Abstract
Protein kinase C (PKC) has been implicated in signal transduction events elicited by several hematopoietic growth factors. Thrombopoietin (TPO) is the major regulator of megakaryocytic lineage development, and its receptor, c-Mpl, transduces signals for the proliferation and differentiation of hematopoietic progenitors. In this study we have examined the effect of TPO on the subcellular distribution of PKC (a measure of enzyme activation) in a growth factor-dependent pluripotent hematopoietic cell line that was engineered to express the c-Mpl receptor (UT-7/mpl). In addition, we have assessed the significance of this activation for the induction of both mitogenesis and differentiation. Using a PKC translocation assay, TPO was found to stimulate a time- and dose-dependent increase in the total content of PKC activity present in the membrane fraction of UT-7/mpl cells (maximum increase = 2.3-fold above basal level after 15 minutes with 40 ng/mL TPO, EC50 = 7 ng/mL). Accordingly, a decrease of PKC content in the cytosolic fraction was observed. Immunoblot analysis using PKC isotype-specific antibodies showed that TPO treatment led to a marked increase of the Ca2+/diacylglycerol-sensitive PKC isoforms α and β found in the membrane fraction. In contrast, the subcellular distribution of these isoforms did not change after treatment with granulocyte-macrophage colony-stimulating factor (GM-CSF). Exposure of UT-7/mpl cells to the selective PKC inhibitor GF109203X completely inhibited the PKC activity associated to the membrane fraction after TPO treatment, and blocked the mitogenic effect of TPO. In contrast, GF109203X had no effect on the TPO-induced expression of GpIIb, a megakaryocytic differentiation antigen. Downregulation of PKC isoforms α and β to less than 25% of their initial level by treatment with phorbol 12,13-dibutyrate also abolished the TPO-induced mitogenic response, but had no significant effect when this response was induced by GM-CSF. Taken together, these findings suggest that (1) TPO stimulates the activation of PKC, (2) PKC activation mediates the mitogenic action of TPO, and (3) PKC activation is not required for TPO-induced expression of megakaryocytic surface markers.
THROMBOPOIETIN (TPO) is the major regulator of platelet production.1,2 This hematopoietic growth factor stimulates the proliferation of megakaryocyte progenitor cells, promotes megakaryocyte terminal differentiation, and is essential for the production and maintenance of normal levels of thrombopoiesis.3-8 In addition, TPO stimulates the proliferation of erythroid progenitors,9,10 plays a role in the production of progenitor cells for other myeloid lineages,11-13 and may suppress apoptosis.14,15TPO is produced by the liver3 and exhibits significant homology to erythropoietin (EPO),3 the lineage-specific growth factor that regulates erythropoiesis. The receptor for TPO, a member of the cytokine-receptor superfamily, is encoded by the proto-oncogene c-mpl.16,17 c-Mpl is expressed on primitive hematopoietic progenitor cells and cells of the megakaryocytic lineage.18 Binding of TPO to c-Mpl induces tyrosine phosphorylation of various signaling proteins,19-26 including Shc and Jak2, which in turn respectively engage the RAS/MAP kinase and Jak/STAT signaling cascades.27
Ligand binding to various members of the cytokine receptor superfamily, including those for interleukin-3,28erythropoietin,29 and prolactin,30 has been shown to induce the activation of protein kinase C (PKC). PKC is a family of phospholipid-dependent ser/thr kinases activated by second messenger action,31,32 and thought to participate in the transmission of signals for growth and differentiation in many cell types, including hematopoietic cells.28,33-36Tumor-promoting phorbol esters, which mimic the action of the second messenger diacylglycerol, bind and activate PKC directly,37and stimulate concomitantly the translocation of cytosolic PKC isoforms to membrane sites.33 These agents have been shown to induce megakaryocytic differentiation in bone marrow cultures38and in hematopoietic cell lines,39 suggesting that PKC mediates signals for megakaryocyte development. Based on the above knowledge, we hypothesized that in addition to stimulating tyrosine phosphorylation, TPO could also modulate the activity of PKC. To test this hypothesis, we used a growth factor–dependent hematopoietic cell line that was engineered to express the c-Mpl receptor (UT-7/mpl). Like parental UT-7 cells,40 41 UT-7/mpl cells depend on granulocyte-macrophage colony-stimulating factor (GM-CSF) or EPO for growth and survival, but in addition, they proliferate and differentiate in the presence of TPO. Here we report that in UT-7/mpl cells, TPO stimulates the translocation of individual PKC isoforms, from the cytosol to a membrane compartment. We also show that PKC is required by TPO for the induction of a mitogenic response but not for the induction of differentiation antigens.
MATERIALS AND METHODS
Materials.
Phorbol esters were purchased from Sigma Chemical Co (Poole Dorset, UK). The bisindolylmaleimide GF109203X (2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)-maleimide) was purchased from Calbiochem-Novabiochem (Nottingham, UK). Stock solutions (2 mmol/L) of phorbol esters were made in ethanol and stored at −20°C. GF109203X (10 mmol/L) was dissolved in dimethylsulfoxide and stored at 4°C. [methyl-3H]Thymidine (80 Ci/mmol) and [γ-32P]adenosine triphosphate (ATP) (>5,000 Ci/mmol) were purchased from Amersham International (Amersham, UK). Tissue culture media and additives were from Gibco Life Technologies (Paisley, UK). Human recombinants TPO and GM-CSF were from R&D Systems (Oxon, UK). Other reagents were from standard suppliers or as listed in the text.
Retroviral infection of UT-7 cells.
Stable cell clones expressing the c-Mpl receptor were obtained by retroviral infection of the human pluripotent cell line UT-7 as previously described.42 Briefly, the retroviral vector pBTZen-c-mpl-SVNeo, containing a 1,500-bp fragment of the human c-mpl cDNA (Sal 1-Not 1 fragment), was transfected into the amphotropic packaging cell line Gp+envAm12.43 Individual geneticin-resistant clones were derived in the presence of 1 mg/mL G418, and then supernatants were tested for retroviral production on NIH3T3 cells. Cell clones with the highest retroviral production (4 × 105c-mplinfectious retroviral particles per milliliter of supernatant) were selected. UT-7 cells were then infected by coculturing for 48 hours on subconfluent irradiated (10 Gy) Gp+envAm12-c-mpl cells in the presence of GM-CSF. Nonadherent cells were then plated in a semisolid medium consisting of 1% methylcellulose (Fluka, Saint-Quentin Fallavier, France) in α-minimum essential medium without deoxyribonucleotides (α-MEM) supplemented with 10% fetal calf serum (FCS), 2.5 ng/mL GM-CSF, and 1 mg/mL G418. Individual colonies were picked up 10 days later and expanded in liquid medium in the presence of 2.5 ng/mL GM-CSF and 1 mg/mL G418. c-Mpl expression was assessed by flow cytometry using the anti-human Mpl mouse monoclonal antibody (MoAb) M1, and by Northern blot analysis using aPvuII-PvuII fragment of c-mpl c-DNA, as previously described.18 Three independent UT-7/mpl clones (5.3, 5.1, and 1101C) expressing relatively low, intermediate, and high levels of c-Mpl, respectively, were examined in the present study. However, for most experiments, UT-7/mpl 5.1 was chosen as the representative clone.
Cell culture.
UT-7/mpl cells were maintained in exponential growth (between 1 and 5 × 105 cell/mL) in α-MEM supplemented with 10% FCS, 2 ng/mL GM-CSF, and 0.5 mg/mL G418 (referred to below as culture medium), under 5% CO2/95% air in a humidified incubator. Parental UT-7 cells were grown under the same conditions except for the omission of G418 in the culture medium. For experiments involving measurements of PKC, before stimulation, cells were rendered quiescent by washing twice in culture medium lacking GM-CSF, followed by incubation for 18 hours in this medium, as described by Pallard et al.23 CMK cells were maintained in RPMI supplemented with 10% FCS. CMK cells were rendered quiescent by incubation for 18 hours in RPMI containing 0.5% FCS. Cells were judged to be quiescent based on [3H]thymidine incorporation measurements. These measurements showed a greater than 25-fold reduction in the rate of DNA synthesis compared with cells growing in complete media (data not shown). Quiescent cells were stimulated at 37°C as described in the respective figure legends, and then harvested as follows.
Preparation of cytosolic and membrane fractions.
Aliquots of 5 × 106 cells were washed with ice-cold Dulbecco's phosphate-buffered saline (PBS), resuspended in 0.5 mL ice-cold homogenization buffer (5 mmol/L EDTA, 10 mmol/L EGTA, 0.3% β-mercaptoethanol, 1 mmol/L phenylmethylsulfonyl fluoride, 10 mmol/L benzamidine and 20 mmol/L Tris-HCl, pH 7.5), and disrupted by sonication (3 cycles of 5 seconds at 22 Hz with intervals of 15 seconds) using a Soniprep 150 Ultrasonic Disintegrator (MSE Scientific Instruments, Nottingham, UK). The resulting lysates were centrifuged at 100,000g for 45 minutes at 4°C and supernatants were collected (cytosolic fraction). Pellets were resuspended in 0.5 mL homogenization buffer (as above) containing 0.5% Brij 58 to solubilize membrane-associated proteins. After standing on ice for 30 minutes with occasional vortexing, detergent-insoluble proteins were removed by centrifugation as above, and supernatants were collected (membrane fraction). The cytosolic and membrane fractions were placed on ice until assayed for PKC activity.
Assay of PKC activity.
Total PKC activity in the membrane and cytosolic fractions was determined using a commercially available assay system (Biotrak, Amersham, UK), as described by the manufacturer. This assay system is based upon the PKC-catalyzed transfer of the [32P]phosphate group from [γ-32P]ATP into a PKC-specific peptide substrate (amino acids 651-658 of the epidermal growth factor receptor with the phosphorylation site on Thr-654), in the presence of Ca2+, phosphatidylserine, and phorbol 12-myristate 13-acetate (PMA). Reactions were carried out at 37°C for 15 minutes, using dilutions of each fraction equivalent to 1 × 105 cells. Reactions were performed in duplicate and the results averaged. Radioactivity values resulting from the phosphorylation of endogenous substrates were subtracted from all determinations. One U of PKC was defined as the amount of enzyme that catalyzes the transfer of 1 pmol 32P from [γ-32P]ATP into the PKC substrate per minute at 37°C.
Immunoblot analysis of protein kinase C isoforms.
The distribution of individual PKC isoforms in the cytosolic and membrane fractions was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblotting using PKC isotype-specific mouse MoAbs (Transduction Laboratories, Lexington, KY). Briefly, after boiling in sodium dodecyl sulfate (SDS)-sample buffer (2.5% SDS, 1 mmol/L EDTA, 2% β-mercaptoethanol, 5% glycerol, 10 mmol/L Tris/HCl, pH 6.8), solubilized proteins were resolved on SDS-polyacrylamide gels (7.5% acrylamide) and transferred to Immobilon PVDF membranes (Millipore, UK). Prestained molecular weight markers and individual PKC isoforms (Transduction Laboratories), used as positive controls, were run in parallel. Membranes were blocked for 1 hour at room temperature with 5% nonfat dried milk in PBS/0.1% Tween 20, and then incubated for 2 hours at room temperature with the indicated anti-PKC MoAbs diluted (1:1,200 to 1:2,500) in PBS/0.1% Tween 20. Immunoreactive bands were detected using horseradish peroxidase-labeled rabbit anti-mouse IgG (Amersham) and enhanced chemiluminescence (ECL, Amersham). The relative intensity of the bands was quantified by scanning densitometry using a UVP Gel Documentation System (Ultra-Violet Products, Ltd, Cambridge, UK). For assessment of PKC isoform downregulation, PBS-washed cell pellets (1.5 × 106 cells) were solubilized in 0.2 mL 2× SDS sample buffer without prior fractionation, and total cellular proteins were electrophoresed and immunoblotted as described above.
Cell proliferation assay.
For measurement of [3H]thymidine incorporation into DNA, UT-7/mpl cells were washed twice in culture medium lacking GM-CSF, resuspended in this medium at 1 × 105 cell/mL, and dispensed in aliquots of 0.1 mL into round bottom 96-well tissue culture plates (Falcon, London, UK). After preincubation for 4 hours at 37°C under 5% CO2/95% air, growth factors were added at the appropriate dilution, and then the plates were incubated for a further 48 hours. At the end of this period, and unless otherwise indicated, cells were pulsed with 0.05 μCi [3H]thymidine (1 μCi/nmol) for 6 hours, and then harvested onto glass fiber filtermats (Skatron Instruments, Suffolk, UK) as previously described.44 All assays were performed in triplicate.
Analysis of megakaryocytic differentiation.
To study megakaryocytic differentiation, UT-7/mpl cells were washed free of GM-CSF and grown for 4 days with 10 nmol/L TPO, in culture medium lacking GM-CSF. Alternatively, cells were incubated in culture medium supplemented with GM-CSF in the presence of 10 nmol/L PMA. Antigen expression was determined by flow cytometry using the fluorescein isothiocyanate (FITC)-conjugated MoAb Tab, which recognizes the platelet glycoprotein (Gp)IIb (CD41a), a generous gift from Dr S. Burstein (Oklahoma City, OK). Cells (1 to 2 × 106) were harvested by centrifugation at 150g for 5 minutes, washed with ice-cold PBS containing 1% FCS, and incubated in 0.1 mL PBS/1% FCS with a 1:50 dilution of FITC-conjugated Tab or an equivalent amount of control FITC-conjugated mouse IgG1 (Becton Dickinson, Le Pont-de-Claix, France) for 30 minutes at 4°C. Then, cells were washed once and resuspended in 0.5 mL PBS/1% FCS. Antigen expression was determined using a FACSort instrument (Becton Dickinson, Mountain View, CA). Data were acquired and analyzed using the Cell Quest software (Becton Dickinson). 7-Amino actinomycin D staining was used to eliminate dead cells from the analysis. Relative antigen expression was estimated on live cells using the median fluorescent intensity. Nonspecific fluorescence of cells stained with the control antibody was subtracted from all determinations. Ploidy was determined by flow cytometric analysis of DNA content using propidium iodide. After harvesting, aliquots of 1 to 2 × 106 cells were fixed in 80% ethanol for at least 4 hours at −20°C, then washed twice with ice-cold PBS, and permeabilized for 5 minutes with 0.25% Tween 20 in PBS at 4°C. Finally, samples were incubated in PBS containing 50 μg/mL propidium iodide (Sigma) and 0.1 mg/mL RNAse A (Merck, Damstadt, Germany) for at least 2 hours in the dark. DNA fluorescence distributions were analyzed as previously described,45 using pulse processing to exclude cell clumps.
Statistical analysis.
Experiments were performed at least three times and unless otherwise indicated, results from one representative experiment are shown. Where indicated, levels of statistical significance were determined using the Student's t-test.
RESULTS
Stimulation of PKC translocation by TPO.
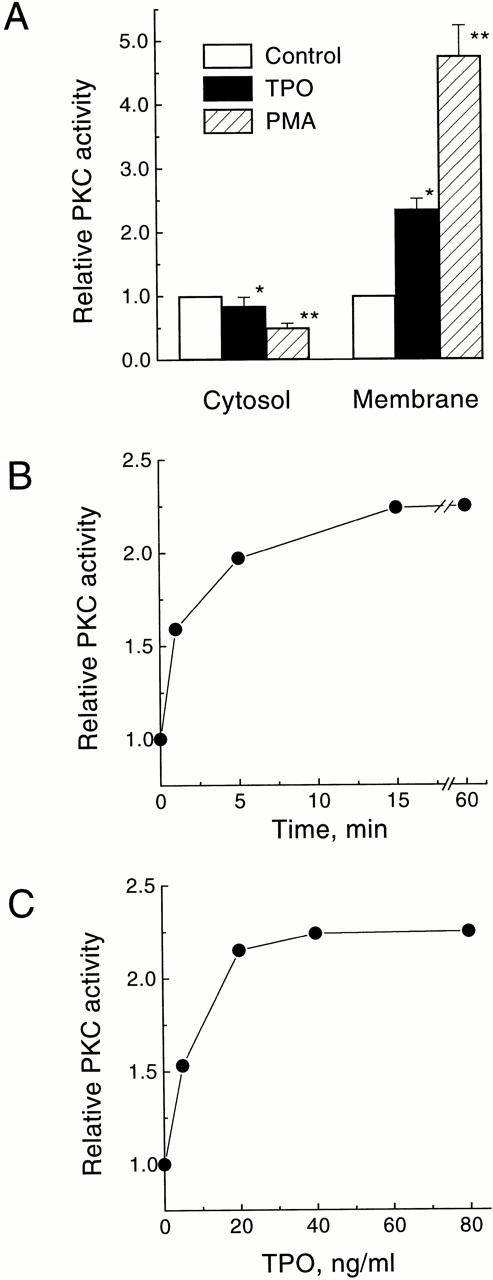
To assess the effect of TPO on the stimulation of PKC in intact cells we measured the “translocation” of the enzymatic activity from the cytosolic to the membrane-rich subcellular fraction. This redistribution is regarded as one of the hallmarks of PKC activation.33 The levels of total PKC activity found in the cytosolic and membrane fractions of quiescent UT-7/mpl 5.1 cells were 19.8 ± 1.8 U/105 cells and 2.7 ± 0.2 U/105cells, respectively (average of four independent determinations ± SD). Treatment of UT-7/mpl 5.1 cells for 15 minutes with PMA resulted in a larger than fourfold increase in the total content of PKC activity in the membrane fraction, and a corresponding decrease in the cytosolic fraction (Fig1A). A similar, albeit less pronounced, effect was observed when these cells were treated with 40 ng/mL TPO (Fig 1A). The TPO-induced rise of PKC activity in the membrane fraction was detectable as early as 1 minute after addition of TPO, reached a maximum (2.3-fold) after 15 minutes, and persisted for at least 60 minutes (Fig 1B). This effect of TPO was dose-dependent, with a half maximal stimulation observed at 7 ng/mL, and maximal stimulation seen above 40 ng/mL (Fig 1C).
Stimulation of PKC translocation by TPO in UT-7/mpl 5.1 cells. (A) Effect of TPO or PMA on the subcellular distribution of total PKC activity. Quiescent cells were treated for 15 minutes with 40 ng/mL TPO or 50 nmol/L PMA and then PKC activity was measured in the membrane and cytosolic fractions. Values represent the mean ± SD of four independent experiments. (□), control; (▪), TPO; (▨), PMA. *P < .05, **P < .01. (B) Time course of TPO-induced increase in membrane-associated PKC activity. Cells were treated with 40 ng/mL TPO for the indicated times and then PKC activity was measured in the membrane fraction. (C) Dose response for the increase in membrane-associated PKC activity induced by TPO. Cells were treated with the indicated concentrations of TPO for 15 minutes and then PKC activity was measured in the membrane fraction. PKC activity is expressed relative to the level present in unstimulated cells incubated in parallel. All other experimental details were as described in Materials and Methods.
Stimulation of PKC translocation by TPO in UT-7/mpl 5.1 cells. (A) Effect of TPO or PMA on the subcellular distribution of total PKC activity. Quiescent cells were treated for 15 minutes with 40 ng/mL TPO or 50 nmol/L PMA and then PKC activity was measured in the membrane and cytosolic fractions. Values represent the mean ± SD of four independent experiments. (□), control; (▪), TPO; (▨), PMA. *P < .05, **P < .01. (B) Time course of TPO-induced increase in membrane-associated PKC activity. Cells were treated with 40 ng/mL TPO for the indicated times and then PKC activity was measured in the membrane fraction. (C) Dose response for the increase in membrane-associated PKC activity induced by TPO. Cells were treated with the indicated concentrations of TPO for 15 minutes and then PKC activity was measured in the membrane fraction. PKC activity is expressed relative to the level present in unstimulated cells incubated in parallel. All other experimental details were as described in Materials and Methods.
To address the possibility that the effect of TPO on PKC might have been an artificial characteristic of a particular clone, we examined two additional independent UT-7/mpl clones that express different levels of c-Mpl on their surface. As shown in Table1, TPO was unable to significantly affect the subcellular distribution of PKC in parental UT-7 cells, which do not express sufficient amounts of c-Mpl.18 In contrast, this factor stimulated a marked translocation of PKC in all the c-Mpl–expressing clones. Furthermore, there was a positive correlation between the degree of TPO-induced PKC translocation and the relative level of c-Mpl expressed on the surface of each clone (Table 1). To extend this finding to cells that express an endogenous c-mpl,we also examined the effect of TPO on the translocation of PKC in CMK cells. This cell line was previously reported to display a functional response to TPO.4 Table 1 shows that in CMK cells, TPO stimulated the translocation of PKC to an extent that was similar to that found in UT-7/mpl cells. Thus, the TPO-induced translocation of PKC was a common feature of every c-Mpl–expressing cell examined, including a cell line that expressed the endogenous gene.
Effect of TPO on the translocation of individual PKC isoforms.
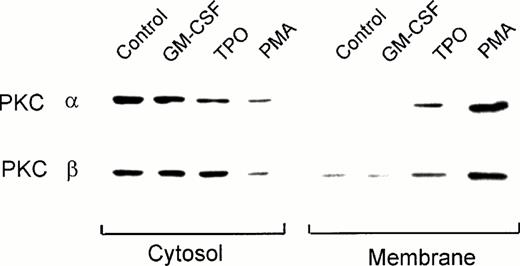
PKC consists of a family of at least 11 different isoforms, classified in three groups according to their cofactor requirements.32 33 Immunoblot analysis using PKC isotype-specific MoAbs showed that UT-7/mpl 5.1 cells expressed moderate to high levels of the conventional isoforms α and β, the novel PKC isoforms δ and θ, and the atypical isoform ι (data not shown). These cells also expressed the PKC isoform μ (also known as PKD), which respond to phorbol esters but cannot be ascribed to any of the above three groups (data not shown). Except for PKC η, which was not tested, other isoforms (γ, ε, ζ, and λ) were either absent or barely detectable. In the present study we characterized the effect of TPO on the translocation of the classical isoforms α and β. Figure 2 shows that in untreated UT-7/mpl 5.1 cells these isoforms were found preferentially in the cytosolic compartment, their presence in the membrane fraction being hardly detectable. This figure also shows that treatment with PMA resulted in the translocation of PKC-α and β from the cytosol to the membrane compartment. Similarly, a marked increase (threefold to sixfold) of these isoforms was observed in the membrane fraction after the cells were treated with TPO (Fig 2). A corresponding decrease of immunoreactivity in the cytosolic fraction was noticeable in the case of PKC-α, but not in the case of PKC-β. In contrast to the effects of PMA or TPO, when the cells were treated with GM-CSF, the subcellular distribution of PKC-α and β was not affected (Fig 2).
Effect of TPO on the subcellular localization of PKC-α and -β isoforms as detected by immunoblotting. Quiescent UT-7/mpl 5.1 cells were treated for 15 minutes with 40 ng/mL TPO, 2 ng/mL GM-CSF or 50 nmol/L PMA as indicated. Cytosolic and membrane fractions were prepared from each sample and aliquots equivalent to 5 × 105 cells in case of the membrane fraction, or half that amount in case of the cytosolic fraction, were analyzed by immunoblotting using MoAbs against the indicated PKC isoforms, as described in Materials and Methods.
Effect of TPO on the subcellular localization of PKC-α and -β isoforms as detected by immunoblotting. Quiescent UT-7/mpl 5.1 cells were treated for 15 minutes with 40 ng/mL TPO, 2 ng/mL GM-CSF or 50 nmol/L PMA as indicated. Cytosolic and membrane fractions were prepared from each sample and aliquots equivalent to 5 × 105 cells in case of the membrane fraction, or half that amount in case of the cytosolic fraction, were analyzed by immunoblotting using MoAbs against the indicated PKC isoforms, as described in Materials and Methods.
Effect of GF109203X on the membrane-associated PKC activity translocated by TPO.
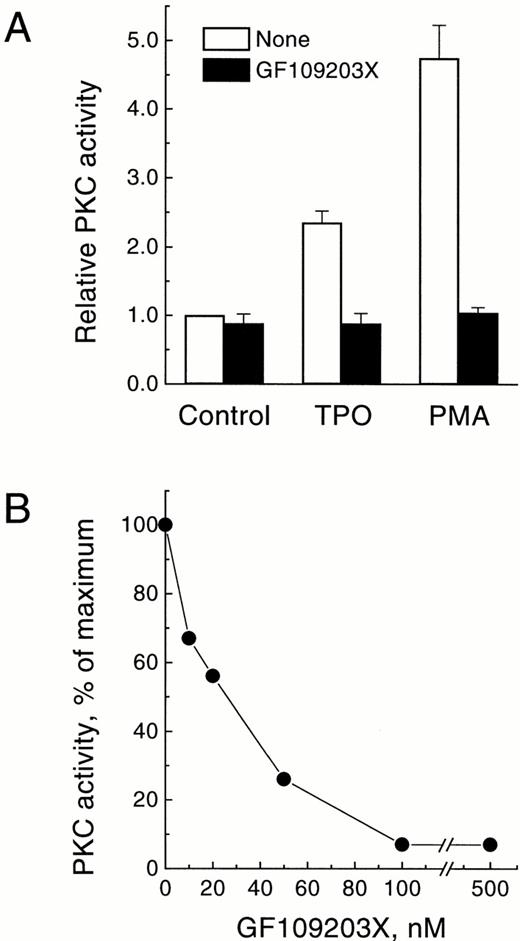
To obtain further evidence that the activity elevated in the membrane fraction following treatment with TPO belonged to PKC, we tested the effect of the bisindolylmaleimide GF109203X, a specific protein kinase inhibitor that acts selectively against the PKC family, both in cell-free systems and in intact cells.46 As shown in Fig3A, addition of 3 μmol/L GF109203X to intact UT-7/mpl 5.1 cells resulted in a complete inhibition of the enzymatic activity measured in the membrane fraction, following treatment of the cells with either TPO or PMA. Furthermore, addition of GF109203X directly to the PKC assay inhibited this activity at nanomolar concentrations (IC50 = 23 nmol/L; average from two experiments; range, 19 to 26 nmol/L), with a maximal effect observed at 100 nmol/L (Fig 3B). These results were entirely consistent with previous reports showing a selective and specific effect of this inhibitor on PKC.46
Effect of GF109203X on the membrane-associated PKC activity translocated by TPO. (A) Inhibition of PMA-dependent or TPO-dependent membrane-associated PKC activity by GF109203X in intact cells. Quiescent UT-7/mpl 5.1 cells were preincubated for 15 minutes in the absence (□) or presence (▪) of 3 μmol/L GF109203X, and then treated for another 15 minutes with 40 ng/mL TPO or 50 nmol/L PMA. At the end of this period PKC activity was measured in the membrane fraction as described in Materials and Methods. Results represent the mean of four independent experiments ± SD. PKC activity is expressed relative to the level present in unstimulated cells incubated in parallel. (B) Inhibition of membrane-associated PKC activity as a function of GF109203X concentration in the assay. Cells were treated for 15 minutes with 40 ng/mL TPO and then PKC activity was measured in the membrane fraction in the presence of the indicated concentrations of GF109203X. Results are expressed as the percentage of PKC activity in the sample with no GF109203X added.
Effect of GF109203X on the membrane-associated PKC activity translocated by TPO. (A) Inhibition of PMA-dependent or TPO-dependent membrane-associated PKC activity by GF109203X in intact cells. Quiescent UT-7/mpl 5.1 cells were preincubated for 15 minutes in the absence (□) or presence (▪) of 3 μmol/L GF109203X, and then treated for another 15 minutes with 40 ng/mL TPO or 50 nmol/L PMA. At the end of this period PKC activity was measured in the membrane fraction as described in Materials and Methods. Results represent the mean of four independent experiments ± SD. PKC activity is expressed relative to the level present in unstimulated cells incubated in parallel. (B) Inhibition of membrane-associated PKC activity as a function of GF109203X concentration in the assay. Cells were treated for 15 minutes with 40 ng/mL TPO and then PKC activity was measured in the membrane fraction in the presence of the indicated concentrations of GF109203X. Results are expressed as the percentage of PKC activity in the sample with no GF109203X added.
Correlation between the stimulation of PKC activity by TPO and the induction of mitogenesis.
GF109203X was also used to investigate the role of PKC in the mitogenic action of TPO. Mitogenesis was examined by [3H]thymidine incorporation into DNA. As shown in Fig 4A,GF109203X inhibited the TPO-induced mitogenic response. This inhibitory effect was dose-dependent (IC50 = 0.5 μmol/L), with complete inhibition observed at 3 to 4 μmol/L. Figure 4B shows the dose response for the stimulation of mitogenesis induced by TPO in the absence or presence of GF109203X. TPO stimulated mitogenesis in UT-7/mpl 5.1 cells in a dose-dependent manner with an EC50of 2 ng/mL (Fig 4B, ○). An optimal dose of GF109203X (3 μmol/L) shifted this dose-response curve to the right (Fig 4B, •). Thus, at concentrations of TPO below 10 ng/mL, GF109203X caused a complete suppression of the mitogenic response, whereas above 10 ng/mL TPO, the inhibition reached 70%.
Inhibition of the TPO-induced mitogenic response by GF109203X. (A) Inhibition of the TPO-induced mitogenic response as function of GF109203X concentration. UT-7/mpl 5.1 cells were treated for 48 hours with 5 ng/mL TPO and the indicated concentrations of GF109203X. [3H]Thymidine incorporation into DNA was measured as described in Materials and Methods. Results are expressed as a percentage of the response observed in the cells treated with TPO alone. (B) Effect of GF109203X on the dose response for the induction of mitogenesis stimulated by TPO. Cells were treated for 48 hours with various concentrations of TPO, in the absence (○) or presence (•) of 3 μmol/L GF109203X. [3H]Thymidine incorporation into DNA was measured as described in Materials and Methods. Results are expressed as a percentage of the maximal response. Mean values from one representative experiment performed in triplicate are shown in each case. Error bars (SDs), which were smaller or equal to the size of the symbol, are not visible.
Inhibition of the TPO-induced mitogenic response by GF109203X. (A) Inhibition of the TPO-induced mitogenic response as function of GF109203X concentration. UT-7/mpl 5.1 cells were treated for 48 hours with 5 ng/mL TPO and the indicated concentrations of GF109203X. [3H]Thymidine incorporation into DNA was measured as described in Materials and Methods. Results are expressed as a percentage of the response observed in the cells treated with TPO alone. (B) Effect of GF109203X on the dose response for the induction of mitogenesis stimulated by TPO. Cells were treated for 48 hours with various concentrations of TPO, in the absence (○) or presence (•) of 3 μmol/L GF109203X. [3H]Thymidine incorporation into DNA was measured as described in Materials and Methods. Results are expressed as a percentage of the maximal response. Mean values from one representative experiment performed in triplicate are shown in each case. Error bars (SDs), which were smaller or equal to the size of the symbol, are not visible.
Another powerful approach to study the role of PKC in cellular responses is to downregulate its level by prolonged pretreatment with high concentrations of phorbol esters.28,33 Hence, PKC downregulation was also used to obtain further evidence for the role of this enzyme in the mitogenic action of TPO. Figure5A shows that in UT-7/mpl 5.1 cells a 6-hour pretreatment with 500 nmol/L phorbol 12,13-dibutyrate (PDBu) was sufficient to cause a substantial downregulation of PKC isoforms α and β (>75%). A longer pretreatment reduced PKC levels even further. However, because prolonged incubations of hematopoietic cells with phorbol esters induce terminal differentiation,39 47 and hence, irreversibly arrest proliferation, the shorter 6-hour preincubation time was chosen. Figure5B shows that this short pretreatment with PDBu inhibited the TPO-induced mitogenic response by greater than 70% (P = .01). In contrast, the same pretreatment had a marginal, statistically nonsignificant effect on the mitogenic response induced by GM-CSF (P > .05). Therefore, this result showed that the inhibition of the mitogenic response to TPO was not the result of a toxic effect or of a more general inhibition of mitogenesis.
Inhibition of the TPO-induced mitogenic response by PKC downregulation. (A) Time course for the downregulation of PKC isoforms α and β. UT-7/mpl 5.1 cells were incubated in culture medium lacking GM-CSF, in the absence or presence of 500 nmol/L PDBu, for the indicated lengths of time. At the end of the incubations whole cell lysates were prepared and aliquots equivalent to 0.3 × 106 cells were analyzed by immunoblotting using MoAbs against the indicated PKC isoforms, as described in Materials and Methods. (B) Effect of PKC downregulation on the TPO-induced or GM-CSF-induced mitogenic response. After a pretreatment of 6 hours with 500 nmol/L PDBu, cells were washed three times with culture medium lacking GM-CSF to remove the PDBu, and then incubated for a further 48 hours with 10 ng/mL TPO or 2 ng/mL GM-CSF in culture medium containing 0.1 μCi/mL [3H]thymidine. At the end of this period cells were harvested onto glass fiber filtermats as described in Materials and Methods. Radioactivity values represent the mean ± SD of three replicates. (□), Untreated cells; (▪), cells treated with PDBu.
Inhibition of the TPO-induced mitogenic response by PKC downregulation. (A) Time course for the downregulation of PKC isoforms α and β. UT-7/mpl 5.1 cells were incubated in culture medium lacking GM-CSF, in the absence or presence of 500 nmol/L PDBu, for the indicated lengths of time. At the end of the incubations whole cell lysates were prepared and aliquots equivalent to 0.3 × 106 cells were analyzed by immunoblotting using MoAbs against the indicated PKC isoforms, as described in Materials and Methods. (B) Effect of PKC downregulation on the TPO-induced or GM-CSF-induced mitogenic response. After a pretreatment of 6 hours with 500 nmol/L PDBu, cells were washed three times with culture medium lacking GM-CSF to remove the PDBu, and then incubated for a further 48 hours with 10 ng/mL TPO or 2 ng/mL GM-CSF in culture medium containing 0.1 μCi/mL [3H]thymidine. At the end of this period cells were harvested onto glass fiber filtermats as described in Materials and Methods. Radioactivity values represent the mean ± SD of three replicates. (□), Untreated cells; (▪), cells treated with PDBu.
Effect of GF109203X on the induction of megakaryocytic differentiation.
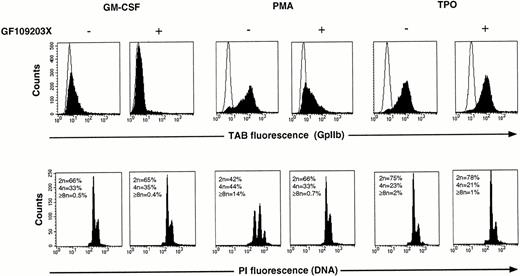
Previously we have shown that GF109203X can be used as a tool to assess the involvement of PKC in phorbol-ester–induced megakaryocytic differentiation.35 Therefore, in the present study this inhibitor was also used to investigate the significance of PKC activation for TPO-induced differentiation of UT-7/mpl cells. As shown in Fig 6 (top), treatment of UT-7/mpl 1101C cells for 4 days with either PMA or TPO resulted in a marked increase (30- and 25-fold, respectively) in the expression of GpIIb, a surface marker of megakaryocytic differentiation. Addition of 3 μmol/L GF109203X almost completely suppressed the increase in GpIIb expression induced by PMA, but had no significant effect when expression of this antigen was induced by TPO. Similar results were obtained in UT-7/mpl 5.1 cells (data not shown). Analysis of DNA content (Fig 6, bottom) revealed that PMA treatment caused a marked increase in the proportion of polyploid cells (cells with DNA content greater than 4n). This response was also inhibited by GF109203X. In contrast to PMA, TPO caused only a marginal increase in ploidy. For this reason, it was difficult to ascertain what was the effect of GF109203X on TPO-induced polyploidization. Culture in the presence of TPO for longer than a week caused a more substantial increase in the number of polyploid cells. However, under these conditions the effect of GF109203X could not be assessed, as it induced apoptosis of a large proportion of cells in the culture (data not shown).
Lack of inhibition of the TPO-induced differentiation response by GF109203X. UT-7/mpl 1101C cells were incubated for 4 days with either 2 ng/mL GM-CSF (left), 2 ng/mL GM-CSF + 10 nmol/L PMA TPO (center), or with 10 nmol/L TPO (right), in the absence or presence of 3 μmol/L GF109203X. GpIIb expression and DNA content were analyzed by flow cytometry as described in Materials and Methods. In the top panels the open traces correspond to the fluorescence distributions of samples stained with a control IgG1, and the filled traces correspond to parallel samples stained with the MoAb Tab.
Lack of inhibition of the TPO-induced differentiation response by GF109203X. UT-7/mpl 1101C cells were incubated for 4 days with either 2 ng/mL GM-CSF (left), 2 ng/mL GM-CSF + 10 nmol/L PMA TPO (center), or with 10 nmol/L TPO (right), in the absence or presence of 3 μmol/L GF109203X. GpIIb expression and DNA content were analyzed by flow cytometry as described in Materials and Methods. In the top panels the open traces correspond to the fluorescence distributions of samples stained with a control IgG1, and the filled traces correspond to parallel samples stained with the MoAb Tab.
DISCUSSION
The PKC family has been implicated in intracellular mechanisms that transduce signals for survival, proliferation, and differentiation of hematopoietic cells.28,33-36 48 In this study we have investigated the involvement of PKC in both the mitogenic and the differentiation responses induced by TPO. For this purpose we have used the human pluripotent hematopoietic cell line UT-7, after it has been modified by a retroviral strategy to express the TPO receptor. UT-7/mpl proliferates and differentiates in response to TPO in a similar fashion to hematopoietic progenitors, and thus it provides a useful model system to study the signaling pathways through which TPO might function.
The results reported here show that engagement of the c-Mpl receptor by TPO leads the translocation of PKC from the cytosol to membrane sites. This translocation, which reflects the activation of various isoforms of this enzyme,33 was found not only in each of the threec-mpl–transfected UT-7 clones examined, but also in CMK cells, which express a functional TPO receptor from the endogenous gene.4 Thus, activation of PKC by TPO cannot be attributed to an artifact of the transfection, or to a property of one particular cell line. Furthermore, the TPO-induced translocation of PKC was comparable in magnitude to that seen in other systems in which PKC is known to be activated by physiological stimuli, acting through receptor-mediated mechanisms.49-51 Recently, using a myelin basic protein-derived synthetic peptide substrate of PKC, Kunitama et al52 reported a TPO-stimulated increase of protein kinase activity in low speed supernatants of detergent-made lysates from UT-7/mpl cells. This increase was attributed to stimulation of PKC via diacylglycerol generation. However, since these investigators measured the total cellular content of this kinase activity rather than its translocation, and the assay included all the necessary cofactors to produce full activation of PKC, the meaning of this increase remains unclear.
Hematopoietic cells express several PKC isoforms that may be activated by various independent mechanisms.31,32 In this study we have focused particularly on the Ca2+/diacylglycerol-sensitive isoforms α and β, and we have shown that both are translocated to the membrane following TPO treatment. However, at this stage, we cannot rule out the possibility that other PKC isoforms are also activated. We should also emphasize that the antibody used here to recognize PKC-β cannot distinguish between βI and βII, the two isoforms produced by alternative splicing. The pathway leading from c-Mpl receptor occupancy to PKC activation also remains to be elucidated. Signal transduction mechanisms involving tyrosine phosphorylation couple this receptor to intracellular effectors.19,20,22,23,26,27 Studies with other hematopoietic growth factor receptors have suggested that tyrosine phosphorylation may result in the activation of a phosphatidylcholine-specific phospholipase C that generates the necessary diacylglycerol for PKC activation.36 In the case of c-Mpl, there is some evidence indicating that TPO stimulates the phosphorylation of phospholipase Cγ,19 21 raising the possibility that this enzyme might participate in the pathway leading to PKC activation.
A major finding of this study is that GF109203X blocked the TPO-induced mitogenic response of UT-7/mpl cells (Fig 4). In this context, it is important to note that the IC50 and the optimal dose of GF109203X for the inhibition of TPO-induced mitogenesis were similar to the values reported in other cellular systems for the inhibition of receptor-mediated biological responses in which PKC is known to be involved.46 However, it is also noteworthy that at concentrations of TPO above 10 ng/mL, GF109203X did not block the mitogenic response completely. This suggests that high concentrations of TPO could induce proliferation through an additional signaling pathway which is independent of PKC. This possibility is consistent with the notion that mitogenic signals may be transduced via redundant intracellular pathways.53 Additional support for the involvement of PKC in the mitogenic effects of TPO came from results of PKC downregulation experiments (Fig 5). These experiments clearly showed that a reduction of 75% to 80% in the levels of PKC-α and -β led to a 75% inhibition of the TPO-induced mitogenic response. In this case the lack of complete inhibition could be attributed either to the presence of residual PKC, or to the existence of an additional TPO-stimulated mitogenic pathway that operates independently of PKC, as discussed above. Whatever the reason for the lack of complete inhibition, both approaches to interfere with PKC, namely inhibition of the enzymatic activity or the downregulation of its expression, strongly suggest that the mitogenic response induced by TPO in UT-7/mpl cells is mediated predominantly by a pathway involving the activation of PKC.
As shown in the present study, engagement of the GM-CSF receptor in UT-7/mpl cells did not result in the translocation of PKC-α or -β. Furthermore, downregulation of these isoforms had no significant effect on the mitogenic response induced by GM-CSF. Thus, in UT-7/mpl cells, PKC-α and β do not appear to be required by GM-CSF for the induction of a mitogenic response. In agreement with our findings, Shearman et al54 reported that also in multipotential FDCP-Mix A4 cells, GM-CSF was unable to stimulate the translocation of these isoforms to the membrane. In contrast to these results, several studies had previously indicated that GM-CSF stimulates the activation of PKC in various hematopoietic cells.36 However, in the majority of those cases the individual PKC isoforms were not identified. Thus, our findings are not necessarily incompatible with previous work suggesting that GM-CSF can indeed stimulate PKC activation. It is possible, for example, that GM-CSF activates PKC isoforms other than α or β, and that this depends also on the cell type in question. Support for this interpretation is provided by studies in TF-1 cells, a multipotent human hematopoietic cell line with properties similar to UT-7. In these cells, PKC-δ55 and PKC-ε,56 but not PKC-α,56 were reported to be involved in the signal transduction pathways activated by GM-CSF. Thus, taken together, our findings and those of other laboratories suggest that in UT-7/mpl cells, GM-CSF and TPO would require different PKC isoforms to mediate a proliferative response.
The present study clearly shows that in contrast to its inhibitory effect on TPO-induced mitogenesis, GF109203X did not affect the induction of GpIIb expression by TPO (Fig 6). Therefore, our data indicate that in UT-7/mpl cells, PKC is not required by TPO for transmission of signals that regulate the induction of megakaryocytic differentiation antigens. This conclusion is compatible with previous findings suggesting that c-Mpl–generated signals for differentiation are transmitted via Shc and Grb2, through the Ras-MAP kinase cascade,26 of which PKC is not a component.57On the other hand, our data also show that GF109203X completely prevented PMA-induced differentiation (Fig 6), suggesting that PKC does play a role in the process. These apparently conflicting results can be explained if one considers that MAP kinases can also be activated by PKC via Raf-1, independently of Ras activation.58 In addition, evidence has now been provided that MAP kinases are downstream mediators of PMA-induced megakaryocytic differentiation.59 Thus, taken together, our findings and those of other laboratories fit a model in which megakaryocytic differentiation may be activated through PKC-dependent and PKC-independent pathways converging on the MAP kinase cascade. Recently, it has been shown that nuclear polyploidization and cytoplasmic maturation may be regulated independently.60Therefore, the evidence provided here showing that PKC is not involved in the induction of GpIIb expression by TPO does not exclude the possibility that a PKC-dependent mechanism might be involved in TPO induction of polyploidization. The fact that TPO caused a very small increase in the number of polyploid cells made it impracticable to assess this possibility fully. Nonetheless, consistent with the inhibitory effect of GF109203X on TPO-induced DNA synthesis (Fig 4), it would appear that this compound also somewhat reduced the small increase in ploidy induced by TPO (Fig 6 and data not shown). Whether PKC plays a role in TPO-induced events associated with polyploidization, other than DNA replication, remains to be explored. Although the present work clearly shows the dominant role of a PKC-independent pathway in the induction of megakaryocytic differentiation antigens by TPO in UT-7/mpl cells, it does not exclude the possibility that PKC might play a role in TPO-induced differentiation of primary hematopoietic progenitors.
Supported in part by Research Grant No. PG/95001 from the British Heart Foundation.
Address reprint requests to Jorge D. Erusalimsky, PhD, Cruciform Project, Rayne Institute, University College London, 5 University Street, London WC1E 6JJ, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 4. Inhibition of the TPO-induced mitogenic response by GF109203X. (A) Inhibition of the TPO-induced mitogenic response as function of GF109203X concentration. UT-7/mpl 5.1 cells were treated for 48 hours with 5 ng/mL TPO and the indicated concentrations of GF109203X. [3H]Thymidine incorporation into DNA was measured as described in Materials and Methods. Results are expressed as a percentage of the response observed in the cells treated with TPO alone. (B) Effect of GF109203X on the dose response for the induction of mitogenesis stimulated by TPO. Cells were treated for 48 hours with various concentrations of TPO, in the absence (○) or presence (•) of 3 μmol/L GF109203X. [3H]Thymidine incorporation into DNA was measured as described in Materials and Methods. Results are expressed as a percentage of the maximal response. Mean values from one representative experiment performed in triplicate are shown in each case. Error bars (SDs), which were smaller or equal to the size of the symbol, are not visible.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/3/10.1182_blood.v91.3.813/3/m_blod4032804.jpeg?Expires=1768400298&Signature=vFTbB-ga1LrlrYGPKlT3uymc0FAS~7uX9hhMl6DthQPj4jzRVUncKT54e~uo-N7nzA-BE4fhuEZVnmvYsgHHM2j~XTpxIwphnBKO9MWWtul9wm7VugUe9giVFP1ZF7EZtDbSlKA7NYsq5U1m9GDw5XB1rVlSaqtftKdaVceJyl-mkzNQ6yz4z9yi1NS~~AQP5O5EEvjtXdryZp~sFz2nOpuz~~F54rFakostYwTGxCxFpNkjPRv7IhYxNrFjYD2rVNYW75l-LE5lvwsdikSkG6MDGX5PRmK6l-80sZf9TTh6weEnOp0mdlmG4-rD6QT9HbvqS3toqTwW~ibzUIohMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Inhibition of the TPO-induced mitogenic response by PKC downregulation. (A) Time course for the downregulation of PKC isoforms α and β. UT-7/mpl 5.1 cells were incubated in culture medium lacking GM-CSF, in the absence or presence of 500 nmol/L PDBu, for the indicated lengths of time. At the end of the incubations whole cell lysates were prepared and aliquots equivalent to 0.3 × 106 cells were analyzed by immunoblotting using MoAbs against the indicated PKC isoforms, as described in Materials and Methods. (B) Effect of PKC downregulation on the TPO-induced or GM-CSF-induced mitogenic response. After a pretreatment of 6 hours with 500 nmol/L PDBu, cells were washed three times with culture medium lacking GM-CSF to remove the PDBu, and then incubated for a further 48 hours with 10 ng/mL TPO or 2 ng/mL GM-CSF in culture medium containing 0.1 μCi/mL [3H]thymidine. At the end of this period cells were harvested onto glass fiber filtermats as described in Materials and Methods. Radioactivity values represent the mean ± SD of three replicates. (□), Untreated cells; (▪), cells treated with PDBu.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/3/10.1182_blood.v91.3.813/3/m_blod4032805.jpeg?Expires=1768400298&Signature=F0IKk-DZmpe8ZL93LKl3irL-VwxYk4cri5elD8n56IIpvRqVcTM05R~8SSIvee60eyqSq0YZ-8ixjR7rNMiIKtPQwNrgloFlHRXoYU6sRcRac3L52l0trtZe3bVQ2uPTOBhPv3vSrAaoeLTBzZ4KGCac2WFpxh62wgWEEhkoT0jJU5642cdh7xZW-UO64e7xvVrdw2RYROPXhgDUT3lWhm4png0qfLslwoYL1zlQJE7VYGeBVP7kl179XD4BSBlUdXaK0YOUM2XXrub69Lu47Dq9sQ0D1HPhmQQ4xcPS~CP2DtdxDGZLHgNv~dvFCJX0WQdCCz-gg5iDaUzMv3Sc5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)