Abstract
Erythrocyte protein 4.2 (P4.2) is an important component of the erythrocyte membrane skeletal network with an undefined biologic function. Presently, very little is known about the expression of the P4.2 gene during mouse embryonic development and in adult animals. By using the Northern blot and in situ hybridization techniques, we have examined the spatial and temporal expression of the P4.2 gene during mouse development. We show that expression of the mouse P4.2 gene is temporally regulated during embryogenesis and that the P4.2 mRNA expression pattern coincides with the timing of erythropoietic activity in hematopoietic organs. P4.2 transcripts are first detected in embryos on day 7.5 of gestation and are localized exclusively in primitive erythroid cells of yolk sac origin. These erythroid cells remain to be the only source for P4.2 expression until the switch of the hematopoietic producing site to fetal liver. In mid- and late-gestation periods, P4.2 mRNA expression is restricted to the erythroid cells in fetal liver and to circulating erythrocytes. Around and after birth, the site for P4.2 expression is switched from liver to spleen and bone marrow, and P4.2 transcripts are only detected in cells of the erythroid lineage. These results provide the evidence for specific P4.2 expression in erythroid cells. In addition, the timing and pattern of expression of the P4.2 gene suggest the specific regulation of the P4.2 gene.
THE ERYTHROCYTE protein 4.2 (P4.2) is one of the major components in the erythrocyte membrane skeletal network (for a review, see Becker and Benz1). P4.2 binds to the cytoplasmic domain of the anion exchanger band 3 and interacts with ankyrin in red blood cells (RBCs).2-5 Although the exact function of P4.2 is not defined, patients with P4.2 deficiency in their RBC membranes suffer from spherocytosis and various degrees of hemolytic anemia, suggesting a role for P4.2 in maintaining the stability and flexibility of RBCs (reviewed in Cohen et al6and Yawata7). The results from recent biophysical and electron microscopic experiments indicate that P4.2 may serve as an accessory linking protein to strengthen the interaction between the integral membrane protein, such as band 3, and the cytoskeletal network.8-10
Molecular cloning of the human P4.2 cDNA from a reticulocyte cDNA library shows that the amino acid sequence of P4.2 has significant homology with the transglutaminase family of cross-linking enzymes.11,12 Two human P4.2 cDNA isoforms of about 2.4 and 2.5 kb, respectively, were isolated, consistent with the presence of multiple species of P4.2 RNAs in human reticulocytes.12 The two cDNA isoforms differ by a 90-bp in-frame insertion. As a result of this difference, the long cDNA isoform encodes a protein of 721 amino acids, whereas the short isoform encodes a protein of 691 amino acids. Using genomic cloning and sequencing analysis, we showed that the two human P4.2 cDNA isoforms are generated by alternative splicing.13 By using isoform-specific antibodies, the protein products of the two P4.2 cDNA isoforms in human erythrocyte membranes were identified.13 The relative molecular mass of the protein encoded by the short P4.2 cDNA isoform on the sodium dodecyl sulfate (SDS)-polyacrylamide gel is typically 72 kD, existing as a major P4.2 species in human erythrocyte membranes. The relative molecular mass of the long cDNA isoform-encoded protein is approximately 75 kD. The ratio of the 72-kD to the 75-kD P4.2 protein estimated from the Western blot analysis is about 15:1.13When human reticulocyte cDNAs were amplified by polymerase chain reaction (PCR) with primers flanking the two alternatively spliced isoforms in the human P4.2 cDNA, two other smaller cDNA isoforms were also detected.14 15 However, the protein products of these small human P4.2 cDNA isoforms have not been identified. In addition, the functional significance of all these different human P4.2 isoforms is presently unknown.
Recently, we16 and others15,17 also cloned the mouse P4.2 cDNA. Similar to the human protein, the mouse P4.2 amino acid sequence shows homology with those of transglutaminases. Although there was a discrepancy on the estimated size of the mouse P4.2 RNA reported by the three groups, only a single P4.2 cDNA that encodes a protein of 691 amino acids (identical to the size of the major 72-kD human P4.2 protein) was found in the mouse reticulocyte library. Using genomic cloning and PCR analysis, we showed that the mouse reticulocyte P4.2 RNA does not exhibit alternative splicing in the regions identified in the human P4.2 RNA.16 Presently, the significance for the difference in P4.2 isoform expression between mouse and human is not known.
In addition to the P4.2 protein in erythrocytes, immunoreactive forms of P4.2 with the molecular weight of 72 kD, or larger or smaller than 72 kD, have been detected in nonerythroid cells and tissues.6,18-20 Presently, the exact structures of these proteins are not known. Immunologic cross-reactivity between the erythrocyte P4.2 protein and other cellular proteins has been reported.6,18,21 In light of the complexity found in other erythrocyte membrane proteins,22-35 whether these immunoreactive analogs of erythrocyte P4.2 represent true P4.2 protein isoforms or are just related proteins in nonerythroid cells remains to be determined.
Presently, very little is known about the expression of the P4.2 gene during embryogenesis and in adult animals. Previous in vivo pulse-labeling studies showed the asynchronous synthesis of membrane proteins of the mature erythrocyte, with those of proteins 4.1 and 4.2 finishing last.36,37 By a two-phase liquid culture system using erythroid burst-forming unit in normal human peripheral blood, the P4.2 protein can be detected in cells equivalent to the stage of early erythroblasts.7 In addition, changes in mRNA expression and synthesis of membrane proteins, including α- and β-spectrins, and ankyrin have been noted during murine erythroid differentiation38,39 and erythropoiesis.40
In this report, we describe a comprehensive analysis of mouse P4.2 RNA expression during development. Our results show that expression of the mouse P4.2 gene is temporally regulated during embryogenesis and that the P4.2 mRNA expression pattern matches the timing of erythropoietic activity in hematopoietic organs. In addition, P4.2 expression is detected only in the erythroid cell-producing organs and circulating erythrocytes during mouse embryonic development and in adult animals.
MATERIALS AND METHODS
Northern blot analysis.
A mouse embryo Northern blot and a mouse multiple tissue Northern blot were purchased from Clontech Laboratories, Inc (Palo Alto, CA). Each lane in the blots contains 2 μg of poly A+ RNA from the specific tissue as indicated. RNAs from various mouse tissues were also isolated as described before16 and 50 μg of each tissue RNA was used in Northern blot analysis.41 The mouse P4.2 cDNA clone pBS(+)/mP4.2-Ust containing the 2.2-kbEcoRI-fragment of the mouse P4.2 cDNA16 was digested with EcoRI and BamHI enzymes. A 714-bpBamHI-EcoRI cDNA fragment containing the 3′ portion of the P4.2-coding region was isolated, labeled with 32P by the random priming technique (Amersham, Arlington Heights, IL), and used as the probe. The blots were hybridized and washed according to standard procedures41 as described previously.16 The hybridization signal was visualized by autoradiagraphy. Also, the same blots were stripped to remove the previously hybridized radioactivity and rehybridized with a32P-labeled β-actin cDNA probe.42
Sample preparation.
FVB/N mice were purchased from Harlan Sprague Dawley, Inc (Indianapolis, IN) and maintained in our animal facility. Embryos and whole deciduas were obtained from the natural mating of FVB/N mice. Gestational age was designated as embryonic day 0.5 (E0.5) on the day the vaginal plug was found. Organs from mice at various times after birth also were dissected. Samples were fixed in 10% neutral buffered formalin (Surgipath, Richmond, IL) at 4°C overnight and processed for paraffin embedding. Five-micron tissue sections were prepared, mounted onto Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA), and stored at −80°C until hybridization.
For preparing bone marrow sections, the femurs from mice at various times after birth were paraffin-embedded, decalcified with Cal-EX decalcifying solution (Fisher Scientific), and processed for tissue sections as described before.
Probe preparation.
Both antisense and sense riboprobes derived from the mouse P4.2 cDNA were prepared for in situ hybridization studies. Briefly, the same 714-bp BamHI-EcoRI mouse P4.2 cDNA fragment containing the 3′ P4.2-coding region as that used in Northern blot analysis was isolated from plasmid pBS(+)/mP4.2-Ust and cloned into pBS(+) (Stratagene, La Jolla, CA). For preparing the P4.2 antisense riboprobe, the resulting transcription plasmid was linearized with BamHI and used in the in vitro transcription reaction41 with T7 RNA polymerase in the presence of33P-UTP (Amersham). For preparing the P4.2 sense riboprobe, the transcription plasmid was digested with EcoRI and the linearized plasmid DNA was transcribed with T3 RNA polymerase as described above.
In situ hybridization.
Slides containing embryo or tissue sections were allowed to warm to room temperature and then dewaxed with xylene. Mounted sections were immersed in 0.2 N HCl for 5 minutes, incubated in 1 μg/mL proteinase K for 5 minutes at room temperature, and then treated with 0.25% (vol/vol) acetic anhydride in 0.1 mol/L triethanolamine-HCl, pH 8.0, for 5 minutes. For hybridization, the slides were first incubated with the prehybridization solution (Novagen, Madison, WI) for 1 hour at 50°C and then hybridized overnight with either the P4.2 antisense or sense riboprobe at 50°C. Subsequently, the slides were sequentially washed in 2× SSC at 50°C for 30 minutes, in RNase buffer (0.5 mol/L NaCl, 10 mmol/L dithiothreitol [DTT], and 20 μg/mL RNase A) at 37°C for 30 minutes in buffer containing 0.1× SSC, 50% formamide, and 10 mmol/L DTT at 50°C for 30 minutes, and finally in buffer containing 1× SSC, 14 mmol/L DTT, and 0.07% sodium pyrophosphate at 50°C for 30 minutes. For exposure to autoradiographic emulsion, the slides were dipped into Kodak NTB-2 emulsion (Eastman Kodak, Rochester, NY) exposed in the dark at 4°C for 1 to 2 weeks, and then developed with Kodak D-19 developer and Fixer. For counterstaining, the slides were treated with 0.1% crystal violet. Autoradiographic images of the embryo and tissue sections were visualized under a Zeiss Axiophot microscope and photographed with Kodak Technical Pan films.
For comparison of histologic cell types, adjacent sections were dewaxed and processed for standard hematoxylin-eosin staining.
RESULTS
Expression of the P4.2 mRNA in adult mouse tissues and during mouse embryogenesis.
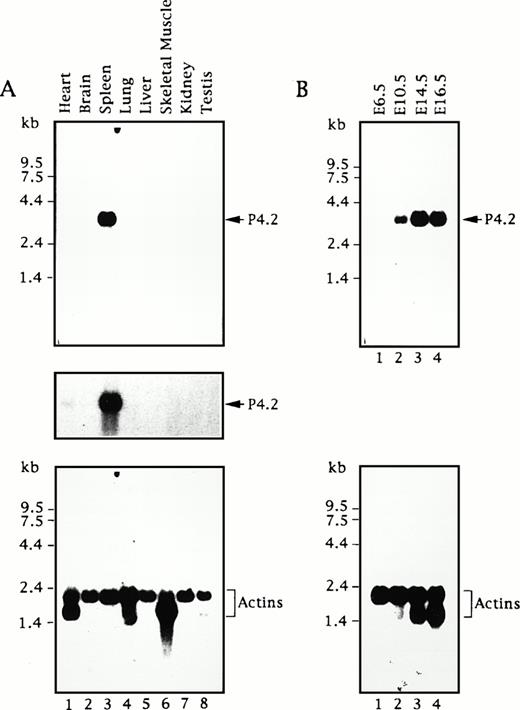
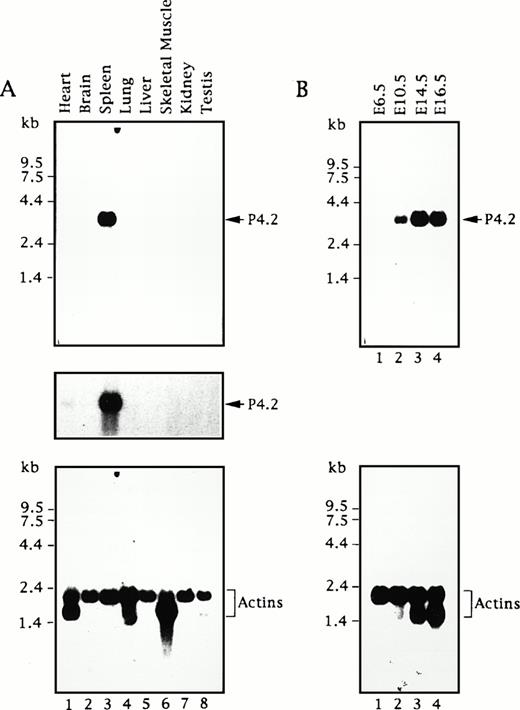
To assess the expression of the P4.2 gene, we first analyzed poly A+ RNAs from various adult mouse tissues by Northern blot analysis using a 714-bp mouse P4.2 cDNA fragment containing the 3′ portion of the P4.2-coding region as the probe. A single 3.5-kb P4.2 transcript was detected at relatively high levels in spleen (Fig1A), analogous to our previous findings using a 2.2-kb P4.2 cDNA probe containing the entire coding region.16 In contrast, little or no P4.2 hybridization was seen in other tissues examined. Upon longer exposure of the autoradiogram, a very small amount of the P4.2 message was detected in heart, whereas no hybridization signal was seen in brain, lung, liver, skeletal muscle, kidney, or testis. As the control, hybridization of the same RNA blot with the probe for actins showed comparable intensity of a 2.1-kb mRNA for cytoskeletal actin and/or a 1.7-kb muscle actin mRNA43 44 (eg, in heart and skeletal muscle) in each sample (Fig 1A), indicating the intactness and relatively similar quantity of mRNAs in each sample.
P4.2 mRNA expression during mouse development. Northern blot analysis of RNAs from adult mouse tissues (A) and embryos at various embryonic ages (B) was conducted as described in the Materials and Methods. A 714-bp mouse P4.2 cDNA fragment containing the 3′ portion of the P4.2-coding region was used as the probe. The P4.2-hybridization signal was visualized by autoradiography (top). A longer exposure of the blot in (A) is shown in the middle. As the control, the same blots were stripped and rehybridized with a32P-labeled β-actin cDNA probe (bottom).
P4.2 mRNA expression during mouse development. Northern blot analysis of RNAs from adult mouse tissues (A) and embryos at various embryonic ages (B) was conducted as described in the Materials and Methods. A 714-bp mouse P4.2 cDNA fragment containing the 3′ portion of the P4.2-coding region was used as the probe. The P4.2-hybridization signal was visualized by autoradiography (top). A longer exposure of the blot in (A) is shown in the middle. As the control, the same blots were stripped and rehybridized with a32P-labeled β-actin cDNA probe (bottom).
Subsequently, we performed a similar Northern blot analysis of poly A+ RNAs from mouse embryos at different stages of development using the same P4.2 cDNA probe. As shown in Fig 1B, no P4.2 hybridization was detected in mRNAs from E6.5 embryos. The expected 3.5-kb P4.2 mRNA was seen in E10.5 embryos and its intensity increased in the embryos at later ages (E14.5 and E16.5). Similarly, when the actin probe was used as the control, the 2.1-kb cytoskeletal actin band of about equal intensity was detected in all embryo mRNAs (Fig 1D). In addition, the 1.7-kb muscle actin message was detected in E14.5 and E16.5 embryos, consistent with its timely expression during myogenesis.45 These results indicate that expression of the mouse P4.2 gene is temporally regulated during embryogenesis and that P4.2 transcripts are primarily detected in hematopoietic tissues such as the spleen in the adult animal.
P4.2 gene expression during early gestation embryos.
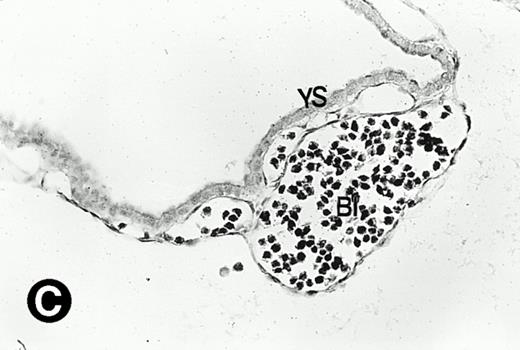
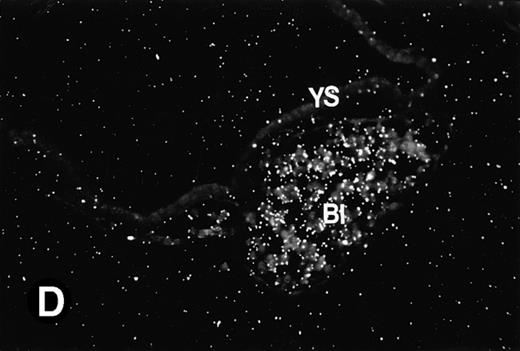
To determine if the mouse P4.2 mRNA expression pattern coincided with particular developmental events, in situ hybridization analysis on sections derived from embryos corresponding to various developmental stages was performed. Because the 714-bp mouse P4.2 cDNA probe containing the 3′ portion of the coding region specifically detected the 3.5-kb P4.2 message in Northern blot analysis, the same cDNA fragment was cloned into pBS(+) and the resulting transcription plasmid was used to synthesize both the antisense and sense riboprobes for in situ hybridization. In agreement with the results from Northern blot analysis, no P4.2-specific labeling was detected in sections of embryos at E6.5 days when hybridized with the P4.2 antisense riboprobe (data not shown). A P4.2 hybridization signal, albeit of weak intensity, was first detected in E7.5 embryos (Fig 2). By comparing the dark-field image with the bright-field embryo structure, P4.2-specific labeling was found to be localized in primitive erythroid cells, such as in the early heart region, of E7.5 embryos (Fig 2A and B). At this embryonic stage, the primitive erythroid cells are large nucleated RBCs and derived from the yolk sac, which is the initial site of erythropoiesis.46-48 The yolk sac consists of an endodermal epithelium and underlying mesoderm within which blood islands and vessels develop. Blood islands are the only source of embryonic erythroid cells until E11.5 days. These primitive erythroid cells are transported into the embryo for circulation via blood vessels. P4.2 expression was also detected in the blood islands and vessels of yolk sac at E7.5 and E8.5 days (Fig 2C and D), consistent with that detected in primitive erythroid cells in the early heart region. In contrast, no hybridization signal was seen when P4.2 sense riboprobe was used (data not shown). Similar hybridizations were performed with the sense probe for sections of embryos or tissues at various time points as described below and were found to be similarly negative.
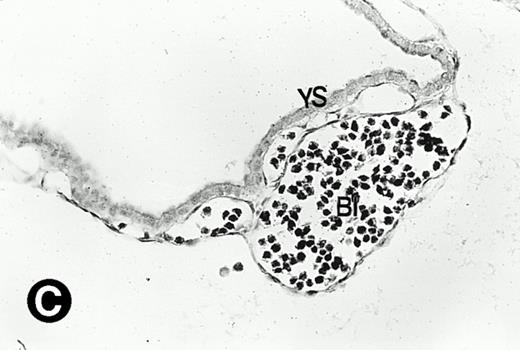
P4.2 gene expression during early gestation embryos. In situ hybridization analysis was used to detect P4.2 expression in mouse embryos at E7.5 (A and B) and E8.5 days (C and D). Sagittal sections of embryos within the deciduas were hybridized with the P4.2 antisense riboprobe. (A and C) Bright-field illumination; (B and D) dark-field illumination. Upon examination of multiple embryo sections, P4.2-specific labeling was detected in erythroid cells in the early heart region and blood vessels (indicated by arrowheads in A and B). NT, neural tube. The blood islands (BI) of the visceral yolk sac (YS) in (C) and (D) also show P4.2 hybridization. Micrographs were taken with a 20× (A and B) or 40× (C and D) objective.
P4.2 gene expression during early gestation embryos. In situ hybridization analysis was used to detect P4.2 expression in mouse embryos at E7.5 (A and B) and E8.5 days (C and D). Sagittal sections of embryos within the deciduas were hybridized with the P4.2 antisense riboprobe. (A and C) Bright-field illumination; (B and D) dark-field illumination. Upon examination of multiple embryo sections, P4.2-specific labeling was detected in erythroid cells in the early heart region and blood vessels (indicated by arrowheads in A and B). NT, neural tube. The blood islands (BI) of the visceral yolk sac (YS) in (C) and (D) also show P4.2 hybridization. Micrographs were taken with a 20× (A and B) or 40× (C and D) objective.
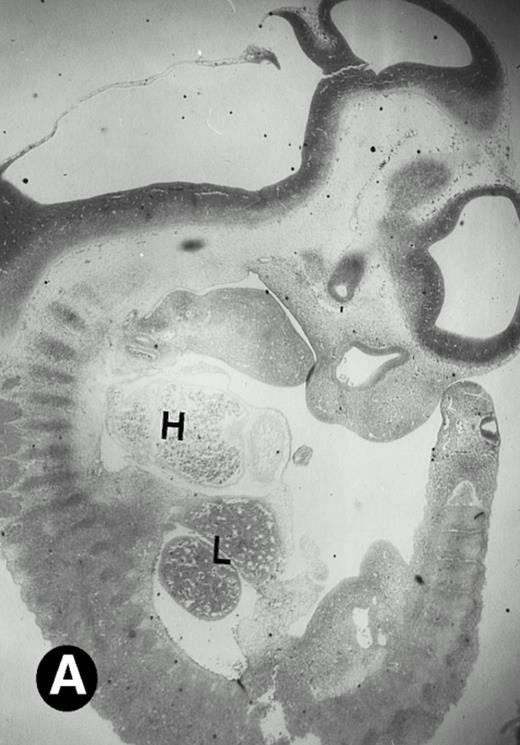
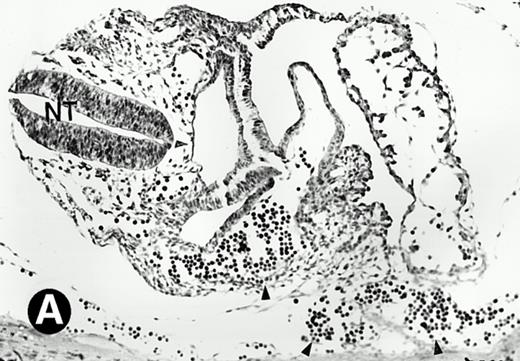
After 8.5 days of gestation, the embryo undergoes accelerated organogensis. By E9 days, the four-chambered heart is developed and the circulation is established.48 Analysis of embryos at gestation ages from E8.5 to E10.5 days showed that P4.2-specific labeling in the heart region remains to be seen. P4.2 hybridization signal with increasing intensity was detected only in the heart and blood vessels of E10.5 embryos (Fig 3A and B). Specifically, P4.2 expression in the heart region was confined to circulating erythroid cells but was not seen in cardiomyocytes (Fig 3C and D). Upon higher magnification of E10.5 embryo sections, P4.2 hybridization signal with weak intensity was observed in the liver (Fig 3E and F), localized notably in erythroid cells in the sinusoids and vessels (indicated by arrowheads). The remaining organs, such as brain, showed no hybridization. These results indicate that the erythroid cells of yolk sac origin are the only source for P4.2 expression during early embryonic development.
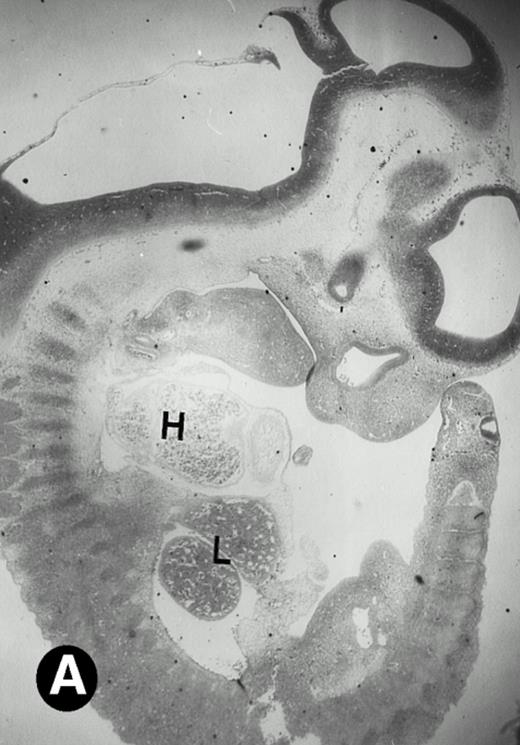
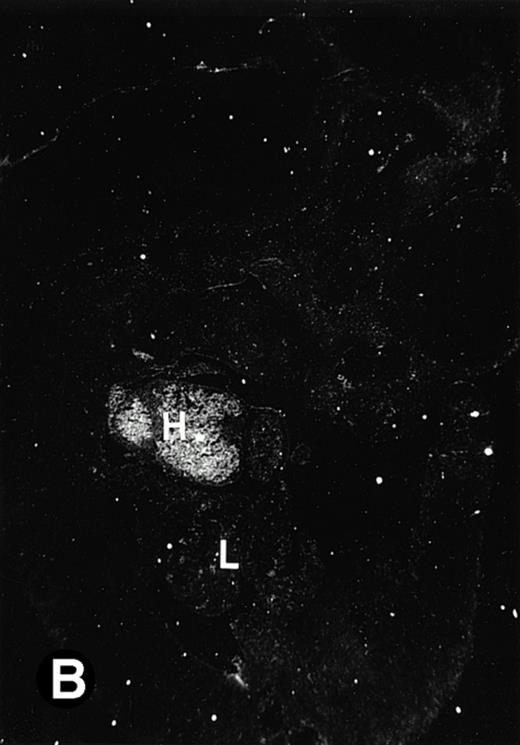
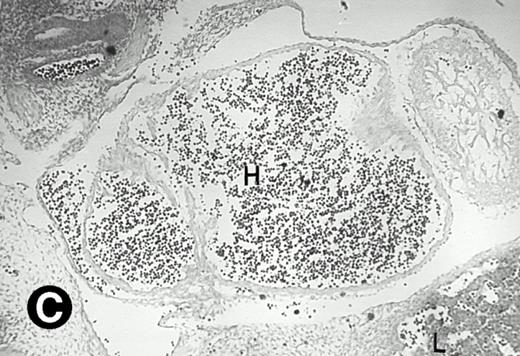
P4.2 gene expression in E10.5 embryos. Bright-field and dark-field views are displayed of hybridization of sagittal sections of embryos at E10.5 days with the P4.2 antisense riboprobe (A and B). Higher-power views of the heart (C and D) or liver (E and F) region are also shown. P4.2 hybridization signal was observed in the erythroid cells in the sinusoids and vessels of the liver (representatives are marked with arrowheads). (A, C, and E) Bright-field illumination; (B, D, and F) dark-field illumination. H, heart; L, liver. Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.
P4.2 gene expression in E10.5 embryos. Bright-field and dark-field views are displayed of hybridization of sagittal sections of embryos at E10.5 days with the P4.2 antisense riboprobe (A and B). Higher-power views of the heart (C and D) or liver (E and F) region are also shown. P4.2 hybridization signal was observed in the erythroid cells in the sinusoids and vessels of the liver (representatives are marked with arrowheads). (A, C, and E) Bright-field illumination; (B, D, and F) dark-field illumination. H, heart; L, liver. Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.
P4.2 gene expression during mid- and late-gestation embryos.
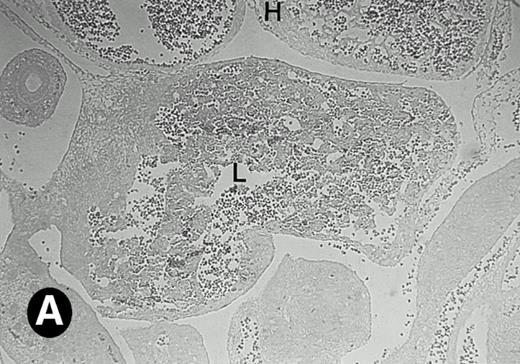
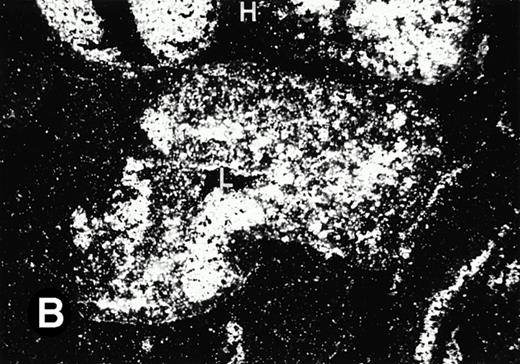
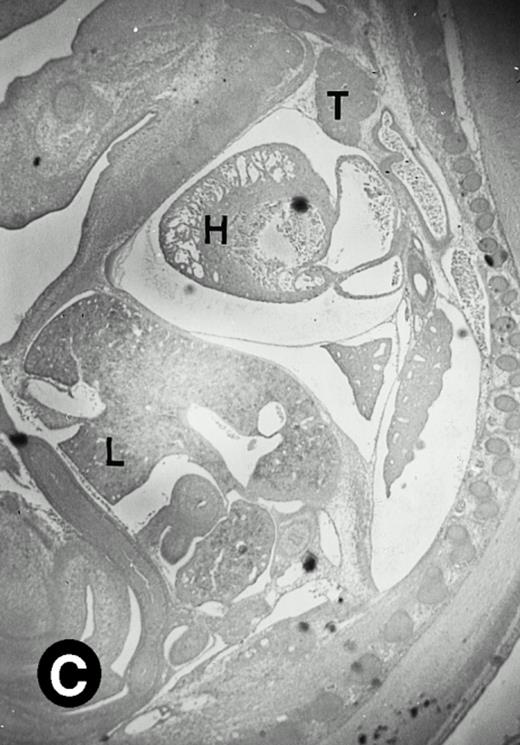
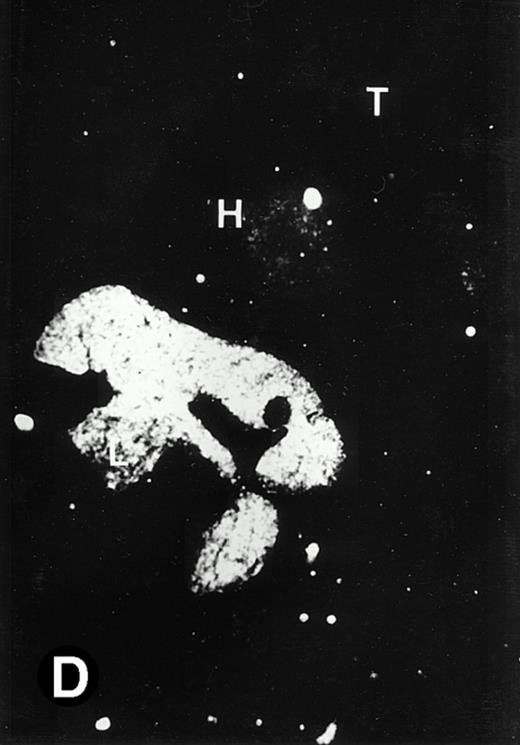
Starting from E12.5 days, there is a switch in the hematopoietic producing sites, from the yolk sac to the fetal liver. Upon the colonization of the multipotential stem cells from the yolk sac to the liver structure at E10.5 days, the fetal liver begins to take over and becomes the almost exclusive hematopoietic organ from E12.5 to E16.5 days. In contrast to primitive erythroid cells derived from the yolk sac, definitive erythrocytes produced from the fetal liver are smaller and enucleated. As soon as the hematopoietic activity appears in fetal liver, P4.2 expression could be seen in this organ. As shown in Fig4, P4.2 hybridization signal could be detected in the liver, heart, and blood vessels of E12.5 embryos (Fig4A and B). Other tissues remained negative for P4.2 hybridization. Similar to those observed in embryos at earlier ages (Figs 2 and 3), P4.2-specific labeling in the heart was detected only in erythroid cells inside the heart chambers (Fig 4C and D). In addition, some P4.2 hybridization signal was seen in circulating erythroid cells in blood vessels (Fig 4E and F). It should be mentioned that, at this stage, only about 1% of erythroid cells in circulation are enucleated and derived from the fetal liver.46-48
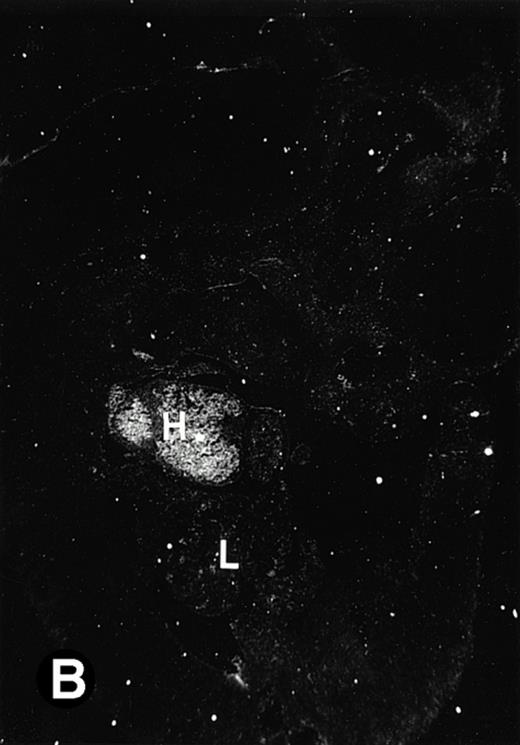
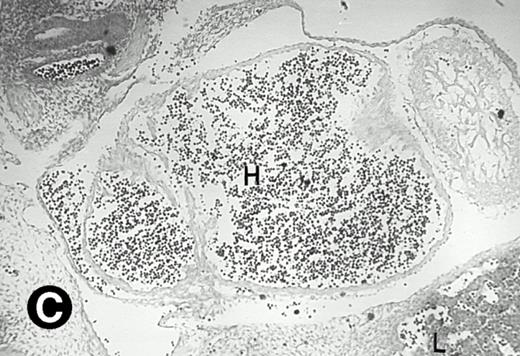
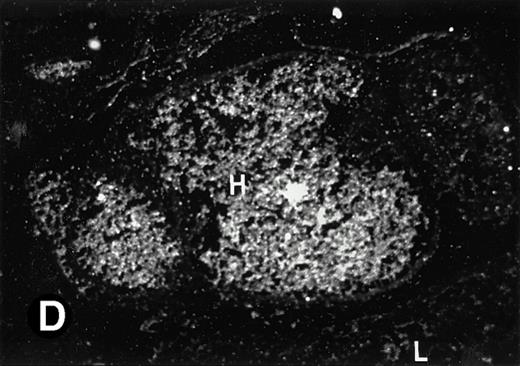
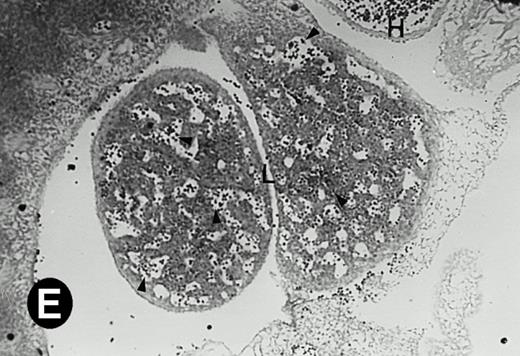
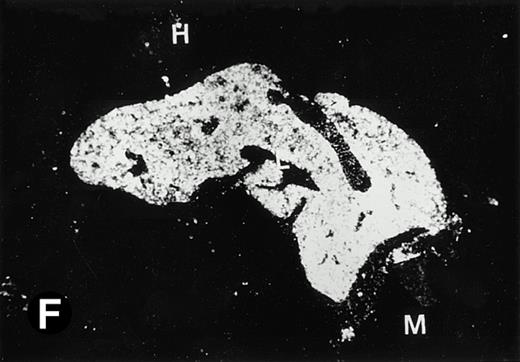
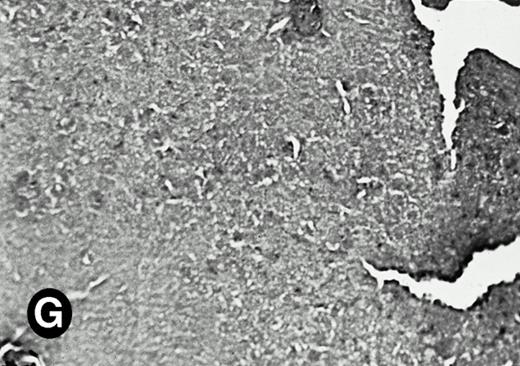
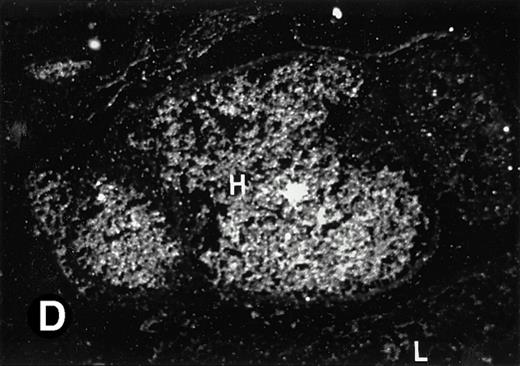
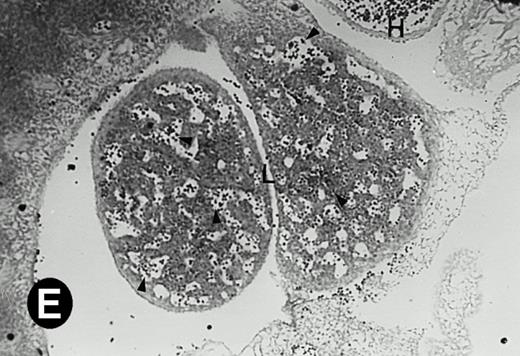
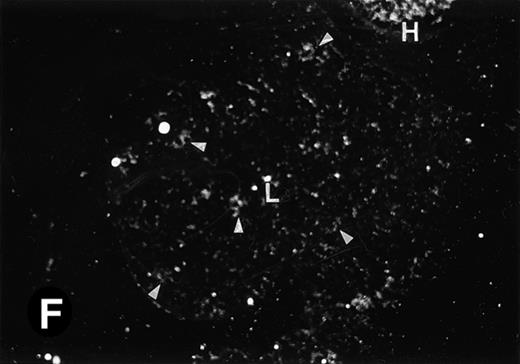
P4.2 gene expression in E12.5 embryos. Sagittal sections of embryos at E12.5 days were hybridized with the P4.2 antisense riboprobe and photographed under bright-field (A) or dark-field (B) illumination. P4.2 hybridization signal was detected in the heart, liver, and blood vessels. H, heart; L, liver; DA, dorsal aorta. Higher-power views of the heart ([C] bright-field illumination and [D] dark-field illumination) or dorsal aorta ([E] bright-field illumination and [F] dark-field illumination) region are also shown. Arrowhead points to P4.2-specific labeling in erythroid cells in the dorsal aorta (E and F). Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.
P4.2 gene expression in E12.5 embryos. Sagittal sections of embryos at E12.5 days were hybridized with the P4.2 antisense riboprobe and photographed under bright-field (A) or dark-field (B) illumination. P4.2 hybridization signal was detected in the heart, liver, and blood vessels. H, heart; L, liver; DA, dorsal aorta. Higher-power views of the heart ([C] bright-field illumination and [D] dark-field illumination) or dorsal aorta ([E] bright-field illumination and [F] dark-field illumination) region are also shown. Arrowhead points to P4.2-specific labeling in erythroid cells in the dorsal aorta (E and F). Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.
As compared with that in E10.5 embryos (Fig 3), P4.2 expression in the liver was greatly enhanced in E12.5 embryos (Fig 5A and B). By E14.5 days, the liver showed the strongest P4.2 hybridization signal among all organs (Fig 5C and D). P4.2-specific labeling was also detected in the heart and blood vessels, but their intensity was greatly reduced (compare Fig 5 with Fig 4), consistent with the dramatic increase of fetal liver-derived, enucleated erythrocytes in circulation. In contrast, as the main source of T-lymphocyte differentiation, the thymus showed no P4.2 hybridization in E14.5 embryos (Fig 5C and D). This lymphogenic organ remained negative for P4.2 expression even after birth (data not shown).
Expression of the P4.2 transcript during liver development. Shown are bright-field (A, C, E, and G) and dark-field (B, D, F, and H) views of hybridization to the P4.2 antisense riboprobe. (A and B) The liver from an E12.5 (A and B) embryo section; (C and D) the abdominal region containing the liver from an E14.5 (C and D) or E16.5 (E and F) embryo; (G and H) a section of liver from the mouse at 4 weeks after birth. T, thymus; H, heart; L, liver; M, metanephros. Micrographs were taken with a 20× (A, B, G, and H) or 2.5× (C, D, E, and F) objective.
Expression of the P4.2 transcript during liver development. Shown are bright-field (A, C, E, and G) and dark-field (B, D, F, and H) views of hybridization to the P4.2 antisense riboprobe. (A and B) The liver from an E12.5 (A and B) embryo section; (C and D) the abdominal region containing the liver from an E14.5 (C and D) or E16.5 (E and F) embryo; (G and H) a section of liver from the mouse at 4 weeks after birth. T, thymus; H, heart; L, liver; M, metanephros. Micrographs were taken with a 20× (A, B, G, and H) or 2.5× (C, D, E, and F) objective.
In E16.5 embryos, P4.2-specific labeling remained strong in erythroid cells of the hepatic mesoderm (Fig 5E and F). A weak P4.2 hybridization signal was detected in the heart and blood vessels, because at this time about 99% of circulating erythrocytes are enucleated. Little or no P4.2-specific labeling was seen in metanephros, which later develop into kidneys (Fig 5E and F). After E16.5 days, hepatocytes begin to speed up in proliferation and differentiation with the biliary duct as center, developing a mature lobular format; simultaneously, the hematopoietic cells in the liver begin to recede in hematopoietic function and their proliferation decreases, with hepatocytes gradually replacing the hematopoietic cells. In correlation, P4.2 hybridization signal was greatly reduced in the liver after E16.5 days (Fig 5), being almost undetectable after birth (Fig 5G and H). These results indicate that P4.2 expression is restricted to hematopoietic cells in the fetal liver during mid- and late-gestation embryos.
P4.2 gene expression in spleen and bone marrow.
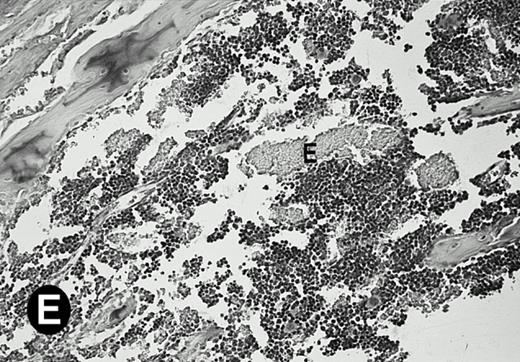
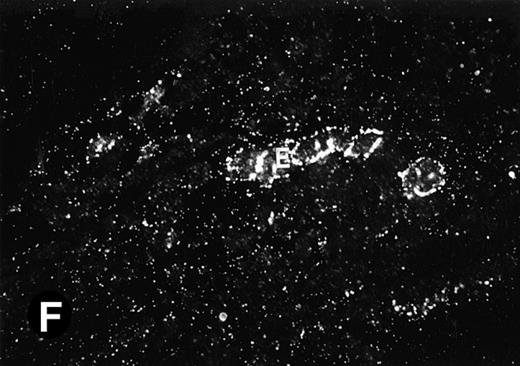
Because around and after birth, the spleen together with the bone marrow become the major hematopoietic organs in mice, we examined P4.2 expression in these hematopoietic organs by in situ hybridization of tissue sections, as described above. In newborn mice, P4.2-specific labeling was detected in specific areas of the spleen (data not shown). Significant P4.2 expression was seen in the emerging red pulp, which later makes up the largest part of the organ and is the center for erythropoiesis and megakaryocyte production. As the components of the spleen become more distinguished, increasing P4.2 hybridization signal was still confined to the red pulp on postnatal day 7 and day 15 (Fig6A and B). No P4.2-specific labeling was seen in the white pulp, which consists of germinal centers for lymphocytes, plasma cells, and macrophages. In older animals, P4.2 expression remains localized exclusively in the red pulps (data not shown). Upon higher magnification of areas containing red pulps from spleen sections of 15-day-old mice, P4.2-specific labeling was detected in erythroid cells, particularly at higher intensity in areas with more mature erythroid cells (lightly stained by crystal violet; Fig 6C and D). In contrast, P4.2 hybridization signal was not detected in megakaryocytes (easily identified as giant cells with a polylobulated nucleus surrounded by a large cytoplasm; Fig 6C and D). Histologic identification of all these cell types was confirmed by standard hematoxylin-eosin staining of adjacent tissue sections.
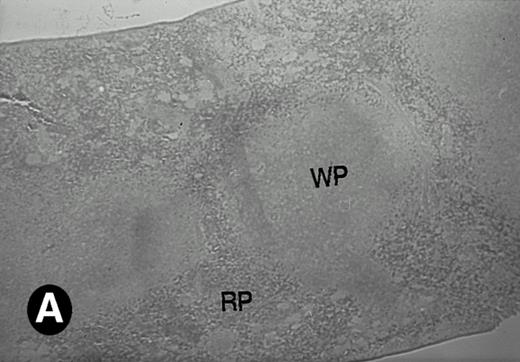
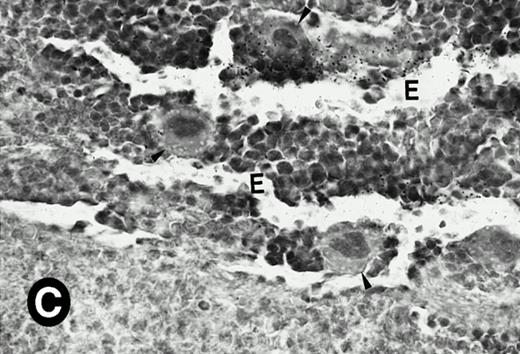
P4.2 gene expression in the mouse spleen and bone marrow. Shown are in situ hybridization analysis of serial spleen sections from a 15-day-old mouse (A through D) using the P4.2 antisense riboprobe. A representative region of red pulp (RP) or white pulp (WP) in the spleen is indicated in (A) and (B). Higher-power views of a red pulp region are shown in (C) and (D). The lightly stained areas (labeled with “E”) enrich more mature erythroid cells and show substantial P4.2 hybridization. Arrowheads indicate isolated megakaryocytes. Sections containing bone marrows from a 7-day-old mouse (E and F) were also hybridized with the P4.2 antisense riboprobe. A representative erythroid colony in the bone marrow is labeled with “E.” Micrographs were taken with a 20× (A and B) or 40× (C through F) objective. (A, C, and E) Bright-field illumination; (B, D, and F) dark-field illumination.
P4.2 gene expression in the mouse spleen and bone marrow. Shown are in situ hybridization analysis of serial spleen sections from a 15-day-old mouse (A through D) using the P4.2 antisense riboprobe. A representative region of red pulp (RP) or white pulp (WP) in the spleen is indicated in (A) and (B). Higher-power views of a red pulp region are shown in (C) and (D). The lightly stained areas (labeled with “E”) enrich more mature erythroid cells and show substantial P4.2 hybridization. Arrowheads indicate isolated megakaryocytes. Sections containing bone marrows from a 7-day-old mouse (E and F) were also hybridized with the P4.2 antisense riboprobe. A representative erythroid colony in the bone marrow is labeled with “E.” Micrographs were taken with a 20× (A and B) or 40× (C through F) objective. (A, C, and E) Bright-field illumination; (B, D, and F) dark-field illumination.
To examine what types of cells in bone marrows expressed P4.2 transcripts, we conducted in situ hybridization of bone marrow sections from mice at various times after birth. Similar to that observed in the spleen, P4.2-specific labeling was detected only in erythroid cells and colonies in the bone marrow from 7-day-old mice (Fig 6E and F). Similar results were obtained in bone marrow of older animals (data not shown). In addition, no P4.2 hybridization signal was seen in cells of nonerythroid lineage, including megakaryocytes. These results indicate that P4.2 message was specifically expressed in cells of the erythroid lineage in postnatal hematopoietic organs.
DISCUSSION
We describe here the temporal and spatial expression pattern of the erythrocyte protein 4.2 gene during mouse embryonic development. We show that expression of the mouse P4.2 gene is temporally regulated during embryogenesis and that the P4.2 mRNA expression pattern coincides with the timing of erythropoietic activity in hematopoietic organs, suggesting a role for this gene in the differentiation of erythroid cells.
Erythropoiesis is a complicated differentiation process during which pluripotent hematopoietic stem cells are orchestrated to become the differentiated erythrocytes.46,47 49 In mice, this process is performed in various hematopoietic organs at different stages of development: yolk sac (E7.5-11.5 days), fetal liver (E12.5-16.5 days), and bone marrow and spleen (around and after birth). Our in situ hybridization results show that P4.2 expression follows the ebb and flow of the erythropoietic activity in each hematopoietic organ at specific developmental stages.
Consistent with the detection of the P4.2 protein in cultured erythroid cells corresponding to the stage of early erythroblasts,50P4.2 transcript was first detected in nucleated erythroid precursors derived from the yolk sac at E7.5 days. During embryonic stage, nucleated erythroid cells derived from the yolk sac progress from pre-type 1 to type 3 erythroblasts during E10.5 to 14.5 days, whereas fetal liver cells, primarily pro- and basophilic erythroblasts at E12.5 to 14.5 days, differentiate predominantly into erythrocytes in the peripheral blood by E16.5 days.40 49 By comparing with the hybridization signal of circulating erythroid cells in the heart and vessels of E10.5 embryos, P4.2 expression appears to be increased at E12.5 embryos and then decreased at 14.5 days, indicating the up-and-down change of P4.2 mRNA levels after the differentiation of yolk sac-derived erythroblasts. Similarly, the up-and-down expression pattern of P4.2 is also observed in the liver from E10.5 days to after birth. By comparing P4.2 expression in circulating erythroid cells in the heart with that in fetal liver of E16.5 embryos, mature erythrocytes released from the fetal liver show much less P4.2 expression. Analogously, we note that circulating erythrocytes derived from the spleen and bone marrow after birth gave rise to very weak P4.2 hybridization signal.
The P4.2 gene is mapped to human chromosome 15 at bands q15-2113,51 and to mouse chromosome 2.52 The chromosomal location of the mouse P4.2 gene was found to be near the mouse pallid (pa) mutation. Pallid was found in a mouse caught in the wild,53,54 which shows dilution of coat color,55 increased bleeding time, and abnormal lysosomal enzyme secretion.56,57Pallid is one of several independent mouse mutations that are models of the platelet storage pool disease.58 In addition, Pallid mice have been proposed as a model of genetic α1-antitrypsin deficiency.59 Using Southern and Northern blot analyses, White et al52 previously suggested that pa is a mutation in the P4.2 gene. However, patients with P4.2 deficiency do not have the platelet storage pool deficiency seen in pa/pamice and the mutant mice do not have the hemolysis and spherocytosis seen in the patients. Recently, Gwynn et al60 conducted additional analyses of P4.2 in pallid mice and concluded that P4.2 and pa are distinct loci and changes in P4.2 inpallid mice are not responsible for the pallidmutation.60
In agreement with our previous findings,16 the Northern blot analysis detected the expression of a single 3.5-kb mouse P4.2 message in the spleen. Little or no P4.2 expression was found in other tissues examined when either commercially available RNAs or RNAs that we isolated from various mouse tissues were used. These results are different from those reported by White et al.52 They previously reported the detection of P4.2 transcripts in tissues other than spleen, including kidney, heart, brain, and liver, and showed that the heart expressed a larger mRNA than the brain, liver, and kidney in normal mice. Also, the kidney in pallid mice, an affected organ by the pallid mutation, expressed a smaller transcript.52 Intriguingly, we detected no P4.2 transcript in the brain, lung, liver, skeletal muscle, kidney, and testis of adult mice. Similar results were also obtained when a 2.2-kb mouse P4.2 cDNA containing the entire coding region was used as the probe (our unpublished results). In addition, this P4.2 expression pattern was further confirmed by the in situ hybridization analysis, which detects an erythroid tissue-restricted expression for the P4.2 gene. Presently, the reason for the discrepancy is not known. It is possible that the probe used may affect the specificity of detection, because we noted that the 3′ untranslated region of the mouse P4.2 cDNA appears to cross-react with other genomic sequences. Alternatively, the stringency for hybridization and washing conditions in the analysis may need to be considered.
Our Northern blot analysis also detected a very small amount of the same 3.5-kb P4.2 transcript in the heart. The results from in situ hybridization analysis showed that, during embryonic development, P4.2 expression in the heart was detected only in erythroid cells inside the heart chambers but not in cardiomyocytes. Similarly, we did not detect any P4.2 expression in the heart muscle at various ages after birth. We only observed very weak P4.2 hybridization signal in circulating erythrocytes in the heart and blood vessels. Thus, it is likely that the low level of P4.2 expression detected in this organ in Northern blot analysis may represent residual P4.2 mRNA in circulating erythrocytes.
Immunoreactive forms of the erythrocyte P4.2 protein have been detected in nonerythroid tissues such as brain, kidney, and platelets and cell lines including HeLa and HT-29.6,18-20 However, the results presented in this report show that P4.2 mRNA expression is restricted to cells of the erythroid lineage. No P4.2 message was detected in nonerythroid organs or megakaryocytes. In addition, by the reverse transcription-PCR61 we have not been able to detect any P4.2-specific cDNA product using RNAs isolated from HeLa or HT-29 cells. Taken together with the observation that the mouse P4.2 gene only express a 3.5-kb message,15-17 these results indicate that the immunoreactive forms of P4.2 detected in nonerythroid tissues or cells are likely not P4.2 protein isoforms; yet, they may be P4.2-related proteins. It should be noted that proteins related to the erythrocyte protein 4.1, another erythrocyte membrane protein, have been identified in nonerythroid cells.32 35 Alternatively, the immunoreactive forms of P4.2 detected in nonerythroid cells may represent differences in P4.2 expression among different species or due to immortalization/transformation (eg, in certain cell lines). Further characterization of the molecular nature of these P4.2-immunoreactive analogs will be needed to show their identity. In addition, we are presently producing mouse P4.2-specific antibodies to examine the P4.2 protein expression.
Nevertheless, the erythroid cell-restricted P4.2 expression pattern in mice suggests the existence of specific regulation of the P4.2 gene. Our transcription mapping and sequencing analyses show that the upstream regulatory regions of both the mouse and human P4.2 genes contain multiple transcription factor-binding sites, including those of the erythroid transcription factors GATA-1, TAL-1, and EKLF (Karacay and Chang, manuscript in preparation). It has been suggested that these transcription factors, by regulating the cell-type specific expression of genes, can control the differentiation status of the hematopoietic cells.62 63 Experiments are in progress to examine how the P4.2 gene is regulated in the erythroid environment.
ACKNOWLEDGMENT
The authors are grateful to Drs Vincent V. Hamparian and Philip T. Nowicki for critical reading of the manuscript and to members of the Chang Laboratory for helpful discussions.
Supported by research grants from American Heart Association and Children's Hospital Research Foundation.
Address reprint requests to Long-Sheng Chang, PhD, Department of Pediatrics, Children's Hospital, The Ohio State University, W230, 700 Children's Dr, Columbus, OH 43205-2696.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




![Fig. 4. P4.2 gene expression in E12.5 embryos. Sagittal sections of embryos at E12.5 days were hybridized with the P4.2 antisense riboprobe and photographed under bright-field (A) or dark-field (B) illumination. P4.2 hybridization signal was detected in the heart, liver, and blood vessels. H, heart; L, liver; DA, dorsal aorta. Higher-power views of the heart ([C] bright-field illumination and [D] dark-field illumination) or dorsal aorta ([E] bright-field illumination and [F] dark-field illumination) region are also shown. Arrowhead points to P4.2-specific labeling in erythroid cells in the dorsal aorta (E and F). Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.695/3/m_blod4023804a.jpeg?Expires=1768502696&Signature=lsHdXNYIWJSyn9Ys~6iGcs~cJMW2BNHlpADg~hFXruD0sCNITGDRTMpX2qloN75O2jRK70mnmRPvrLe7HTavw39bVP9L1u19hTPCZr6dJkoKFsUFmX4IkjelYz0IDnEbfGF~ZYi8WBSkVLxPCBThyVChU1dFtJhw3i4wG-Vybsj2uZb3X8SclZR7fja-Xhh-ihgpD9nHzCyKCjn7~RcQIziyu9s2fwaOPzl5WEtMFvaxCfIxWljIR1dqlbfBmrs1y1JrKALJf9yKo6AnLVDRXbQ1hFzYh62KHOmhacH14TkOOUL5qQ44cjl-FeTbf6YMsVh~0Lm9Qv4u8yKDLwl5HA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. P4.2 gene expression in E12.5 embryos. Sagittal sections of embryos at E12.5 days were hybridized with the P4.2 antisense riboprobe and photographed under bright-field (A) or dark-field (B) illumination. P4.2 hybridization signal was detected in the heart, liver, and blood vessels. H, heart; L, liver; DA, dorsal aorta. Higher-power views of the heart ([C] bright-field illumination and [D] dark-field illumination) or dorsal aorta ([E] bright-field illumination and [F] dark-field illumination) region are also shown. Arrowhead points to P4.2-specific labeling in erythroid cells in the dorsal aorta (E and F). Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.695/3/m_blod4023804b.jpeg?Expires=1768502696&Signature=I8LZ-xm0PITjsSabtA3b99w7DxEE-TEzPjExmFkULe5-~9nbkqlt0w~D3188rkvluw8PxqtsVOAgVqpCwI~l2Mq4naVlDEHHBdXzcyroPGbpAPV2marsL4RPXoaKptW09e6umwi3XbUoCfq1guIpdanepxbXs5m5mZYJWkE-8cj8f-L93~EqxvLIUn062Rzdr-hGeiA7u7gUS99ItQABNcbAk3JdAVka6CwevCHFzobrf2TBh8ye6AJDfp4kjPgz3fgvqrjrf4LsqiwAMjTaGKUx0ZZtkViwvL8CTeBxSkvgydzHWdt-CD7eSf5doymFp0WkKauRMxGm6wg41qwLUA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. P4.2 gene expression in E12.5 embryos. Sagittal sections of embryos at E12.5 days were hybridized with the P4.2 antisense riboprobe and photographed under bright-field (A) or dark-field (B) illumination. P4.2 hybridization signal was detected in the heart, liver, and blood vessels. H, heart; L, liver; DA, dorsal aorta. Higher-power views of the heart ([C] bright-field illumination and [D] dark-field illumination) or dorsal aorta ([E] bright-field illumination and [F] dark-field illumination) region are also shown. Arrowhead points to P4.2-specific labeling in erythroid cells in the dorsal aorta (E and F). Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.695/3/m_blod4023804c.jpeg?Expires=1768502696&Signature=CHYsVFGi3YGTfNuArKLes014F96n9JhMZvG77Sxa-vYYXWvvg9qLkCMKIsq4aAgZZ03z2FtizsyoH6UTnMQwCOZfOkVuMDHwG4AjGttZFGSu1UxJKy5ybSTSOXF27OlLujysWRazjy7HUdGgiQcmjvPZYxPkFegWOEs14hF0ZJFKVTPMjx0Pw12xzM-vUoi~VMTY~oVjGiShknDCgAReOOTIFeq6Jzb8xA06pPSnh4AxZ0jUTO9tLxKgLrKXkBYGK-D5G8V-PwRqnZ1RHNnqXe99uzxoU4AXR8YAs3wKPxS9PPrBLpCC5fTUJhrtV-JenzoEQPNcoDyzMp5pzZ3TSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. P4.2 gene expression in E12.5 embryos. Sagittal sections of embryos at E12.5 days were hybridized with the P4.2 antisense riboprobe and photographed under bright-field (A) or dark-field (B) illumination. P4.2 hybridization signal was detected in the heart, liver, and blood vessels. H, heart; L, liver; DA, dorsal aorta. Higher-power views of the heart ([C] bright-field illumination and [D] dark-field illumination) or dorsal aorta ([E] bright-field illumination and [F] dark-field illumination) region are also shown. Arrowhead points to P4.2-specific labeling in erythroid cells in the dorsal aorta (E and F). Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.695/3/m_blod4023804d.jpeg?Expires=1768502696&Signature=4cIseV3wqaO1CPlz4U4kcvYYWW586xL-1p03B7UmiYXXbFIuAMvIYmEII1v3JDtF~NhUPJQ~r7PmcgubmDh4HeNQ-7dIyTOMsNr6~wae02Cmt5zkuKH-oU-5L0xD1Hz1tbcz~ZmKBb~vugOqWYbMQXlo8wPu6O8qc7TP-6q2XH5Y-WqUv3lB9UDCVGrxkR7NAey5jbwrsHb-l0VtokKEPw~JjmQAQk3pHZrKsp67cPhSW6KzY1-KqSk7A-U9YyR3ujitOKDy94H3VwroZ~8ye3c14O1wuNdUmvOGLXQ~Aa0EGR6K-DbuUpdp8bmCyY8c7LwoKQgfgNg80~qjVuudIQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. P4.2 gene expression in E12.5 embryos. Sagittal sections of embryos at E12.5 days were hybridized with the P4.2 antisense riboprobe and photographed under bright-field (A) or dark-field (B) illumination. P4.2 hybridization signal was detected in the heart, liver, and blood vessels. H, heart; L, liver; DA, dorsal aorta. Higher-power views of the heart ([C] bright-field illumination and [D] dark-field illumination) or dorsal aorta ([E] bright-field illumination and [F] dark-field illumination) region are also shown. Arrowhead points to P4.2-specific labeling in erythroid cells in the dorsal aorta (E and F). Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.695/3/m_blod4023804e.jpeg?Expires=1768502697&Signature=sldGJTmj3obp1-R06HGtYIvLVpQwZtKv094pXy5w5wmLehg~Id4Miyy2A-PCzZxXGx8Bziu3FtNMI3yczxPDG8AELGIl5WR~iZWB1HlS72xlmLUD3miveyuXb5x230W-3~tA6nwEBjd3gYKfL4WX-kXLxDUEEK5FgOYrtKYasJWwGb-yGPUbTkgHdnPVd08On7GyVPR21xh3OnZCogAoOT3T59fS~9wh8ZwZCy6r9U~J7KkNf6PHgaUieuSbu7pPG265Phttz0OHrFaVIyzFwqfJXfP4ISfIcGRn805cwFPlcuLUaY2ghj9mJeIfIB6xtTXRUGrkDWhmpc2wfWoBlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. P4.2 gene expression in E12.5 embryos. Sagittal sections of embryos at E12.5 days were hybridized with the P4.2 antisense riboprobe and photographed under bright-field (A) or dark-field (B) illumination. P4.2 hybridization signal was detected in the heart, liver, and blood vessels. H, heart; L, liver; DA, dorsal aorta. Higher-power views of the heart ([C] bright-field illumination and [D] dark-field illumination) or dorsal aorta ([E] bright-field illumination and [F] dark-field illumination) region are also shown. Arrowhead points to P4.2-specific labeling in erythroid cells in the dorsal aorta (E and F). Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.695/3/m_blod4023804f.jpeg?Expires=1768502697&Signature=sjOeDk9Jh5EWLrjV8fbf~80IBE-BC1X3dlX0ZwrrnHCnIXYsEfOMe8kv3xAZFv6Qb27hmGF-UhKYOUho88FBSwqM~18yYTXts8Lgi5AgMvBCFRFr4Hjupfu~vK8CigLjXF~4CnhMXMR1WEEsH~3oCeJlyU0f1ToXqHGSI0nSaG~1hks9ihrgMQRYuxmMTmoUit-22sJeu174fKcLYsevZ3hTo0XaztK87c82Tw4GGe7-NkIbOt7YoYRfVaMOOk5XRqja-hE4mEoydNdIe7Y1L93dXCs3PV8TfzHmCohgGOMto3XxB4eMb5N8MJq2HiQcoR5UAI8fQfAsuuUA44MpCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)







![Fig. 4. P4.2 gene expression in E12.5 embryos. Sagittal sections of embryos at E12.5 days were hybridized with the P4.2 antisense riboprobe and photographed under bright-field (A) or dark-field (B) illumination. P4.2 hybridization signal was detected in the heart, liver, and blood vessels. H, heart; L, liver; DA, dorsal aorta. Higher-power views of the heart ([C] bright-field illumination and [D] dark-field illumination) or dorsal aorta ([E] bright-field illumination and [F] dark-field illumination) region are also shown. Arrowhead points to P4.2-specific labeling in erythroid cells in the dorsal aorta (E and F). Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.695/3/m_blod4023804a.jpeg?Expires=1768502697&Signature=sBpnSHi15aWh9jRsN8ETVmGXZzQD0OODC0XWU0emBOIzCNlhCodGEF2RNkWVi9KXTWZ1kNdsOcnObtXjuhnJwzY3n9GzA6IrPX-dt7Z3yr1~yq0Oskadx~Y1pTVEQvOPMPbMEs9bos1Ka7ezwCjjFYfAY5a2VFFYUh-5kffwXD2wP6uU-bgSwRNBPSAiG6G8Ke6w4B9Trn2CDGozy35p9Xk4E3l-2cHWzm-9fvghCc6kAvD-rDi656vG566hhfploNGO2zb2I7wY5Q95NyGdbJMZDkGg9~WXXEGq~JNfqNkFuzs7C7QsWbEGOyYYy4vCIR88d3Kv1DU8lT6Pj96v~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. P4.2 gene expression in E12.5 embryos. Sagittal sections of embryos at E12.5 days were hybridized with the P4.2 antisense riboprobe and photographed under bright-field (A) or dark-field (B) illumination. P4.2 hybridization signal was detected in the heart, liver, and blood vessels. H, heart; L, liver; DA, dorsal aorta. Higher-power views of the heart ([C] bright-field illumination and [D] dark-field illumination) or dorsal aorta ([E] bright-field illumination and [F] dark-field illumination) region are also shown. Arrowhead points to P4.2-specific labeling in erythroid cells in the dorsal aorta (E and F). Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.695/3/m_blod4023804b.jpeg?Expires=1768502697&Signature=awOiIA9lKjLMLeQscukSwklFQWP14KCjmlhwl15APDlOdasrFNAS5KUBA9IwTj3TNTIA9Q3Bm2jIojfpi6nGKFsX8ViXJyOPr4bE9mgo9yd3qH8M-tbDvDgZ4ji~hoo1GT4hbaWE2M1VqXpHWuS84vV4d0sdpH9~8kwcVtT~j4LLT5sKML-W78Gp7WPKleYpsWADpe9Z6oTD~kff29WVKIG7r9R7xwCJGqHDUUtul8Jnt10euLJNZyzSap5U3XqrnaCF29e7fPoIU-JDTheIlzv20B7KCGygk5LNs2B2XNishZzeLwX-mohE0YBLJk3OjgM5722CZ7NGqXMFsADhuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. P4.2 gene expression in E12.5 embryos. Sagittal sections of embryos at E12.5 days were hybridized with the P4.2 antisense riboprobe and photographed under bright-field (A) or dark-field (B) illumination. P4.2 hybridization signal was detected in the heart, liver, and blood vessels. H, heart; L, liver; DA, dorsal aorta. Higher-power views of the heart ([C] bright-field illumination and [D] dark-field illumination) or dorsal aorta ([E] bright-field illumination and [F] dark-field illumination) region are also shown. Arrowhead points to P4.2-specific labeling in erythroid cells in the dorsal aorta (E and F). Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.695/3/m_blod4023804c.jpeg?Expires=1768502697&Signature=G0sxkLmHC4vLwJLRAH9oI1Jrpp30GQRTinb8anfNXDOqo-X0X1h1UdIZ0BpzekgZkh1REIK~X9T1MLLiz0~vHVhUARXAPX6ksBx6ku~SVvYqFn9Aac0PaZuJHwNks9OkO9EzvEzi-v9tack9FUn91jNL9snD-snT-zIFwlR--fN5DoiOMwviWtgi6GZgG136mWpccsEB1dLtO1HmsHE7mQafSacM11Mwt0s-P-vgTXlKPhsgfnmPQKmyy3PsM9LDnK-oXa0pVVaNNNGlpQHcyO1esj1XCQoYTWFMAljUsoYjn2YUWv-N4GQHm~JFCBokVaYXA54JHpU4AOIG05Lc7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. P4.2 gene expression in E12.5 embryos. Sagittal sections of embryos at E12.5 days were hybridized with the P4.2 antisense riboprobe and photographed under bright-field (A) or dark-field (B) illumination. P4.2 hybridization signal was detected in the heart, liver, and blood vessels. H, heart; L, liver; DA, dorsal aorta. Higher-power views of the heart ([C] bright-field illumination and [D] dark-field illumination) or dorsal aorta ([E] bright-field illumination and [F] dark-field illumination) region are also shown. Arrowhead points to P4.2-specific labeling in erythroid cells in the dorsal aorta (E and F). Micrographs were taken with a 10× (A and B) or 20× (C, D, E, and F) objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/2/10.1182_blood.v91.2.695/3/m_blod4023804d.jpeg?Expires=1768502697&Signature=I3Vgr0k2SBGjRoiHuR3DYdAvT83HpCAl5DScnG3--VecRcnwZhCKQV3EnmQRwUM8otJFtqSGIPVAzb8pzPYCUwUkPPZT8uhzCMbze2Oj5gzccttsD9l3NeNp5Tt32fFgcAY5wdGywa-axMKesEe7V4FV6lC8~vCoTseP5CKWLekzOWrk~KWkNDwxxf9Hfg4QThWoflH8C3c59c9svf0k4Ht7SWFYqDjozWshSayhyD5T8nf-UUmOUrj7k6CFjfnTotxUVGpQItIVtWoBGyyhwTI19JFCpHIRIhVG9b3HAx8ddN-5MKHSIw7O4w7JVTkEmru4OYnCKEfUuXJQnLzh3A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)