Abstract
Myeloablative conditioning associated with hazardous immediate and late complications is considered as a mandatory first step in preparation for allogeneic blood or marrow transplantation (allogeneic BMT) for the treatment of malignant hematologic disorders and genetic diseases. Immune-mediated graft-versus-leukemia (GVL) effects constitute the major benefit of allogeneic BMT. Therefore, we have introduced the use of relatively nonmyeloablative conditioning before allogeneic BMT aiming for establishing host-versus-graft tolerance for engraftment of donor immunohematopoietic cells for induction of GVL effects to displace residual malignant or genetically abnormal host cells. Our preliminary data in 26 patients with standard indications for allogeneic BMT, including acute leukemia (n = 10); chronic leukemia (n = 8), non-Hodgkin's lymphoma (n = 2), myelodysplastic syndrome (n = 1), multiple myeloma (n = 1), and genetic diseases (n = 4) suggest that nonmyeloablative conditioning including fludarabine, anti–T-lymphocyte globulin, and low-dose busulfan (8 mg/kg) is extremely well tolerated, with no severe procedure-related toxicity. Granulocyte colony-stimulating factor mobilized blood stem cell transplantation with standard dose of cyclosporin A as the sole anti-graft-versus-host disease (GVHD) prophylaxis resulted in stable partial (n = 9) or complete (n = 17) chimerism. In 9 patients absolute neutrophil count (ANC) did not decrease to below 0.1 × 109/L whereas 2 patients never experienced ANC <0.5 × 109/L. ANC ≥ 0.5 × 109/L was accomplished within 10 to 32 (median, 15) days. Platelet counts did not decrease to below 20 × 109/L in 4 patients requiring no platelet support at all; overall platelet counts >20 × 109/L were achieved within 0 to 35 (median 12) days. Fourteen patients experienced no GVHD at all; severe GVHD (grades 3 and 4) was the single major complication and the cause of death in 4 patients, occurring after early discontinuation of cyclosporine A. Relapse was reversed by allogeneic cell therapy in 2/3 cases, currently with no residual host DNA (male) by cytogenetic analysis and polymerase chain reaction. To date, with an observation period extending over 1 year (median 8 months), 22 of 26 patients (85%) treated by allogeneic nonmyeloablative stem cell transplantation are alive, and 21 (81%) are disease-free. The actuarial probability of disease-free survival at 14 months is 77.5% (95% confidence interval, 53% to 90%). Successful eradication of malignant and genetically abnormal host hematopoietic cells by allogeneic nonmyeloablative stem cell transplantation represents a potential new approach for safer treatment of a large variety of clinical syndromes with an indication for allogeneic BMT. Transient mixed chimerism which may protect the host from severe acute GVHD may be successfully reversed postallogeneic BMT with graded increments of donor lymphocyte infusions, thus resulting in eradication of malignant or genetically abnormal progenitor cells of host origin.
MYELOABLATIVE COMBINATIONS of high-dose chemo-radiotherapy followed by rescue with autologous or allogeneic bone marrow transplantation (BMT) blood or marrow-derived stem cell transplantation are common modalities to treat various hematologic malignancies resistant to conventional doses of chemotherapy. For patients relapsing after conventional front-line chemotherapy protocols or for patients that are considered at high risk to relapse as well as for patients with genetic diseases, alloBMT is the treatment of choice. The use of myeloablative doses of chemotherapy with or without total body irradiation (TBI) followed by rescue with allogeneic BMT offers an important advantage in the form of alloreactivity against host leukemia cells, the so-called graft-versus-leukemia (GVL) effects,1,2 and perhaps even graft-versus-tumor (GVT) effects,3,4 which may be associated with undesirable graft-versus-host disease (GVHD). For patients with underlying malignancies, the transplant procedure is considered mostly as a rescue procedure following myeloablative treatment to eradicate the basic malignancy by the cytoreductive agents given within the short period of conditioning before autologous or allogeneic BMT. In patients with genetic disorders, myeloablative doses of cytoreductive agents are used to eradicate genetically abnormal stem cells before allogeneic BMT. Attempts to improve the disease-free survival by increasing the intensity of the conditioning regimen, thereby eradicating host-derived stem cells more effectively, have resulted in unacceptable toxicity. It seems unlikely that a substantial improvement in the treatment of high-risk hematologic malignancies, which may require eradication of all tumor cells, may be accomplished merely by increasing the intensity of the conditioning based on the well known “log-dose” relationship between the dose of cytoreductive agents and the degree of tumor cell kill. Moreover, by comparing numerous protocols comprising a wide range for intensities for each of the cytoreductive components used for over 20,000 transplants reported to the International Bone Marrow Transplant Registry, no difference or clear advantage could be documented for different regimens administered as preparation for autologous BMT or allogeneic BMT, including or excluding TBI.5 The potential benefits of more intensive chemo-radiotherapy may be further exploited in the future by using targeted chemotherapy or targeted radiolabeled tumor-seeking compounds.
Over the years, the importance of immune reactions between donor-derived immunocompetent T lymphocytes and host-type tumor cells has been recognized to be of major therapeutic importance, accounting for the significantly better antitumor effects induced by allogeneic BMT compared with autologous BMT and transplants from an identical twin.1,2 Moreover, we have shown in early 1987, as confirmed in many transplant centers worldwide since, that relapse after allogeneic BMT in patients considered incurable can be frequently reversed by donor lymphocyte infusion (DLI).6-10 All this indicates that the main therapeutic component of allogeneic BMT may be ascribed to T-cell–mediated GVL effects rather than to physical elimination of all tumor cells by high doses of cytoreductive agents given as part of the conditioning before transplantation. The possibility to completely eradicate tumor cells by adoptive allogeneic cell therapy induced by DLI in patients failing all alternative modalities suggests that alloreactive T lymphocytes of donor origin may be the strongest tool available against tumor cells of hematopoietic origin. Hence, the main role of the transplant procedure may be in the induction of a state of host-versus-graft tolerance giving donor-derived T lymphocytes the opportunity to recognize and eradicate host-derived tumor cells or abnormal stem cells without immunosuppressive treatment as anti-GVHD prophylaxis. This working hypothesis prompted us to develop a new approach to the treatment of diseases generally referred to conventional allogeneic BMT, focusing on the use of donor T cells to eradicate both nonmalignant and malignant cells of host origin, thus avoiding the need for myeloablative conditioning, to improve the immediate and long-term outcome of the patients. Our working hypothesis is based on experiments in animal models of lymphocytic leukemia/lymphoma11-13 and acute myeloid leukemia14 and on clinical observations over the past 10 years, documenting that the efficacy of allogeneic cell therapy induced by the allograft is the key element in accomplishing the benefits the alloBMT procedure.8 9 A protocol was designed based on minimizing the intensity of the conditioning regimen to the range of nonmyeloablative treatment, followed by infusion of granulocyte colony-stimulating factor (G-CSF)-mobilized donor stem cells enriched with circulating T lymphocytes collected by apheresis using Baxter's CS 3000plus. The main focus is on intensive short-term immunosuppression with fludarabine and anti–T-lymphocyte globulin (ATG) with low-dose oral busulfan before infusion of blood stem cells. GVL effects are mediated by the large number of donor-derived immunocompetent T lymphocytes given together with donor stem cells. GVL effects may be increased later on, by allogeneic cell therapy with DLI on an outpatient basis. As will be shown below, our preliminary experience seems promising and suggests that allogeneic nonmyeloablative stem cells may result in complete elimination of malignant or abnormal host cells with no or minimal procedure-related toxicity. This approach may be safely offered to patients of all age groups with low anticipated incidence of immediate and long-term complications.
PATIENTS AND METHODS
A cohort of 26 consecutive patients undergoing allogeneic nonmyeloablative stem cell transplantation is presented, all of whom were given a combination of fludarabine and ATG. Patients' characteristics are described in Table 1.All patients would have been considered eligible candidates for conventional myeloablative allogeneic BMT, including 6 with chronic myelogenous leukemia in first chronic phase (CML/CP); 1 with CML in accelerated phase (CML/AP); 1 with juvenile CML (JCML); 7 with acute myelogenous leukemia (AML) in first complete remission (CR), 1 of whom with secondary leukemia (AML, M5) 3 years after treatment for carcinoma of the ovary and 1 with AML in second CR; 1 with acute lymphoblastic leukemia (ALL) in first CR and 1 in second CR; 2 with non-Hodgkin's lymphoma (NHL) resistant to chemotherapy; 1 with myelodysplastic syndrome (MDS) with excess blasts; and 1 with multiple myeloma (MM). The series also included 4 patients with nonmalignant disorders including 1 child with severe β-thalassemia major, 1 child with Fanconi's anemia, 1 child with Gaucher's disease, and 1 adult with Blackfan Diamond syndrome (Table 1). Patient age ranged between 1 and 61 (median, 31) years. Conditioning before infusion of allogeneic stem cells included immunosuppressive treatment with six daily infusions of fludarabine (Fludara; Schering AG, Berlin, Germany) 30 mg/m2 (in adults the dose was adjusted to ideal body weight) for 6 consecutive days (days −10 to −5); oral busulfan 4 mg/kg/d for 2 consecutive days (days −6 to −5); and anti–T-lymphocyte globulin (ATG-Fresenius AG, Munich, Germany) 10 mg/kg/d for 4 consecutive days (days −4 to −1). One patient (unique patient number [UPN] 111 with Fanconi's anemia) received cytoxan 10 mg/kg instead of busulfan. G-CSF–mobilized blood stem cells were collected once after 5 days of administration of G-CSF 10 μg/kg/d. HLA-A,B,C,DR,DRB1 matched siblings were used as donors, with one exception: UPN 1109 was grafted with A and C locus mismatches with positive mixed lymphocyte reaction in the direction of host-versus-donor. The total number of nucleated cells infused on day 0 ranged between 3.38 and 16.39 (mean, 8.60) × 108/kg. Prophylaxis against GVHD included cyclosporine A (CSA) 1.5 mg/kg twice daily intravenously starting on day −1, switching to an oral dose of 3 mg/kg twice daily as soon as the patients were off intravenous therapy or as soon as they were discharged, with early tapering off, starting as soon as engraftment with no GVHD was confirmed (around 4 to 6 weeks) and the patient's condition stabilized. Prophylaxis againstPneumocistis carinii included trimethoprim/sulfamethoxazole (10 mg/kg/d trimethoprin) administered pretransplantation (days −10 to −1) and as soon as the absolute neutrophil counts (ANCs) exceeded 0.75 × 109/L (7 mg/kg/d trimethoprim) twice weekly. Prophylaxis against Herpes simplex virus included low-dose oral acyclovir 200 mg ×3/d starting on day −10 for 4 months.
Chimerism was assessed by standard cytogenetic analysis in male/female donor-recipient combinations and in patients with CML, searching for the proportion of Ph+ cells, to assess the actual proportions of host and donor cells in marrow aspirates, representing hematopoietic cells in mitosis. Residual male cells in female to male chimeras were detected by the amelogenine gene method as previously described in detail.15 In sex-matched donor-recipient combinations, the various number of tandem repeats (VNTR)-polymerase chain reaction (PCR) test with a sensitivity of detection of 5% was used to assess the presence of residual host or donor cells as previously described.16
Statistical evaluation.
The Kaplan-Meier method was used to calculate the probability of disease-free survival as a function of time as well as for determining the time to recovery of hematopoietic reconstitution.
RESULTS
The allogeneic nonmyeloablative stem cell transplantation protocol was much better tolerated in comparison with the anticipated side effects following a standard myeloablative regimen. As can be seen in Table2, listing common procedure-related toxic manifestations, following allogeneic nonmyeloablative stem cell transplantation no grade 3 or 4 toxicity (World Health Organization [WHO] criteria) were observed in any of the recipients. Grade 2 mucositis was documented in only 2 cases. All patients maintained oral intake throughout the procedure, with 8 (31%) never requiring any parenteral caloric supplements. Septic fever episodes with positive blood cultures were observed in 4 cases, whereas 22 patients experienced no evidence of severe culture-positive systemic infection. Severe veno-occlusive disease (VOD) of the liver was observed in 2 cases whereas 11 developed mild to moderate manifestations of VOD and 13 patients showed no evidence of hepatic abnormality. No pulmonary toxicity was observed in this cohort of patients.
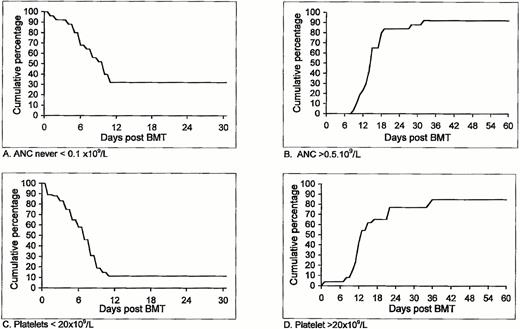
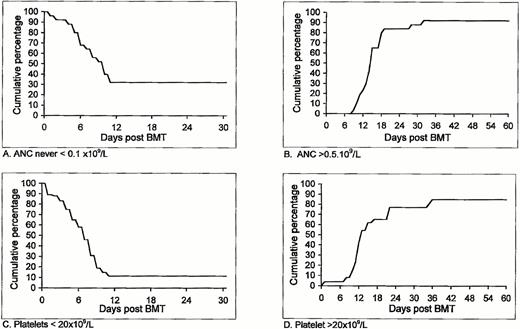
Additional important clinical parameters following allogeneic nonmyeloablative stem cell transplantation in the first cohort of patients who entered our study is shown in Table 3 and Fig1. In 9 patients (34%) ANC did not decrease to below 0.1 × 109/L, and for the entire group it took a median of 10 days for the ANC to drop below 0.1 × 109/L (Fig 1A). Two patients never experienced ANC <0.5 × 109/L (Table 3). The number of days with ANC <0.1 × 109/L in the remaining 17 patients ranged between 0 and 20, with a median of 4 days. ANC >0.5 × 109/L was accomplished within 10 to 32 (median, 15) days (Fig 1B). Platelet counts did not decrease to below 20 × 109/L in 4 patients (Table 3), therefore requiring no platelet support at all. Among the remaining 22 patients, a decrease of platelet count to below 20 × 109/L was observed after a median of 7 days, with 11% probability of remaining with a low (<20 × 109/L) platelet count after day 11 (95% confidence interval of 3% to 27%) (Fig 1C). Spontaneous platelet counts >20 × 109/L were achieved within 0 to 35 (median 12) days. Unsupported platelet counts >20 × 109/L was observed within 36 days in 85% of the patients (95% confidence interval, 69% to 95%) (Fig 1D).
Duration and degree of pancytopenia and engraftment of HLA-identical G-CSF–mobilized blood stem cell allografts after nonmyeloablative conditioning, expressed as cumulative percentage of patients who never featured ANC < 0.1 × 109/L (A) or ANC < 0.5 × 109/L; cumulative percentage of patients with platelet counts below 20 × 109/L (C) and patients with platelet counts never going below 20 × 109/L that never required any platelet transfusion (D).
Duration and degree of pancytopenia and engraftment of HLA-identical G-CSF–mobilized blood stem cell allografts after nonmyeloablative conditioning, expressed as cumulative percentage of patients who never featured ANC < 0.1 × 109/L (A) or ANC < 0.5 × 109/L; cumulative percentage of patients with platelet counts below 20 × 109/L (C) and patients with platelet counts never going below 20 × 109/L that never required any platelet transfusion (D).
GVHD ≥ grade 1 was observed in 12 of 26 patients (Table 3). Severe GVHD (grades 3 and 4) was the single major complication, diagnosed in 6 cases (25%) and was the only cause of mortality in 4 patients, all of whom developed the first signs of disease while off of CSA. Interestingly, acute GVHD developed in only 4 patients while on regular CSA maintenance therapy (only 1 patient with grade 3 GVHD, UPN 1109, currently alive and well). In 8 cases initiation of GVHD was observed only following sudden discontinuation of CSA in an attempt to enhance engraftment or displace residual host cells, documented by molecular or cytogenetic analysis. The 4 patients who died from severe GVHD, considered protocol failure, account for all the losses observed in the entire series, with an observation period exceeding 1 year (median 8 months). In one of the patients (UPN 1093) who died of grade 4 GVHD, which developed while she was off of CSA in a foreign country, access to follow-up or further CSA treatment was denied; therefore, her death may not be considered a protocol failure. The second patient (UPN 1124) died of GVHD grade 4 that developed immediately after discontinuation of CSA and re-infusion of a second inoculum of mobilized stem cells enriched with blood T cells given intentionally without CSA on day +22 in an attempt to enhance delayed granulocyte engraftment. Subsequently, granulocyte counts increased within less than a week after the second inoculum and reached ANC > 0.5 × 109/L on day +35, suggesting that stem cell top-up may not only have been redundant, but most likely may have contributed to the severity of GVHD, which could be also due in part to the first BMT procedure, which resulted in unavoidable fatal outcome. The third and forth patients with CML (UPNs 1131 and 1135) also developed grade 4 GVHD after sudden discontinuation of CSA. All the other 8 patients who developed GVHD, of which only 3 manifested >grade 2 GVHD, responded to standard prednisone treatment starting with 2 mg/kg with slow tapering off, as clinically indicated.
Engraftment was documented in all patients by increasing blood counts as shown in Table 2, using either amelogenin-PCR for detection of residual male cells in a female recipient (sensitivity of 1 male cell in 106 female cells) as well as for detectio of mixed chimerism on a semiquantitative basis in sex-mismatched host-donors, because using this assay we could detect the presence of 1% to 30% male cells compared with greater than 30% male cells.15VNTR-PCR was used in sex-matched donor-recipient pairs with a sensitivity of 1 in 20 cells. In 9 of 26 evaluable patients, a transient stage of mixed chimerism was confirmed by documenting minimal residual host cells by cytogenetic analysis, PCR, or disease-specific reverse transcriptase (RT)-PCR (eg, bcr-abl in CML). In confirmed mixed chimeras with no GVHD CSA was rapidly discontinued, within 2 to 4 weeks.
No conclusive data can be given at this point to assess the total incidence of chronic GVHD in allogeneic nonmyeloablative stem cell transplantation–treated recipients because of the relatively short observation period ranging from several months to over 1 year. As can be seen in Table 3, chronic GVHD was diagnosed in 9 patients, in 2 of which (UPNs 1073 and 1098) signs of GVHD appeared only after initiation of allogeneic cell therapy.
Relapse was observed in 2 patients with acute leukemia (UPNs 1073 and 1080) whereas rapidly progressive residual disease was observed in 1 patient with NHL totally resistant to chemotherapy (UPN 1098). Cytogenetic relapse with normal blood counts was diagnosed in 1 patient with CML who developed no spontaneous GVHD even after discontinuation of CSA. The patient is now under allogeneic cell therapy (UPN 1137).
Successful displacement of tumor cells by allogeneic cell therapy was already accomplished in 2 cases (UPNs 1080 and 1098) while 1 patient is still under treatment, too early for evaluation (Table 2). UPN 1080, a male recipient originally treated for ALL in second CR, featured no GVHD after allogeneic nonmyeloablative stem cell transplantation, developed overt hematologic relapse at 4 months with the number of blasts doubling within 1 to 2 days. The large tumor mass was successfully debulked using a combination of cytosine arabinoside 3 g/m2/d in two split doses for 4 days and a single dose of mitoxantron 12 mg/m2, followed by re-infusion of lymphocyte-enriched donor (female) blood stem cells with no CSA. Elimination of detectable male cells was confirmed by PCR analysis in parallel with GVHD (grade 1-2) induced after DLI, with stable CR being maintained to date with no further treatment. Because this patient received chemotherapy for debulking of rapidly developing leukemia cells before DLI, the data should be interpreted with some reservations because chemotherapy, rather than allogeneic cell-mediated immunotherapy, may have played a major role as well. In UPN 1098, an elderly male patient with chemotherapy-resistant NHL, with rapidly progressive malignant lymphoid infiltrate in the marrow and bone neuralgia, with no spontaneous GVHD following allogeneic nonmyeloablative stem cell transplantation, elimination of all disease manifestations was confirmed after allogeneic cell therapy with DLI in parallel with disappearance of host/male DNA by PCR and documentation of 100% female karyotype, in parallel with onset of mild acute GVHD grade 2 that evolved to mild limited chronic GVHD. Another patient (UPN 1073) with AML in second CR relapsed 9 months following allogeneic nonmyeloablative stem cell transplantation. She is currently under combined treatment with chemotherapy and allogeneic cell therapy.
All 4 patients with nonmalignant indication for alloBMT are currently alive and well, with a Karnofsky score of 100% with no evidence of the basic disease, fully reconstituted with donor cells and no residual host cells by male-specific PCR or VNTR-PCR.
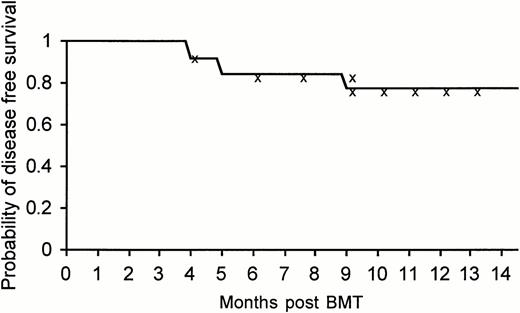
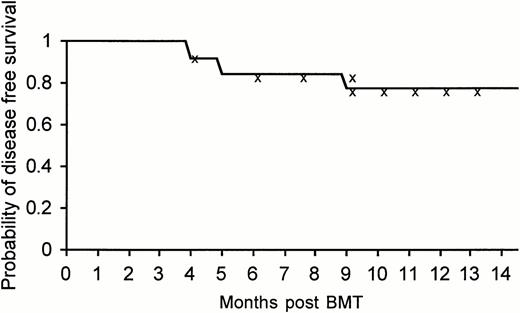
To date, with an observation period extending over 1 year (median, 8 months), 22 of 26 patients (85%) treated by allogeneic nonmyeloablative stem cell transplantation are alive, 21 (81%) disease-free by all measurable criteria, including PCR, with excellent quality of life, and a Karnofsky score of 100%. It is too early to assess the actuarial disease-free survival at the present time, but the actual disease-free survival at a median follow-up of 8 months was 80.7% (at 14 months the actuarial disease-free survival was 77.5% with a 95% confidence interval of 53% to 90%) (Fig2). The data should be carefully interpreted in view of the short observation period available.
Kaplan-Meier actuarial disease-free survival at 14 months of the entire group of 26 patients treated with allogeneic nonmyeloablative stem cell transplantation.
Kaplan-Meier actuarial disease-free survival at 14 months of the entire group of 26 patients treated with allogeneic nonmyeloablative stem cell transplantation.
Mild and limited chronic GVHD developed in 9 of 25 patients with an observation period greater than 100 days, but thus far none have developed clinically significant disease manifestations.
DISCUSSION
Our study shows that elimination of malignant and genetically abnormal hematopoietic cells of host origin can be achieved with a safe and well-tolerated nonmyeloablative conditioning in patients with acute and chronic leukemia, lymphoma, multiple myeloma, and nonmalignant diseases including β-thalassemia major, Blackfan Diamond anemia, Fanconi's anemia, and Gaucher's disease. The principle behind the new allogeneic nonmyeloablative stem cell transplantation protocol, presented here for the first time, is to maximize transient immunosuppression with nonmyeloablative agents rather to attempt to eradicate all tumor cells or genetically abnormal stem cells which are expected to be eliminated over time by alloreactive T cells of donor origin. Thus, we have chosen a combination of fludarabine and anti–T-lymphocyte globulin raised in rabbits against Jurkat cell line with reproducible and stable expression of cell-surface proteins which induce more specific activity against activated T lymphocytes. No procedure-related mortality was reported thus far and all 4 deaths observed were due to acute GVHD, which may have resulted in part from inadequate preventive measures with CSA, including early withdrawal and a possibly redundant top-up with a second blood stem cell allograft with no CSA prophylaxis (UPN 1124). At least 2 of the 4 relapses observed, in patients with rapidly progressive chemotherapy-resistant disease, responded to allogeneic cell therapy, which further supports the important role and efficacy of T-cell–mediated immunotherapy in recipients rendered tolerant to donor cells without interference of immunosuppressive agents such as CSA. It should be taken into consideration that early molecular or cytogenetic relapse within 4 to 6 months of allogeneic nonmyeloablative stem cell transplantation would not be considered a relapse at such an early stage, even after lethal conditioning.17
Because of the short observation period and the relatively small cohort of recipients, our data have to be carefully evaluated with no over-interpretation. However, our working hypothesis, based on earlier observations,6-10 seems to be supported by the encouraging data. Until recently it was believed that the main goal of allogeneic BMT and especially autologous BMT is to enable abrogation of host-derived tumor cells or genetically abnormal hematopoietic cells by administration of maximally tolerated doses of myeloablative chemo-radiotherapy, while the transplant merely serves as a rescue procedure. Hence, over the years the goal has been to maximize the intensity of the conditioning, to kill all or as many tumor cells or genetically abnormal host cells as possible. We suggest that the main role of the transplant procedure is induction of donor-specific unresponsiveness in the host that can be effectively induced by donor stem cells, thereby enabling acceptance of donor-derived immune cells. Development of host-versus-graft tolerance allows optimal secondary adoptive immunotherapy with immunocompetent donor T lymphocytes in case alloreactive donor T cells present in the graft leave tumor cells behind. Although induction of GVL can be initially accomplished by T-lymphocyte–enriched donor-derived stem cells, due to concomitant administration of CSA as mandatory anti-GVHD prophylaxis some of the GVL effect may be suppressed. After induction of donor-specific transplantation tolerance by allogeneic nonmyeloablative stem cell transplantation, pending absence of GVHD following discontinuation of CSA or other immunosuppressive agents, immunocompetent T lymphocytes obtained from the donor can be added in graded increments while controlling for disappearance of tumor/host cells on the one hand and signs of GVHD on the other.
Our working hypothesis is supported by many observations in rodents. The use of allogeneic cell therapy for eradication of tumor cells in tolerant recipients is well established.11-14 Likewise, Quesenberry et al18,19 have already documented the feasibility of engraftment of BM cells with establishment of mixed chimerism in nonmyeloablated recipients when no allogeneic barriers exist, although with a much lower proportion of donor-derived cells. Furthermore, we have more than 10 years of clinical experience with patients relapsing after allogeneic BMT, suggesting that allogeneic cell therapy may displace and eradicate malignant and normal stem cells of host origin, even in patients who were considered incurable until recently.6,8,9 Many of these patients can now be rescued, most probably cured, by DLI or more aggressive allogeneic cell therapy program,9 provided that alloreactive immunocompetent donor-type blood lymphocytes are given to chimeric, tolerant recipients, sometimes even patients failing myeloablative doses of common anti-cancer modalities who receive no immunosuppressive agents such as CSA.6,8,9 The negative effects of CSA on GVL effects were previously documented in experimental animals20 and humans.21 The success rate of allogeneic cell therapy increases if DLI is administered as soon as relapse is apparent, preferably at the stage of minimal residual disease (MRD).9 Because the use of DLI was accepted as a standard rescue procedure6-10 in patients relapsing after allogeneic BMT, it follows that relapse may not only be treated but be more effectively prevented by DLI.22 Thus, prevention rather than treatment of relapse can become the standard goal in treating high-risk hematologic malignancies as soon as remission is established. The present clinical study indicates that allogeneic nonmyeloablative stem cell transplantation may become one of the protocols to accomplish this goal, because treatment of MRD instead of bulky disease is certainly easier, much more promising, and cost-effective.
Recently, we have shown that adoptive allogeneic cell therapy based on DLI may also successfully displace residual hematopoietic cells of host origin in patients undergoing allogeneic BMT for nonmalignant diseases such as β-thalassemia major23 and infantile osteopetrosis (M. Aker, S. Slavin, unpublished observations, 1995). Immunocompetent T lymphocytes, present in mobilized blood stem cells, may also displace residual host-type genetically abnormal hematopoietic cells following allogeneic nonmyeloablative stem cell transplantation. The proportion of donor cells may be increased by allogeneic cell therapy if needed to further displace host-type hematopoietic cells and increase the proportion of donor stem cells. Indeed, as shown here, allogeneic nonmyeloablative stem cell transplantation was successful in all 4 cases attempted (Tables 1 and3), suggesting that this approach may successfully replace the need for aggressive conditioning, especially in infants and children, thus possibly reducing late complications that are inevitable following conventional myeloablative conditioning. Interestingly, as can be seen in Table 3, severe GVHD was not an essential requirement for replacement of all host with donor hematopoietic cells, suggesting that in principle, successful allogeneic BMT may be accomplished with reduced conditioning while avoiding severe GVHD. Attention must now be given to improve the technology by careful prevention of GVHD by more carefully controlling post-BMT immunosuppression, or alternatively by improving the conditioning or the composition of the graft (ie, reducing the T-cell number or inactivating their alloreactive potential).
The protocol described here seems to be effective for all candidates eligible for any standard allogeneic BMT procedure. Furthermore, allogeneic nonmyeloablative stem cell transplantation was well tolerated, certainly better than any standard myeloablative conditioning, with no major procedure-related toxicity. Based on our preliminary experience, that needs to be confirmed in a larger series of patients observed for a longer time period, major advantages are to be expected if it can be confirmed that allogeneic nonmyeloablative stem cell transplantation can safely replace allogeneic BMT. Because of the patients' excellent general feeling throughout the procedure, independence of hyperalimentation and the low incidence of common immediate complications (mucositis; fever due to intercurrent infections with no or shorter period of agranulocytosis; shorter period of platelet dependence; smaller risk of severe veno-occlusive disease of the liver, interstitial pneumonitis, and multi-organ failure resulting from combination of some or all of the above) we anticipate that allogeneic nonmyeloablative stem cell transplantation may eventually become an outpatient procedure. Perhaps even more important, the state of transient or stable mixed chimerism that results from allogeneic nonmyeloablative stem cell transplantation may help design newer strategies for better control of GVHD.
The use of allogeneic nonmyeloablative stem cell transplantation may also help bypass frequent late complications that result from the combined effects of high-dose chemo-radiotherapy in addition to prior conventional treatments, especially in the low- and high-age groups. In the low-age group, in contrast to myeloablative allogeneic BMT, allogeneic nonmyeloablative stem cell transplantation may reduce the incidence of growth retardation and infertility due to the unique sensitivity to chemoradiotherapy of the growth centers in the bones, the gonads, and testicles. Indeed, early recovery of menstrual bleeding observed in a 19-year-old woman (data not shown) was encouraging in this regard. In elderly individuals, who normally may not be eligible for a standard alloBMT, allogeneic nonmyeloablative stem cell transplantation may permit a relatively safe clinical application of a potentially curative procedure based primarily on adoptive immunotherapy rather than high-dose chemo-radiotherapy.
In the long run, induction of a state of mixed chimerism, as shown in Table 3, may help reduce the incidence and severity of GVHD. Based on animal data, mixed chimerism seems to be a reliable recipe for engraftment of allogeneic hematopoietic cells while avoiding GVHD, as was previously shown by our earlier work24,25 and confirmed by others.26,27 Apparently, as suggested by experimental data in mice, host hematopoietic cells can veto donor antihost alloreactivity while donor hematopoietic cells can veto residual alloreactive host cells, hence explaining why mixed chimeras can result in bilateral transplantation tolerance.24-27
Following a similar rationale, the M.D. Anderson group has also attempted the use of low-toxicity regimen, confirming the feasibility to induce chimerism without the use of myeloablative regimen using different compounds in the course of the conditioning.28Their engraftment rate appears significantly lower, most likely due to lack of use of anti–T-lymphocyte Ig, as done in the present series.
Availability of a relatively safe protocol for adoptive cell therapy using matched allogeneic stem cells and T cells may offer treating physicians another therapeutic tool that may be considered with fewer hesitations for a larger number of patients in need at an optimal stage of their disease. Many clinicians would agree that as far as using chemotherapy and other available cytoreductive anticancer agents, whatever cannot be achieved at an early stage of treatment is unlikely to be accomplished later. In addition to preventing the development of resistant tumor cell clones by continuous courses of conventional doses of chemotherapy, clinical application of a final curative modality at an earlier stage of disease may avoid the need for repeated courses of chemotherapy with cumulative multi-organ toxicity, while preventing development of platelet resistance induced by repeated sensitization with blood products and development of resistant strains of various infective agents that frequently develops in the course of antimicrobial protocols given for treatment of infections that are unavoidable during repeated courses of conventional anticancer modalities.
In summary, we propose that immunotherapy mediated by allogeneic lymphocytes in tolerant hosts at an early stage of the disease, for every patient with a fully matched sibling, may result in a significant improvement of disease-free survival, quality of life, and cost-effectiveness for candidates of allogeneic BMT. Once confirmed, these observations may open new avenues for the treatment of hematologic malignancies and genetic diseases at an earlier stage of the disease, avoiding the need for repeated courses of chemotherapy or alternative replacement therapy, respectively. Tumor cells or genetically abnormal stem cells may be effectively eliminated by an optimal combination of intense immunosuppression with relatively low-dose chemotherapy, followed by infusion of donor stem cells enriched with immunocompotent T cells, aiming for induction of bilateral transplantation tolerance, thus enabling gradual elimination of all host-type cells by donor T cells over time, while controlling for GVHD. It remains to be seen whether a similar therapeutic approach can be developed for patients with matched unrelated donor available and whether a similar modality may be extrapolated for a large number of malignancies other than those originating from hematopoietic stem cells.
ACKNOWLEDGMENT
We thank Ryna and Melvin Cohen and Baxter International Corporation and the Rich Foundation for supporting our ongoing basic and clinical research in cell therapy. The work was carried out in the Max Moss Leukemia Research Laboratory established and supported by his devoted wife Adi Moss.
Supported by Schering AG.
Address reprint requests Shimon Slavin, MD, Department of Bone Marrow Transplantation, Hadassah University Hospital, Jerusalem 91120, Israel.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be here-by marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.