in vitro kinase assay and the immunoblot assay described in our previous report18were performed under nonquantitative conditions because of the weak kinase activity detected in peripheral blood cells and the limited availability of clinical samples. Our data in the present study indicate that the flow cytometric analysis is much more quantitative in the detection of BTK expression compared with other methods described in our previous report.18 In the PH domain of BTK, the Arg28 residue has been postulated to be located within a binding site of potential ligands such as phospholipids39 or protein kinase C,40 and in vitro experiments using GST fusion proteins have suggested that the Arg28 mutation diminishes binding capabilities of these potential ligands.39 40 Our observation that the Arg28 (to Pro) mutation reduced the expression of BTK protein measured by the flow cytometric analysis might suggest a new interpretation on the significance of the Arg28 mutation. Consistent with our observation, other investigators observed a decreased expression of the BTK protein harboring the Arg28 to Cys mutation (XID mutation) in murine mast cells (Hata et al, personal communication, October 1997). The Arg28 mutation may lead to a conformational change of BTK that makes the protein unstable, at least in some intracellular conditions within hematopoietic cells. Alternatively, this mutation may disrupt the binding of potential ligands that are necessary for the stabilization of the BTK protein.
We showed that a flow cytometric assay based on monocyte BTK expression is useful to identify the X-chromosomal mosaicism in mothers as obligate carriers. In 35 of 41 families (∼85%), it was found that the mothers exhibited a bimodal or mosaic BTK expression in monocytes. During this study, we had the opportunity to examine 14 sisters of 11 patients with BTK deficiency. The flow cytometric assay showed that, in 9 of 14 sisters (∼64%), bimodal expression of BTK in monocytes was detected, implying XLA carriers. Unexpectedly, an apparently normal profile of monocyte BTK expression in mothers was obtained in 6 families (6, 14, 15, 17, 23, and 41). This was expected in family 6, because the patient showed strong monocyte BTK expression comparable to that of normal patients. Patients in the other 5 families showed the complete BTK deficiency. All of these patients are sporadic cases without any family history. The nonmosaic expression of BTK in their mother's monocytes may be derived from the de novo mutation in oocytes or gonadal mosaicism that have been seen in certain X-linked hereditary diseases.30 Such a kind of inheritance was confirmed in families 14 and 15 by nucleotide sequence analysis of the mother's somatic cells. It has been observed that X-chromosome inactivation may result in skewing in normal cells.31 It is possible that the skewed inactivation of the mutated X-chromosome may result in the single positive pattern of BTK expression in the mother's monocytes. In the course of this study, we determined whether the skewed inactivation of the mutated X-chromosome might occur in monocytes from female XLA carriers. In family 29, although the mother showed mosaic BTK expression in monocytes, the single-positive pattern of monocyte BTK expression was found in the grandmother. Using the genomic analysis, we found that both normal and mutated X-chromosomes were present in somatic cells of the grandmother as well as the mother (unpublished observations, October 1997). Thus, it should be noted that a normal pattern of monocyte BTK expression in the woman does not rule out the possibility that she is an XLA carrier. Of course, the clinical usefulness of a flow cytometric assay for identification of XLA carriers should be validated by further analysis of additional cases in a combination with the genetic analysis.
In conclusion, the present study suggests that most BTKmutations appear to result in deficient expression of the BTK protein in affected individuals. The mosaic expression of the BTK protein in monocytes, as shown in a flow cytometric assay, is potentially useful in the diagnosis of female carriers. With an accumulation ofBTK mutations, atypical or mild XLA has been found among the cases previously diagnosed as common variable immunodeficiency or other immunodeficiencies,4,11,16,18 and the clinically different phenotypes have been observed in siblings with the same BTKmutations.19 The semiquantitative capability of a flow cytometric assay to assess the cellular BTK expression may contribute to an elucidation of the significance of BTK mutations in phenotypic expression of the disease and an understanding of the role of BTK in B-cell development.
ACKNOWLEDGMENT
The authors are very grateful to all families and physicians for the generous cooperation in this study and to Dr Toshiaki Kawakami and his colleagues (La Jolla Institute for Allergy and Immunology, San Diego, CA) for the kind supply of their preliminary data and helpful discussion. We also thank Hitoshi Moriuchi for the excellent technical assistance.
Supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan and by grants from the Ministry of Health and Welfare of Japan and from the Mother and Child Health Foundation.
Address reprint requests to Toshio Miyawaki, MD, PhD, Department of Pediatrics, Faculty of Medicine, Toyama Medical and Pharmaceutical University, 2630 Sugitani, Toyama, Toyama 930-01, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.