Abstract
This study was undertaken to assess the significance of lung-resistance related protein (LRP) expression in plasma cells from untreated multiple myeloma (MM) patients and to determine whether LRP was associated with a poor response and survival in patients treated with different dose regimens of melphalan. Seventy untreated patients received conventional oral dose melphalan (0.25 mg/kg, day 1 to 4) combined with prednisone (MP) or intravenous intermediate-IDM; 70 mg/m2) or high- (140 mg/m2) dose Melphalan (HDM). LRP expression was assessed with immunocytochemistry using the LRP-56 monoclonal antibody. LRP expression was found in 47% of patients. In the MP treated patients, LRP expression was a significant prognostic factor regarding response induction (P < .05), event free survival (P < .003), and overall survival (P < .001). In the intensified dose melphalan treated patients LRP did not have a prognostic value. The response rates of LRP-positive patients to MP and IDM/HDM were 18% versus 81%, respectively (P < .0001). We conclude that LRP is frequently expressed in untreated MM patients and is an independent predictor for response and survival in patients treated with MP. Pretreatment assessment of LRP identifies a subpopulation of patients with a poor probability of response to conventional dose melphalan. Dose intensification of melphalan is likely to overcome LRP-mediated resistance.
ALKYLATING AGENTS and corticosteroids are still the mainstay of therapy for patients with multiple myeloma (MM).1,2 However, only approximately 50% to 60% of patients respond to oral melphalan and prednisone in conventional dose resulting in a median response duration of 1 to 2 years and a median survival of about 3 to 4 years. Dose-intensification studies have shown a dose-response relationship for melphalan3-5 in MM; therefore, high-dose melphalan has been used to improve response rates and survival.
Melphalan, like other alkylating agents, exerts its cytotoxic effect through the covalent linkage of alkyl groups to DNA. Resistance against alkylating agents includes both cellular and extracellular factors. In cell line studies resistance to melphalan has been attributed to a decreased drug uptake caused by alterations in either the number or the affinity of membrane-bound proteins.6 An alternative explanation may be an increased cellular detoxification by glutathione S-transferases.7 So far, studies in hematological malignancies such as MM have failed to show a role of these laboratory findings in clinical specimens.8 Multiple drug resistance (MDR) has been identified as an important path of drug resistance in MM. MDR is the phenomenon of cancer cells developing cross-resistance to a variety of structurally unrelated chemotherapeutic compounds such as vinca-alkaloids, anthracyclines, and epipodophyllotoxins.9 MDR is associated with the expression of the drug transport mediating proteins P-glycoprotein (PgP) and the multidrug resistance–related protein (MRP).10 The increasing evidence of additional mechanisms of MDR led recently to the identification of a novel protein associated with MDR, originally termed the lung-resistance protein (LRP).
The LRP gene has recently been cloned and identified as the human p110 major vault protein.11 Vaults are novel cellular organelles first described by Kedersha and Rome in 1986,12 which are thought to mediate intracellular transport of a wide variety of substrates. LRP has been found to be widely distributed in human normal tissues and in tumors, closely reflecting the susceptibility to chemotherapy of different tumor types.13 Importantly, recent studies in myeloma and other human cancer cell lines relate LRP expression to resistance against the alkylating agent melphalan14 15 (and W.S. Dalton et al, personal communication, July 1997).
In the current study we have assessed LRP expression in myeloma patients, and based on these results we introduce this MDR-related protein as a putative important marker of clinical resistance to the alkylating agent melphalan resulting in an adverse prognosis. Moreover, we describe the overcoming of LRP-related resistance against melphalan by dose intensification.
PATIENTS AND METHODS
The study was performed on nonselected, sequentially stored frozen cytocentrifuge slides prepared from Ficoll-Hypaque–purified bone marrow aspirates obtained from all MM patients with newly diagnosed disease who were treated with melphalan-based regimens between January 1987 and November 1995.
Additionally, bone marrow aspirates of three normal donors for allogeneic bone marrow transplantation (BMT) and the aspirates of five patients with monoclonal gammopathy of undetermined significance (MGUS) were studies for LRP expression.
Patients.
Seventy unselected patients treated at the Departments of Haematology of the University Hospital Utrecht or the University Hospital Rotterdam Dijkzigt were studied. Clinical staging was defined according to the criteria proposed by Salmon and Durie.16 Median age of patients treated with conventional-dose oral melphalan and prednisone was 67 years. Patients treated with intravenous (IV) intermediate- or high-dose melphalan were median 52 years. The performance status was determined according to the criteria of the Eastern Cooperative Oncology Group (ECOG): 0, normal; 1, ambulant with symptoms; 2, bedrest less than 50% of the day; 3, bedrest greater than 50% of the day; 4, bedrest all day. The patient characteristics are summarized in Table1.
Chemotherapy regimens and response evaluation.
Patients received melphalan as first-line treatment, either in combination with prednisone (MP, 38 patients) or as monotherapy in intermediate dose (IDM, 20 patients) or high dose (HDM, 12 patients). Patients under 65 years of age were candidates for IDM or HDM, unless they refused intensive treatment. Patients refusing intensive treatment and patients over 65 years received MP. Performance status was no selection criterium for treatment modality. The intermittent MP regimen consisted of oral melphalan 0.25 mg/kg/d and prednisone 2 mg/kg/d administered for 4 days. Courses were repeated every 6 weeks.
IDM (melphalan 70 mg/m2) was administered by rapid IV infusion. Two courses of IDM were given with an interval of 6 weeks.17 The HDM regimen (140 mg/m2) consisted of a single dose. Response was determined by standard criteria for myeloma response.18 A partial response was defined as a reduction of at least 50% in serum M protein or urinary light chain concentration with no progression of lytic bone lesions, without increase of bone pain or anemia. A complete response (CR) was defined as complete disappearance of myeloma proteins from serum and urine and normalization of the bone marrow. Response in patients treated with MP was determined after 4 courses, or earlier when progression was obvious. When a partial response (≥50% reduction in M protein) was achieved, therapy was continued for at least 1 year. Patients with a minimal response (between 25% and 50% reduction in M protein) received another four courses. Patients unresponsive after four courses (less than 25% reduction in M-protein concentration) and patients with a minimal response after four courses but no further improvement of response after eight courses, continued with second-line chemotherapy, usually a combination of vincristine, adriamycin, and dexamethasone (VAD). Patients treated with IDM or HDM were evaluated 2 months after the second IDM or single-dose HDM, respectively. Nonresponding patients were also treated with VAD.
Immunocytochemical staining of LRP.
LRP expression was determined by an alkaline phosphatase immunocytochemical detection method19 using the specific murine monoclonal antibody (MoAb) LRP-56 (IgG2b) that was obtained after immunization of mice with the non-Pgp multidrug-resistant human nonsmall lung cancer cell line SW-1573/2R120.14 Bone marrow cells were separated by Ficoll-Hypaque, washed twice with minimal essential medium (MEM; GIBCO, Grand Island, NY) and stored at −20°C until use. Cytocentrifuged slides were airdried overnight and fixed in acetone for 10 minutes.
After preincubation for 20 minutes with 10% rabbit serum in phosphate-buffered saline plus 1% bovine serum albumin (PBS/BSA; Sigma Chemical Co, St Louis, MO), cytospins were incubated with LRP-56 (diluted 1:500 in 1% BSA) or with idiotype matched control (nonspecific mouse IgG-1; Cappel: Organon Teknica 50327/36345) for 1.5 hours. Next, rabbit anti-mouse immunoglobulin (RAM; Dakopatts Z 259, DAKO Corp, Glastrup, Denmark) diluted 1:25 for 1 hour was added followed by incubation with alkaline phosphatase substrate (APAAP; Dakopatts D 651, DAKO), diluted 1:50 for 1 hour. Incubations with RAM and APAAP were repeated for 0.5 hour. The color reaction was produced using a Neufuchsin (Merck 4041; Merck, Darmstadt, Germany) substrate incubating for 40 minutes. All incubations were performed at room temperature. Between incubation steps, slides were washed thoroughly in PBS for 10 minutes. Finally, cytospins were counterstained in diluted hematoxilin and washed with tap water. Simultaneously, the LRP-positive fibrosarcoma HT1080 DR4 control cell line20 was stained as control for the immunocytochemical assay.
All slides were examined and scored independently by two observers, blinded to the clinical data. Plasma cells were identified on morphological criteria. At least 250 plasma cells were evaluated. A sample was considered to be LRP-positive if ≥10% of the plasma cells stained with the LRP-56 antibody and the idiotype matched controls were indeed negative. These criteria were based on previous experience with LRP-56 staining in 155 cancer specimens, which indicated that a 10% cut-off value may distinguish two groups of LRP-expressing tumors.13 21
Determination of prognostic factors.
The serum B2-microglobulin level was determined by means of a competitive enzyme immunoassay (Phadezym; Pharmacia, Uppsala, Sweden). The plasma cell labeling index (LI) was measured by the incorporation of bromodeoxyuridine as described previously.22 Serum levels of lactate dehydrogenase (LDH) were measured according to standard methods.
Statistical analysis.
Data analysis was performed using the SPSS statistical software package (SPSS Inc, Chicago, IL).
Prognostic parameters such as age were determined at diagnosis and were retrospectively assessed for their relationship with LRP expression. The response rates were compared between LRP and prognostic factors expression groups. Qualitative variables were analyzed using the chi-squared test. Multivariate analysis was performed using step-wise discriminant analysis. Overall survival was measured in months from the moment of diagnosis, providing 95% confidence intervals. Actuarial survival curves were estimated using the Kaplan-Meier method,23 and differences in survival between subgroups were compared with the log-rank test (Mantel-Cox).24 Also, the hazard rates for each variable were calculated with the Cox-regression model using enter and remove limits of 0.05 and 0.1. Hypotheses were evaluated at a significance level of 0.05. Two-sided statistical tests were used in all analyses.
RESULTS
Frequency and pattern of LRP expression.
LRP was expressed in 47% (33/70) of bone marrow samples of patients with newly diagnosed myeloma. In the MP-treated population 47% (17/38) of patients were LRP positive as compared with 50% (16/32) in the IDM/HDM-treated population. The staining of the LRP-56 MoAb in the LRP-positive myeloma cells was invariably cytoplasmatic in the perinuclear region, in a granular fashion (Fig1). The intensity of the staining was generally strong, but variance in staining intensity was too small to justify objective classification between aspirates. LRP expression in positive bone marrow samples was heterogeneous, typically showing LRP-56 immunoreactivity in the majority of myeloma cells (median 50%, range 10 to 90). There were no samples with LRP expression in less than 10% of the plasma cells. LRP was not expressed in plasma cells of normal donors (0/3) or patients with MGUS (0/5). In the majority of MM patients as well as in normal donors and MGUS patients, LRP expression was found in granulocytic marrow components, irrespective of expression on plasma cells.
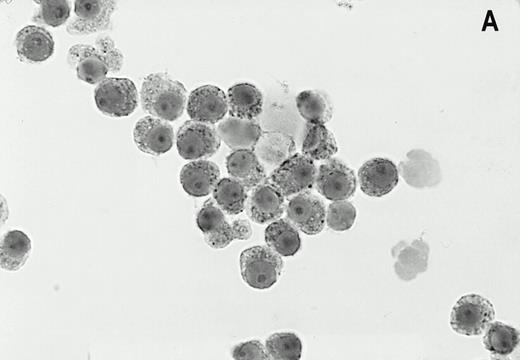
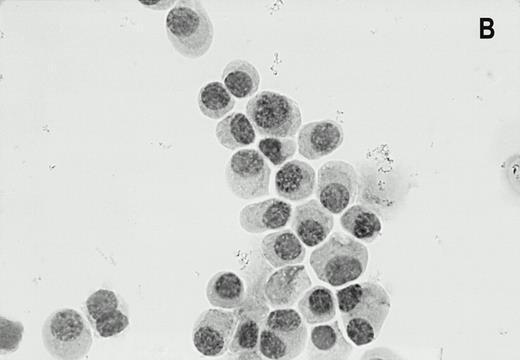
Alkaline phosphatase immunocytohistochemical staining using the MoAb LRP-56 of cytocentrifuged bone marrow cells containing >95% plasma cells of a patient with multiple myeloma. Cytospins were counterstained in diluted hemotoxiline. LRP immunoreactivity in a granular fashion in the cytoplasm is present in almost all plasma cells (A). Isotype control is negative (B).
Alkaline phosphatase immunocytohistochemical staining using the MoAb LRP-56 of cytocentrifuged bone marrow cells containing >95% plasma cells of a patient with multiple myeloma. Cytospins were counterstained in diluted hemotoxiline. LRP immunoreactivity in a granular fashion in the cytoplasm is present in almost all plasma cells (A). Isotype control is negative (B).
Correlation with established prognostic factors.
Using previously defined cut-off levels we studied the distribution of the plasma cell LI%, serum B2-microglobulin level, and serum LDH in relation to plasma cell LRP expression. The cut-off level for LI was ≥2%,25 for B2-microglobulin ≥4 μg/mL,26 and for LDH ≥300 U/L.26 LRP expression was associated with high LDH levels at diagnosis (X2, P = .05). LRP did not correlate with serum B2-microglobulin (P = .1), plasma cell LI% (P = .07), or age (P = .9).
LRP expression and response to melphalan chemotherapy.
The response to chemotherapy consisting of MP or IDM/HDM is summarized in Table 2; in 38 patients treated with standard MP the overall response rate was 37% (14/38). There were no complete remissions obtained with MP. In this group, LRP expression was associated with a poor response to induction treatment. Fifty-two percent (11/21) of the LRP-negative patients achieved a remission as compared with 18% (3/17) of the patients with LRP-positive myeloma at diagnosis (X2, P = .027). By univariate analysis, bone marrow plasma cell LI, serum B2-microglobulin, and serum LDH did not have a significant prognostic value regarding the response to MP therapy (Table 2).
A remission was achieved in 84% (27/32) of the patients treated with intensified dose melphalan, including 8 patients who achieved a CR (25%). No significant difference was found between the response rate in the LRP-negative patients (88%, 14/16) and the LRP positive population (81%, 13/16; P = .285). The subgroups of LRP-positive and LRP-negative IDM/HDM-treated patients showed no statistically significant differences in distribution of LDH, LI%, B2-microglobulin, or age. A comparison of responses between the two regimens in LRP-positive patients showed a significant higher response rate with IDM/HDM as compared with MP (81% v 18%,P < .0001). In LRP-negative patients a better response rate with IDM/HDM (88% v 52%, P = .006) was also observed.
Expression of LRP and survival.
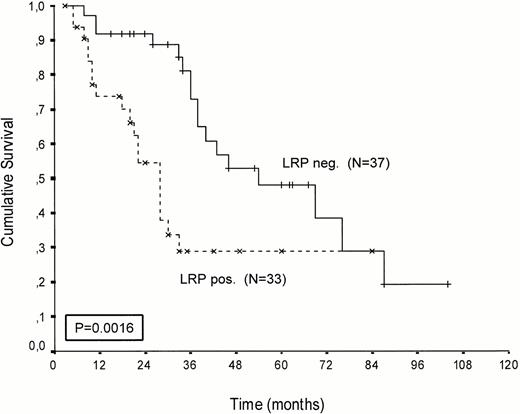
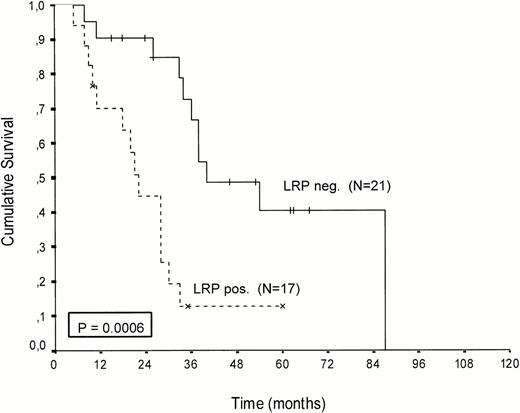
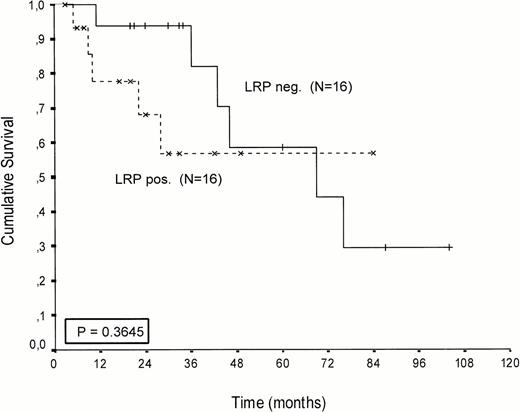
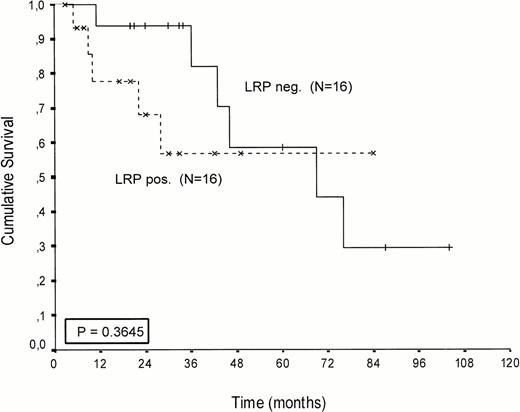
Kaplan-Meier survival curves of LRP-positive and LRP-negative patients are presented in Figures 2-4.An inverse correlation was found between LRP expression and survival duration. In the complete group, the median survival of LRP-positive patients was 28 months (95%-Cl: 23 to 33), whereas the median survival duration of LRP negative patients was 54 months (Cl: 26 to 82;P < .002; hazard ratio [HR] = 2.9 (1.4-5.7); Table 2, Fig2). This difference was likely caused by the 38 MP-treated patients who had a median survival 40 months in LRP-negative (95%-Cl: 41 to 69) and 22 months in LRP-positive patients (95%-Cl: 18 to 26;P = .0006, HR = 4.1 (1.7-9.8); Table 2, Fig 3). In the IDM/HDM-treated patients no significant difference in survival between LRP-positive and LRP-negative patients was observed (median survival 69 months [95%-Cl 14 to 124]) in LRP-negative patients and not reached in LRP-positive patients (P = .365, HR = 1.7 [0.5-5.9; Table 2, Fig 4]).
Patients treated with melphalan, either at conventional dose and combined with prednisone or administered as an intensified (70/140 mg/m2 IV). Probability of survival from the start of treatment.
Patients treated with melphalan, either at conventional dose and combined with prednisone or administered as an intensified (70/140 mg/m2 IV). Probability of survival from the start of treatment.
Patients treated with conventional melphalan and prednisone. Probability of survival from the start of treatment.
Patients treated with conventional melphalan and prednisone. Probability of survival from the start of treatment.
Patients treated with IV intensified melphalan (70/140 mg/m2 IV). Probability of survival from the start of treatment.
Patients treated with IV intensified melphalan (70/140 mg/m2 IV). Probability of survival from the start of treatment.
By univariate analysis LRP (P < .002) was the strongest adverse prognostic marker for survival in the whole population followed by bone marrow plasma LI, serum B2-microglobulin, and serum LDH (Table 2). In MP-treated patients high serum LDH (P = .0001) and LRP expression (P < .0006) both had an adverse effect on survival (Table 2).
Multivariate analysis showed that in the whole population (50 cases available for analysis) only LRP expression was an independent prognostic factor (P = .03). In the subgroup of MP-treated patients (25 cases available) LRP (P = .03) and serum LDH (P = .0001) remained statistically significant for survival. In the patients treated with IDM/HDM none of the prognostic factors affected survival. Age had no prognostic significance for survival by either univariate or multivariate analysis.
In the subgroup of responding patients we performed statistical analysis regarding event-free survival (EFS). Within the responding MP-treated patients, the EFS was remarkable shorter in LRP-positive patients. Three LRP-positive patients treated with MP relapsed after 6, 7, and 15 months, respectively (median 7 months, 95%-Cl: 5 to 9), whereas the EFS of LRP-negative MP-treated patients was median 24 months (n = 11, 95%-Cl; 16 to 32; P < .003). EFS of IDM/HDM-treated LRP-positive patients was median 22 months (n = 14, 95%-Cl: 14 to 30) versus 24 months (n = 13, 95%-Cl: 15 to 33;P = .182) for LRP-negative patients.
DISCUSSION
Our findings indicate that LRP is widely expressed in untreated MM and that it is associated with a low probability of response and a shorter survival in patients treated with a conventional MP regimen. LRP positivity was found in 47% of the patients with newly diagnosed MM. This figure is consistent with data indicating the widespread expression of LRP in untreated human malignancies.13Multivariate analysis showed that LRP was an adverse prognostic marker that was independent for serum B2-microglobulin, bone marrow plasma cell LI, serum LDH, and age. Interestingly, LRP-related resistance to MP may initially be overcome by dose intensification of melphalan as suggested by the outcome of patients treated with IDM and HDM.
These observations add proof to the recent in vitro and clinical studies identifying LRP as an independent predictor for chemoresistance against melphalan. Studies undertaken to assess the in vitro sensitivity of the RPMI, 8226 human myeloma cell line to several cytotoxic drugs showed that by exposure to melphalan an MDR subline emerged, termed 8226 LR5, which is resistant to melphalan and highly upregulates LRP expression in absence of other MDR proteins (showing a drug accumulation defect). Also exposure to mitoxantrone resulted in a highly LRP-positive cell population (8226 MR40), which showed additional resistance to melphalan, again in absence of other MDR-related proteins (W.S. Dalton et al, personal communication, July 1997).
Moreover, in human cancer cell lines derived from 8 cancer types, using immunocytohistochemical detection methods, a significant correlation between LRP expression in these cancer types and in vitro sensitivity to melphalan was found. No correlation was found between the expression of other MDR-related proteins and melphalan sensitivity.15These in vitro results are in line with our clinical finding of an association between LRP expression on myeloma cells in untreated patients and lack of response to oral melphalan chemotherapy in these patients.
Further evidence for the relationship of LRP with chemoresistance to both classical and MDR-related drugs is provided by several recent clinical studies. In patients with adult myeloid leukemia27and patients with FIGO stage III/IV ovarian cancer,21 LRP expression of malignant cells was significantly correlated with inferior response to chemotherapy, including cisplatin and alkylating agents, and with shorter overall survival.
The precise mechanism of LRP-related chemoresistance, however, is still unsolved. To date the biological function of LRP as a major constituent of the human vault protein is unknown. A small fraction of vaults are localized to the nuclear membrane and nuclear pore complexes, raising the possibility that vaults mediate the bidirectional transport of a variety of substrates between the nucleus and the cytoplasm.28 In support of this view, entrapment of drugs in exocytotic vesicles and decreased nuclear to cytoplasmic drug ratios were reported in LRP-overexpressing multidrug resistant cells.29 30 Interestingly, melphalan and cisplatin exert their main cytotoxic effect in the cell nucleus having very similar modes of action on nucleic acids. This makes it tempting to hypothesize that vaults are involved in the nucleo-cytoplasmic exchange of these drugs.
Our findings cannot exclude that LRP is a pleiotropic marker of resistance coexpressed with other (MDR-related) drug resistance genes. In this study we did not assess the expression of other MDR-related proteins, ie, Pgp and MRP. Therefore, no conclusion can be drawn on the individual roles of each resistance protein in these MM samples. However, we and others found that PgP is expressed in very low frequency (<5%) in untreated MM and has no prognostic value at that stage.31,32 In contrast, PgP is highly expressed in VAD-refractory MM,31,33 whereas MRP is not expressed above background values in the majority of MM samples.34Moreover, in in vitro experiments and clinical studies MDR and MRP did not confer resistance to melphalan.15 21
Obviously other nonclassical MDR transport mechanisms of resistance must be involved in MM, which may explain why resistance occurs in LRP-negative patients.
An alternate conclusion from the adverse prognostic value of LRP in patients treated with the MP regimen could be an inverse relation between LRP expression and the sensitivity of myeloma cells to corticosteroids. Previous studies have shown that steroid dose intensity is one of the most important predictors of treatment outcome. Considering this, the use of prednisone in patients receiving oral melphalan is an important difference between the treatment groups. However, recent in vitro studies in acute lymphocytic leukemia using flow cytometry and a methylthiotetrazole (MTT) assay have shown a lack of relation between LRP expression and resistance to prednisolone (M.L. den Boer et al, Department of Paediatrics, Free University Hospital, Amsterdam, The Netherlands, personal communication, January 1997). In addition to a potential role in chemoresistance LRP may be associated with a biologically more aggressive state of MM. This is not only suggested by the fact that EFS of LRP-positive patients tended to be shorter but also by the relation between LRP and elevated LDH levels in untreated disease. LRP did not correlate with serum B2-microglobulin, LI, or age. High LDH levels in untreated MM have been related to high tumor mass, unusual clinical features like extraosseous masses, and hypodiploidy or low RNA content of plasma cells, possibly reflecting a late stage of myeloma transformation and a poor clinical outcome.26 Also in our study MP-treated patients with elevated LDH levels survived significantly shorter. More detailed studies on the relation of LRP with clinipathological and cytogenetic parameters in MM are therefore warranted.
Our findings are clinically important because LRP-related resistance to the MP regimen may be circumvented by dose intensification of melphalan. LRP-positive patients had a significant better response to IDM/HDM as compared with MP. The dose-response relation for melphalan has been widely documented, and dose escalation has been clinically applied in recent years.3-5 In general, previously untreated patients have superior response rates to intensive regimens as compared with MP, and remissions are of good quality.5Our results indicate that assessment of the LRP status might identify a patient population that can initially benefit from dose intensification and in which this regimen could be considered as a first-line treatment above the MP regimen.
In conclusion, in this study we assessed the expression of LRP in untreated myeloma and introduce this novel drug resistance–related protein as a prognostic factor for response to the MP regimen and survival. Moreover, we report that LRP-associated resistance to melphalan may be circumvented by dose intensification, creating the possibility to select patients who benefit from these regimens. Further studies on the expression of LRP and other mechanisms of drug resistance seem warranted to confirm these results and to clarify the functional characterization and the biological role of LRP in myeloma and other tumor types.
Address reprint requests to H.M. Lokhorst, MD, PhD, University Hospital Utrecht, Department of Haematology (G03.647), PO Box 85500, 3508 GA Utrecht, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.