Abstract
Multimerin is a novel, massive, soluble protein that resembles von Willebrand factor in its repeating, homomultimeric structure. Both proteins are expressed by megakaryocytes and endothelial cells and are stored in the region of platelet α-granules resembling Weibel-Palade bodies. These findings led us to study the distribution of multimerin within human endothelial cells. Multimerin was identified in vascular endothelium in situ. In cultured endothelial cells, multimerin was identified within round to rod-shaped, dense-core granules, some of which contained intragranular, longitudinally arranged tubules and resembled Weibel-Palade bodies. However, multimerin was found primarily in different structures than the Weibel-Palade body proteins von Willebrand factor and P-selectin. After stimulation with secretagogues, multimerin was observed to redistribute from intracellular structures to the external cellular membrane, without detectable accompanied secretion of multimerin into the culture media. In early passage endothelial cell cultures, multimerin was associated with extensive, fibrillary, extracellular matrix structures, in a different distribution than fibronectin. Although multimerin and von Willebrand factor are stored together in platelets, they are mainly found within different structures in endothelial cells, indicating that there are tissue-specific differences in the sorting of these soluble, multimeric proteins.
REGULATED SECRETORY pathways provide an important mechanism for responding to external stimuli and for rapidly altering cellular functions.1-6 Platelets and endothelial cells are important for hemostasis and both contain secretory granules that allow sequestered proteins to be released at sites of vessel injury.7-9 In some cells (including platelets), many different proteins are stored in a common secretory granule, whereas in other cells, there are populations of storage granules with different content proteins.1,3,5,6-8 10-15
Platelet α-granules and endothelial cell Weibel-Palade bodies are secretory granules that share similar features: both contain longitudinally aligned tubular structures and their granule membranes are the storage site for P-selectin.7,8,16-24 The tubules found in these granules coincide with the presence of ultra-high molecular-weight von Willebrand factor, and they are absent in severe von Willebrand disease.7-9,17,18,25-31 Yet, in contrast to the many different proteins stored in α-granules,7,8 only von Willebrand factor, P-selectin, histamine, and the lysosomal membrane protein CD63 have been localized to Weibel-Palade bodies.9,18,22-24,26,32 33
Within α-granules, most soluble proteins are found within the central matrix or electron-dense nucleoid.7,8 Recently, multimerin and factor V were localized to the eccentric, α-granular electron lucent zone where von Willebrand factor is stored.34,35Like von Willebrand factor, multimerin is one of the largest proteins found in platelets, and it is composed of variably sized, disulfide-linked homomultimers, most of which are millions of daltons in size.36-38 Similar to von Willebrand factor and its binding of factor VIII,39,40 multimerin also interacts with a coagulation cofactor, factor V. 35 In resting platelets, but not in plasma, all of the biologically active factor V is stored complexed with multimerin.35 However, the complexes of multimerin and factor V dissociate when platelets are activated by thrombin, suggesting multimerin may function as an intragranular factor V–carrier protein.35 Although multimerin resembles von Willebrand factor in its massive homomultimeric structure36,37,41,42 and these proteins bind homologous coagulation cofactors,35,39 the primary structures of multimerin and von Willebrand factor are not related.43-48The functions of multimerin are largely unknown, but its Arg-Gly-Asp-Ser site and similarities to the extracellular matrix protein collagens type VIII and X have suggested multimerin functions as an adhesive or extracellular matrix protein.43
The colocalization of multimerin and von Willebrand factor in the Weibel-Palade body-like region of platelet α-granules,34and multimerin's expression in vascular tissue and endothelial cells43 led us to investigate the storage and biosynthesis of multimerin in human endothelial cells. We report that multimerin is contained within dense-core granules in human endothelial cells and within fibrillary structures in the extracellular matrix of cultured endothelium. However, we found multimerin primarily within different endothelial cell granules than von Willebrand factor, indicating that there are differences in the way endothelial cells and megakaryocytes compartmentalize these multimeric proteins.
MATERIALS AND METHODS
Antibodies.
Antibodies used for immunoprecipitation, ELISA, and immunocytochemistry included monoclonal and polyclonal antimultimerin,38monoclonal, and polyclonal anti-von Willebrand factor (Dako, Carpenteria, CA; electron microscopy studies, Dakopatts, Glostrup, Denmark), polyclonal anti–P-selectin (Cedarlane Laboratories, Hornsby, Ontario, Canada; electron microscopy studies, a gift from Dr Michael Berndt, Victoria, Australia49), monoclonal anti-CD63 (D545 from Dr. S. J. Israels, Winnipeg, Manitoba, Canada50; and a monoclonal antibody from Caltag Laboratories, Burlingame, CA), normal mouse IgG (Zymed Laboratories Inc, San Francisco, CA), polyclonal antifibronectin (Organon Teknika Inc, Scarborough, Ontario) and sheep antihuman factor V (Affinity Biologicals, Hamilton, Ontario). For some immunostaining experiments, polyclonal antimultimerin (0.5 μL) was preadsorbed for 30 minutes with buffer or with 80 mU of soluble, affinity purified multimerin.38 One mU of multimerin was defined as the amount of multimerin antigen in 1 × 106 platelets of pooled platelet lysate.
Secondary antibodies included Texas Red (TR) and fluorescein isothiocyanate (FITC) conjugated goat antimouse IgG, goat antirabbit IgG, and donkey antisheep IgG (Jackson Immuno-Research Laboratories, West Grove, PA; antisera with minimal cross-reactivity against the other species IgG), goat antirabbit IgG coupled to 10 nm and 15 nm colloidal gold, and goat antimouse IgG coupled to 15 nm colloidal gold (Amersham, Les Ullys, France). For some studies, the cell nuclei were counterstained with propidium iodide (2.5 μg/mL; Sigma, St. Louis, MO).
Cell preparation.
Human umbilical vein endothelial cells were isolated from collagenase digested umbilical cord segments and grown in Primaria flasks (VWR Scientific, Toronto, Ontario) containing minimal essential media supplemented with 20% fetal bovine serum (Gibco BRL, Burlington, Ontario), 20 μg/mL endothelial cell growth factor (Boehringer Mannheim, Laval, Quebec, Canada), 12 U/mL porcine heparin, 2 mmol/L L-glutamine, 0.09% sodium bicarbonate, 10 mmol/L HEPES, and 50 μg/mL gentamycin. For cell passage studies, cells from 4 to 6 cords were pooled and passaged every 3 days at 95% to 100% confluence (with trypsin, EDTA for endothelial cells; Sigma) and samples were harvested on day 3 at greater than 80% confluence. For immunoblot, immunoprecipitation, and enzyme-linked immunosorbent assays (ELISA), cells were solubilized in lysing buffer containing 1% Triton X-100 and protease inhibitors (20 mmol/L Tris, 130 mmol/L NaCl, pH 7.4 with 10 mmol/L EDTA, 0.1 μmol/L leupeptin, 0.2 mmol/L phenylmethylsulfonyl fluoride, and 5 mmol/L N-ethyl maleimide; 0.25 mL per T25 flask), scraped from the flask, and Triton-insoluble material was removed by centrifugation (15,000g × 15 min). For some studies, the Triton-insoluble pellet was washed twice in lysing buffer with Triton X-100, then solubilized by boiling in buffer containing 1% sodium dodecyl sulfate (SDS). In some experiments, passage 1 day 2 endothelial cells were cultured in media containing 0 to 12 μg/mL cycloheximide. For investigations of endocytosis, passage 1 day 2 endothelial cells were incubated (30 minutes or 18 hours at 37°C) in complete media with or without 10 μg/mL monoclonal antimultimerin, washed, cultured for a further 0 to 18 hours, fixed, and labeled (permeabilized and nonpermeabilized) with TR-antimouse IgG. Some slides were double-immunolabeled with rabbit antimultimerin.
Metabolic labeling studies (18-hour, 3-day, and pulse-chase studies34) were performed with first passage endothelial cells (T25 flasks, 80% confluent). Cells were incubated for 0.5 hours in methionine-free media and were labeled in methionine-free media (3 mL) containing 0.1 (3-day and 18-hour labeling) or 0.5 (pulse-chase) mCi/mL 35S-methionine, and 2% dialyzed fetal calf serum (18-hour and 3-day studies). For studies of regulated protein secretion, cells were labeled for 3 days in media supplemented with 10%, 20%, or 40% unlabeled methionine, followed by consecutive 18-hour and 3-hour chases in complete medium without label (duplicate T25 flasks for each condition). The labeled cells were washed twice in sterile phosphate-buffered saline, incubated (37°C) in serum-free medium with or without 10 μmol/L ionophore A23187 (Sigma; 0.1% dimethyl sulfoxide final, all flasks) or 1 to 2 U/mL of human thrombin (Enzyme Research Laboratories, South Bend, IN), and the cell lysates and culture media (collected into the same protease inhibitors as the lysates) were harvested at 30 minutes. For other investigations of regulated secretion, the culture media of unlabeled control and secretagogue-treated cells were analyzed by ELISA, immunoprecipitation, and immunoblotting.
Glycoprotein analyses.
The multimerin content of culture media and cell lysates were quantitated (neat and 1/2 dilutions) with an ELISA, and pooled platelet lysate as the standard.51 The multimerin concentrations were expressed as mU/T25 flask. The lower limit of multimerin detection in the ELISA was approximately 5 mU/mL. To compare the multimerin antigen content/mg of cellular protein, the total protein content of platelet and endothelial cell lysates was determined (BioRad DC protein assay reagent, BioRad, Mississauga, Ontario).
Radioimmunoprecipitations (one mL volumes of culture media and cell lysates) were prepared as previously described,34,38 by using JS-1 covalently linked to Sepharose beads (25 μL) or protein A Sepharose (25 μL), preincubated with polyclonal antimultimerin (25 μL) or anti–von Willebrand factor (15 μL). To reduce nonspecific binding, samples were precleared twice with gelatin agarose (30 μL/mL; Sigma), and once by using protein A Sepharose (25 μL/mL); preliminary experiments indicated that this treatment did not remove multimerin. For analyses of cell lysates containining radiolabeled multimerin, samples were immunoprecipitated twice, as described,34 to further reduce nonspecific protein binding. Immunoprecipitates were analyzed with reduced 4% to 8%, or 7% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and nonreduced multimer gels (1.25% agarose/1.5% acrylamide gels).37 For some studies, multimerin was deglycosylated with endoglycosidase H or N-glycosidase F as described,34and analyzed by autoradiography or by immunoblotting. For immunoblot analyses of endothelial cell lysates and culture media, samples were concentrated by immunoprecipitation with 20 μL of JS-1 Sepharose beads, followed by Western blotting with polyclonal antimultimerin and chemiluminescent substrate for protein detection.51
Immunohistochemistry.
Immunoperoxidase histochemistry was performed as described,34 by using monoclonal (JS-1) and polyclonal antimultimerin, frozen sections of normal tissue (tonsil, lung, liver, small bowel, aorta, carotid artery, umbilical cord, placenta, and bone marrow), and cultured endothelial cells. Immunofluorescent labeling experiments were performed by using endothelial cells cultured for 1 to 4 days on gelatin-coated, sterile, glass coverslips. Coverslips were fixed (5 minutes, 3.5% paraformaldehyde), treated with glycine (0.1 mol/L, 5 minutes), and blocked (30 to 60 minutes in phosphate-buffered saline containing 50 μg/mL normal goat IgG and 3% bovine serum albumin) before incubation with antisera in blocking buffer. Nonpermeabilized cells were labeled with primary antibodies at 4°C before fixation. For double immunolabeling experiments, antibody incubations were performed sequentially, by using antimultimerin followed by the relevant fluorescent secondary antibody, before labeling with the second primary antibody. Controls for the primary antisera included no primary antibody, normal mouse IgG, and normal rabbit serum. Controls also included coverslips incubated with a single primary antibody, and both relevant and irrelevant fluorescent secondary antibodies. Double-labeling experiments were performed in parallel with single-labeled coverslips, by using dilutions of primary antisera validated not to give false-positive colocalization by the irrelevant secondary antibody (monoclonal antibodies: 10 μg/mL; anti-CD63: pool of two different antibodies, each at 10 μg/mL; polyclonal antimultimerin: 1/200; monoclonal and polyclonal anti–von Willebrand factor: 1/1000 and 1/5000; 10 μg/mL polyclonal antihuman factor V; polyclonal anti–P-selectin: 1/50; polyclonal antifibronectin: 1/200; fluorescent secondary antibodies: 1/50).
Cells were examined with an Axioplan Universal Microscope (Carl Zeiss), and FITC, TR, and combination filters. Images were acquired with Northern Exposure Image Analysis Software (version 2.90, Empix Imaging Inc, Mississauga, Ontario, Canada), were separated into red and green channel images by using Photoshop 3.0 (Adobe Systems Inc, Mountain View, CA), and imported into Canvas 5 (Deneba Software, Miami, FL). Confocal scanning laser microscopy was performed with a Universal Confocal Laser Scan Research Microscope System (Carl Zeiss), a 100 X objective, and individual excitation lasers and filters for TR and FITC fluorochromes. Single-labeled preparations were used to set the levels for contrast and brightness in the double-labeled preparations, to ensure that there was no crossover of the fluorochrome into the opposite channel.
Electron microscopy.
Endothelial cells (T25 flasks) were fixed in situ with 1% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4; 1 hour, 4°C). The fixed cells were washed three times (0.1 mol/L phosphate buffer), scraped from the flask, and transported (ambient temperature, pressurized aircraft compartment) in 0.1 mol/L phosphate buffer containing 12.3% sucrose. Cells were embedded in glycol methacrylate upon arrival. Thin sections were immunolabeled as previously described17 52 and examined under a Philips CM10 electron microscope. In double-labeled preparations, the sections were labeled with antimultimerin, followed by 10 nm immunogold antirabbit, before labeling the opposite side of the section with antibodies against von Willebrand factor or P-selectin and 15 nm immunogold. Controls included sections labeled with nonimmune antisera, and for the double-labeling studies, replacing the second primary antibody with nonimmune or irrelevant antisera.
Direct-binding studies.
Endothelial cells were grown in 24-well plates for 3 days (first passage, approximately 90% confluent; seeded at 2.5 × 105 cells/well), were treated with 10 μmol/L A23187 or control buffer (0.1% dimethyl sulfoxide final in all samples) in Hank's buffer containing 125I labeled JS-1 (monoclonal antimultimerin; 0.125 to 11 μg/mL) +/- a 100-fold excess of unlabeled JS-1. After a 30-minute incubation, the wells were washed gently four times and the bound counts were solubilized (2% SDS) and counted. Cell pellets prepared from the wash fluids contained less than 1% of the total bound counts and the nonspecific binding of JS-1, at the highest JS-1 concentration, was approximately 16% of the total binding.
RESULTS
Localization of multimerin in cultured endothelial cells and in endothelium in situ.
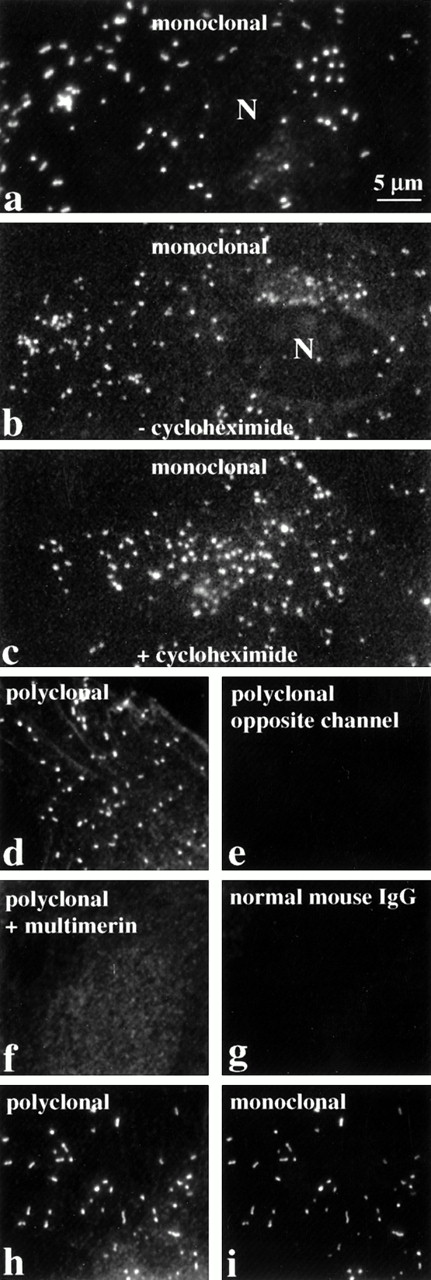
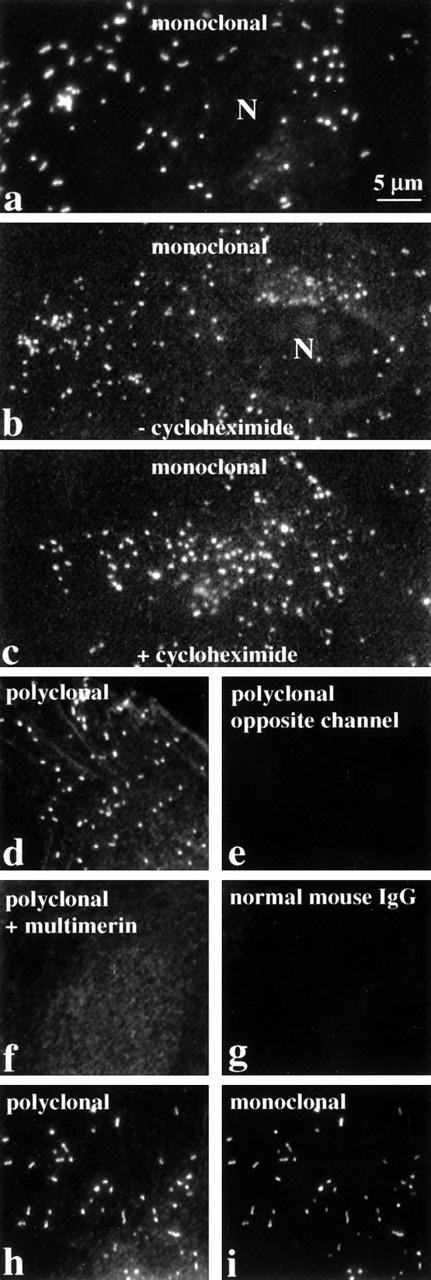
Indirect immunofluorescent labeling studies, with monoclonal and polyclonal antimultimerin, indicated multimerin was contained within round to rod-shaped granules within cultured human umbilical vein endothelial cells, with additional, less intense, perinuclear staining (Figs 1a, b, d, h, and i and 2c). The round-shaped granules were most common (Figs 1b, c, and d and 2c), but more elongated granules were also found in some cells (Fig 1a, h, and i). These structures were not evident when nonpermeabilized endothelial cells were labeled (Fig2a), confirming their intracellular location. No granular labeling was observed with normal mouse IgG (Fig 1g), preimmune rabbit antisera, omission of the primary antibodies, or with polyclonal antimultimerin that was preadsorbed with purified multimerin (Fig 1f).
The intracellular distribution of multimerin in endothelial cells, evaluated by indirect immunolabeling and epifluorescent microscopy. Fixed, permeabilized endothelial cells were labeled with monoclonal and polyclonal antimultimerin, normal mouse IgG, or polyclonal antimultimerin preadsorbed with purified multimerin or with buffer (N indicates cell nuclei). The granular staining was similar in cells cultured for 24 hours with (c) or without (b) the protein synthesis inhibitor cycloheximide. Most of the multimerin-labeled granules were round to slightly elongated (b, c, d), but rod-shaped granules were also seen in some cells (a, h, i). The same cytoplasmic structures in endothelial cells were recognized by monoclonal and polyclonal antimultimerin (h and i show identical fields of a double-labeled cell). No fluorochrome was detected in the opposite channel of single labeled cells (d and e are paired images; same magnification, all panels).
The intracellular distribution of multimerin in endothelial cells, evaluated by indirect immunolabeling and epifluorescent microscopy. Fixed, permeabilized endothelial cells were labeled with monoclonal and polyclonal antimultimerin, normal mouse IgG, or polyclonal antimultimerin preadsorbed with purified multimerin or with buffer (N indicates cell nuclei). The granular staining was similar in cells cultured for 24 hours with (c) or without (b) the protein synthesis inhibitor cycloheximide. Most of the multimerin-labeled granules were round to slightly elongated (b, c, d), but rod-shaped granules were also seen in some cells (a, h, i). The same cytoplasmic structures in endothelial cells were recognized by monoclonal and polyclonal antimultimerin (h and i show identical fields of a double-labeled cell). No fluorochrome was detected in the opposite channel of single labeled cells (d and e are paired images; same magnification, all panels).
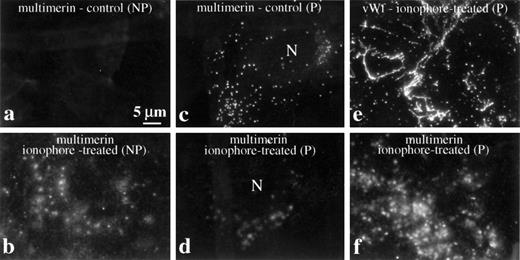
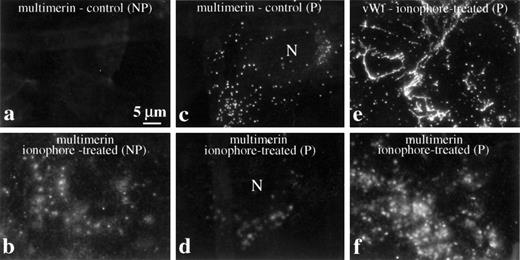
Redistribution of multimerin after treatment of endothelial cells with secretagogues. Cells were washed and resuspended in fresh, serum-free culture media before treatment with and without ionophore A23187. Nonpermeabilized (NP) and permeabilized (P) cells were labeled with antimultimerin (a and b), antimultimerin and anti–von Willebrand factor (e and f), or with antimultimerin and propidium iodide to visualize cell nuclei (c and d show the multimerin-labeled structures associated with cells that contained labeled nuclei). Standard immunofluorescent microscopy images of endothelial cells processed 30 (a-d) and 60 minutes (e and f) after treatment with buffer or ionophore illustrate the redistribution of multimerin in response to secretagogues (N indicates cell nuclei) and the different distributions of multimerin and von Willebrand factor associated with secretagogue-treated endothelial cells (e and f).
Redistribution of multimerin after treatment of endothelial cells with secretagogues. Cells were washed and resuspended in fresh, serum-free culture media before treatment with and without ionophore A23187. Nonpermeabilized (NP) and permeabilized (P) cells were labeled with antimultimerin (a and b), antimultimerin and anti–von Willebrand factor (e and f), or with antimultimerin and propidium iodide to visualize cell nuclei (c and d show the multimerin-labeled structures associated with cells that contained labeled nuclei). Standard immunofluorescent microscopy images of endothelial cells processed 30 (a-d) and 60 minutes (e and f) after treatment with buffer or ionophore illustrate the redistribution of multimerin in response to secretagogues (N indicates cell nuclei) and the different distributions of multimerin and von Willebrand factor associated with secretagogue-treated endothelial cells (e and f).
In quiescent cultures, very little multimerin was detected on the external membrane of nonpermeabilized endothelial cells (Fig 2a). However, after treatment with secretagogues (ionophore A23187 or thrombin) in fresh, serum-free culture media, there was a significant increase in the multimerin immunolabeling of the external cell membrane (Fig 2b), and this coincided with a more diffuse staining pattern of cell-associated multimerin (Fig 2b, d, and f) and the loss of the discrete intracellular, granule staining for multimerin (Fig 2c and d). In parallel with the secretion of von Willebrand factor, multimerin appeared on the external cell membrane 2 to 5 minutes after treatment with secretagogues (not shown). Multimerin remained in diffuse patches on the external cell membrane 30 (Fig 2b and d) to 60 (Fig 2f) minutes after secretion. In preparations counterstained to visualize cell nuclei, “footprints” resembling the distribution of multimerin on the membrane of secretagogue-treated endothelial cells were seen in regions where the endothelial cells had lifted off the coverslip during processing, suggesting at least part of the multimerin secretion was at the basal cell surface. Attempts to determine if multimerin was secreted onto apical and/or basal cell surfaces by using confocal microscopy were unsuccessful, due to bleaching of the diffuse multimerin immunolabel during serial optical sectioning. Striking differences were seen in the distributions of multimerin (Fig 2f) and von Willebrand factor (Fig 2e) in preparations of secretagogue-treated endothelial cells (Fig 2e and f compare their distributions 60 minutes after treatment with secretagogues).
To investigate if the structures containing multimerin could be constitutive secretory vesicles, endothelial cells were cultured for 24 hours with or without high concentrations (12 μg/mL) of the protein synthesis inhibitor cycloheximide. Inhibition of protein synthesis resulted in a loss of perinuclear staining for multimerin (Fig 1c), von Willebrand factor, and a marked reduction in perinuclear staining for the constitutively secreted protein, fibronectin (not shown). However, the granular staining for multimerin (Fig 1c) and von Willebrand factor, and the secretagogue-induced redistribution of these proteins, were similar in control and cycloheximide-treated cells.
To determine if the granules containing multimerin might represent structures containing internalized protein, endothelial cells were cultured (30 minutes or 18 hours) in media containing antimultimerin and observed for 0 to 18 hours for evidence of antibody internalization. Intense labeling of fibrillary structures in the extracellular matrix of cultured endothelium was observed in these studies. The distribution of antimultimerin in permeabilized and nonpermeabilized cells was identical and no antimultimerin was detected in intracellular granules (not shown).
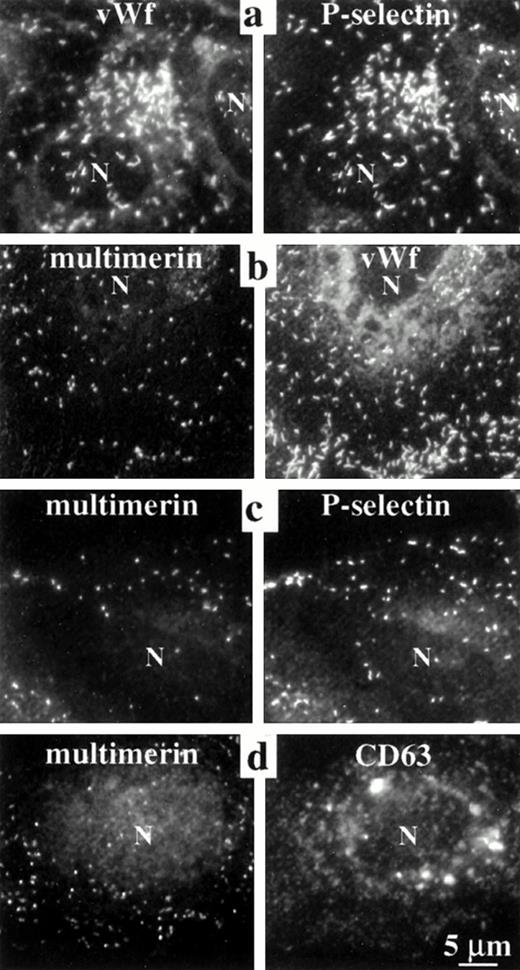
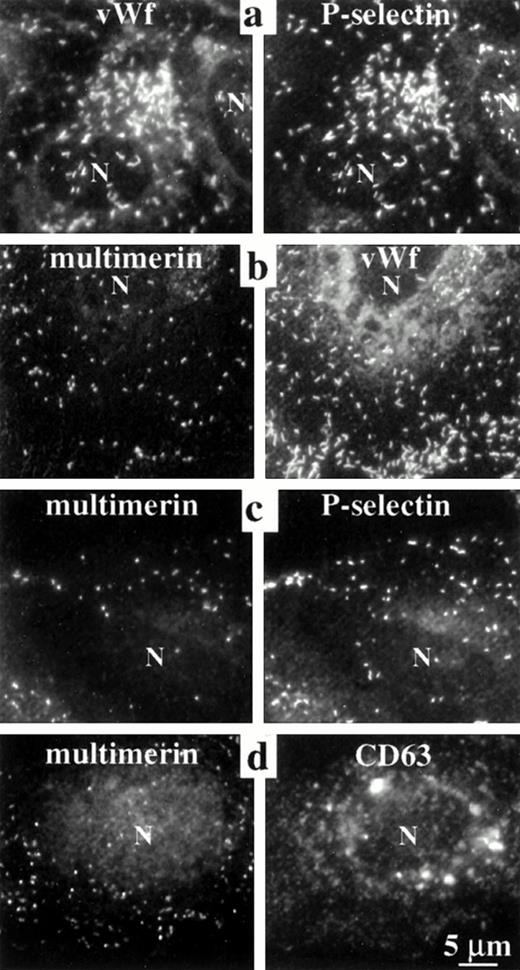
The elongated shape of some multimerin granules in endothelial cells suggested multimerin might be stored in Weibel-Palade bodies16 and perhaps in the same location as von Willebrand factor and P-selectin, two proteins known to be stored within these organelles.9,18,22-24 However, comparison studies indicated that there was more heterogeneity in the number of multimerin granules/cell (0 to >200) and most multimerin granules were not as elongated as the structures containing von Willebrand factor and P-selectin (Figs 1, 2, 3,4, and 8b). To further compare the distributions of multimerin, von Willebrand factor, and P-selectin, double-labeling experiments were performed, with stringent conditions to avoid false-positive colocalization. No fluorescence was detected in the opposite channel of single primary antibody labeled cells, labeled with both relevant and irrelevant secondary antibodies (Fig 1d). When double-labeling was performed with monoclonal and polyclonal antimultimerin, the same intracellular granules were labeled (Fig 1h and i). As anticipated, an identical population of intracellular granules were labeled by antibodies to von Willebrand factor and P-selectin (Fig 3a). However, the distribution of multimerin in endothelial cells was different from von Willebrand factor and P-selectin (Fig 3b and c). To determine if multimerin was stored in lysosomes, the distribution of multimerin was compared to CD63, a protein found in lysosomes and in Weibel-Palade bodies.33CD63 was identified in larger perinuclear structures and in peripheral cytoplasmic structures, but these structures did not contain detectable multimerin (Fig 3d). Although our previous studies indicated multimerin was stored complexed with coagulation factor V in platelets,35 we were unable to detect factor V within cultured human umbilical vein endothelial cells.
Comparisons of the intracellular distribution of multimerin, von Willebrand factor, P-selectin, and CD63. Paired, epifluorescent microscopic images of structures in the cytoplasm of double-immunolabeled, quiescent, permeabilized endothelial cells are shown. Primary antibodies included monoclonal (a, vWf; b and c, multimerin; d, CD63) and polyclonal antisera (a and c, P-selectin; b, vWf; d, multimerin). Single-labeled coverslips, processed in parallel, showed the same pattern of labeling. Identical granules were labeled by antibodies to P-selectin and von Willebrand factor, but multimerin was found in a different distribution than von Willebrand factor, P-selectin, and CD63 (N indicates cell nuclei). Cells labeled with polyclonal antimultimerin (d) showed more background labeling of nongranular structures than the cells labeled with monoclonal antimultimerin (b and c).
Comparisons of the intracellular distribution of multimerin, von Willebrand factor, P-selectin, and CD63. Paired, epifluorescent microscopic images of structures in the cytoplasm of double-immunolabeled, quiescent, permeabilized endothelial cells are shown. Primary antibodies included monoclonal (a, vWf; b and c, multimerin; d, CD63) and polyclonal antisera (a and c, P-selectin; b, vWf; d, multimerin). Single-labeled coverslips, processed in parallel, showed the same pattern of labeling. Identical granules were labeled by antibodies to P-selectin and von Willebrand factor, but multimerin was found in a different distribution than von Willebrand factor, P-selectin, and CD63 (N indicates cell nuclei). Cells labeled with polyclonal antimultimerin (d) showed more background labeling of nongranular structures than the cells labeled with monoclonal antimultimerin (b and c).
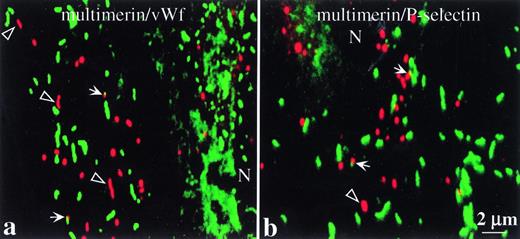
Confocal scanning laser microscopy images comparing the intracellular distributions of multimerin with von Willebrand factor and P-selectin. Cells were prepared as in Fig 3. The images are overlays of matching 250 nm optical sections (taken through the cellular plane showing the maximal intensity granular labeling for both proteins) and show labeled structures in the cytoplasm of endothelial cells. Multimerin is shown in red, and P-selectin and von Willebrand factor are shown in green (N indicates the cell nuclei). Differences are seen in the distributions of multimerin, compared to von Willebrand factor and P-selectin. Some of the elongated multimerin-labeled granules (open arrowheads) did not contain detectable von Willebrand factor or P-selectin immunolabel. Regions of possible overlap in the distributions of multimerin and von Willebrand factor or P-selectin are indicated (solid arrows).
Confocal scanning laser microscopy images comparing the intracellular distributions of multimerin with von Willebrand factor and P-selectin. Cells were prepared as in Fig 3. The images are overlays of matching 250 nm optical sections (taken through the cellular plane showing the maximal intensity granular labeling for both proteins) and show labeled structures in the cytoplasm of endothelial cells. Multimerin is shown in red, and P-selectin and von Willebrand factor are shown in green (N indicates the cell nuclei). Differences are seen in the distributions of multimerin, compared to von Willebrand factor and P-selectin. Some of the elongated multimerin-labeled granules (open arrowheads) did not contain detectable von Willebrand factor or P-selectin immunolabel. Regions of possible overlap in the distributions of multimerin and von Willebrand factor or P-selectin are indicated (solid arrows).
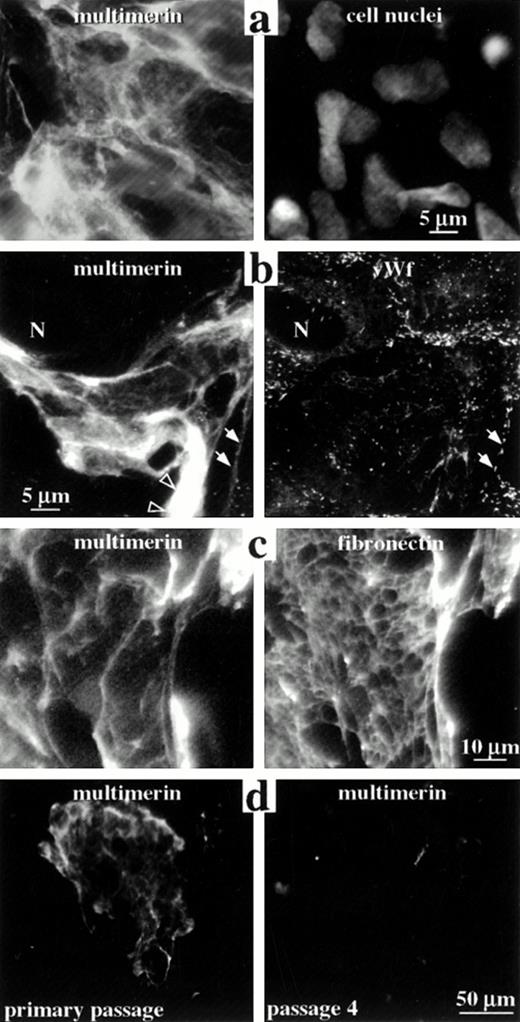
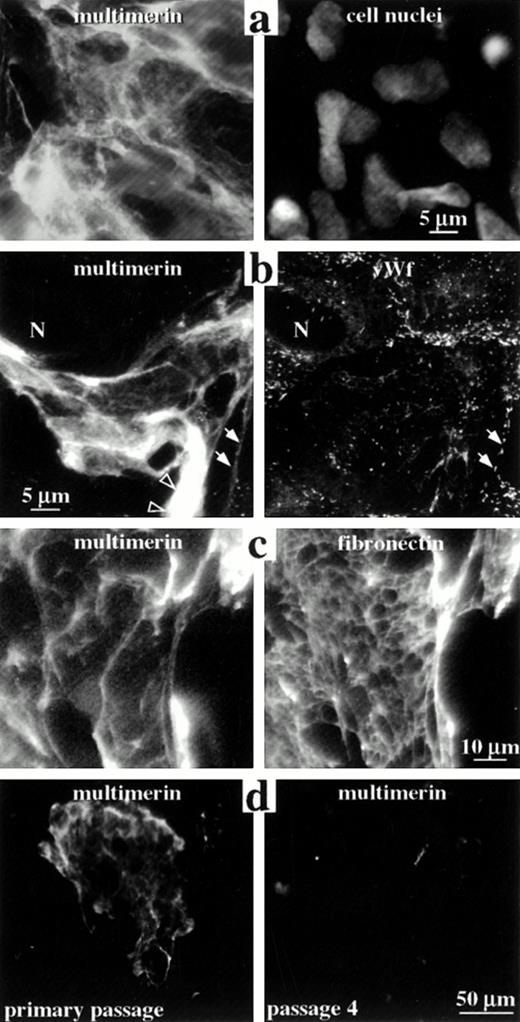
The distribution of multimerin in the extracellular matrix of endothelial cells. Nonpermeabilized (a and c) and permeabilized (b and d) endothelial cells were labeled with monoclonal antibodies to multimerin (a-d), polyclonal antibodies to von Willebrand factor (b), polyclonal antibodies to fibronectin (c), and propidium iodide (to visualize cell nuclei; a), and were examined by epifluorescent microscopy. Panels a to c show paired images of the same field of primary passage endothelial cells. The cells shown in panel b contained few multimerin granules but abundant von Willebrand factor granules (N indicates a cell nucleus). Solid arrows indicate regions where the von Willebrand factor-labeled Weibel-Palade bodies in the cell periphery appeared to follow the margins of the multimerin-containing fibrils. Open arrowheads indicate multimerin in thicker, intensely labeled structures. Multimerin and fibronectin were associated with large fibrillary structures in the extracellular matrix (c), with differences in their distributions. Cell passage in vitro was associated with reduced extracellular matrix staining for multimerin (d).
The distribution of multimerin in the extracellular matrix of endothelial cells. Nonpermeabilized (a and c) and permeabilized (b and d) endothelial cells were labeled with monoclonal antibodies to multimerin (a-d), polyclonal antibodies to von Willebrand factor (b), polyclonal antibodies to fibronectin (c), and propidium iodide (to visualize cell nuclei; a), and were examined by epifluorescent microscopy. Panels a to c show paired images of the same field of primary passage endothelial cells. The cells shown in panel b contained few multimerin granules but abundant von Willebrand factor granules (N indicates a cell nucleus). Solid arrows indicate regions where the von Willebrand factor-labeled Weibel-Palade bodies in the cell periphery appeared to follow the margins of the multimerin-containing fibrils. Open arrowheads indicate multimerin in thicker, intensely labeled structures. Multimerin and fibronectin were associated with large fibrillary structures in the extracellular matrix (c), with differences in their distributions. Cell passage in vitro was associated with reduced extracellular matrix staining for multimerin (d).
Confocal microscopy analyses (250 nm optical sections [Fig 4a and b] and overlays of serial optical sections through the entire cell [not shown]) confirmed multimerin was contained within discrete, round to rod-shaped structures. The serial optical sections indicated that most of the multimerin, von Willebrand factor– and P-selectin–containing granules were at the same cell depth, consistent with the thinness of the peripheral, endothelial cell cytoplasm. The majority of the multimerin granules did not contain detectable von Willebrand factor (Fig 4a) or P-selectin immunolabel (Fig 4b). In cells containing multimerin granules, approximately 1 in 300 of the von Willebrand factor–labeled structures appeared to overlap multimerin-labeled structures (Fig 4a, solid arrows) and similar findings were seen in cells labeled with antibodies to multimerin and P-selectin (Fig 4b, solid arrows). Elongated granules containing multimerin but not von Willebrand factor or P-selectin were identified (Fig 4a and b, open arrowheads).
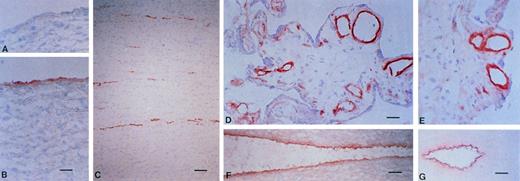
In frozen sections of human tissue, multimerin was identified within the endothelium of different-sized venous and arterial blood vessels, including capillaries, venules, veins, arterioles, arteries, and vaso vasorum (Fig 5). The most intense multimerin immunolabeling was observed within the cell layer adjacent to the vessel lumen, with less intense staining of the subendothelium (Fig 5). Most vessels examined showed staining for multimerin, but occasional vessels without multimerin labeling were observed. No staining of endothelium was observed with the negative controls, processed without the monoclonal antibody or with preimmune rabbit IgG (Fig 5A). Apart from platelets, megakaryocytes, endothelium, and adjacent structures, no other cells or structures exhibited staining for multimerin.
The distribution of multimerin in normal blood vessels. Frozen sections of normal tissue, including aorta (A and B), small bowel (C), placenta (D and E), carotid artery (F), and umbilical cord (G; vein is shown) were immunolabeled with monoclonal antimultimerin (B, C, D, F, and G), polyclonal anti-multimerin (E), or the preimmune polyclonal antiserum (A). Bars indicate 30 μm (C and G) and 10 μm (A, B, D, E, and F).
The distribution of multimerin in normal blood vessels. Frozen sections of normal tissue, including aorta (A and B), small bowel (C), placenta (D and E), carotid artery (F), and umbilical cord (G; vein is shown) were immunolabeled with monoclonal antimultimerin (B, C, D, F, and G), polyclonal anti-multimerin (E), or the preimmune polyclonal antiserum (A). Bars indicate 30 μm (C and G) and 10 μm (A, B, D, E, and F).
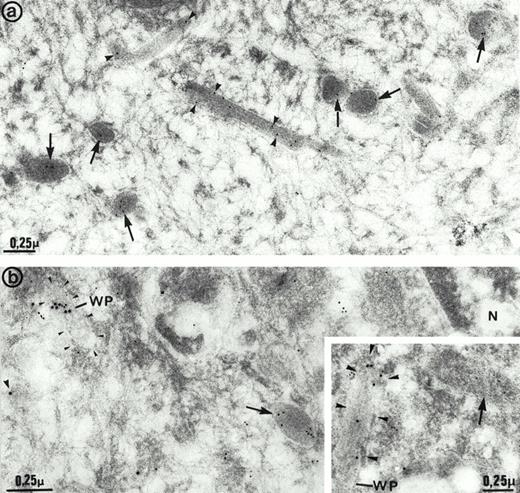
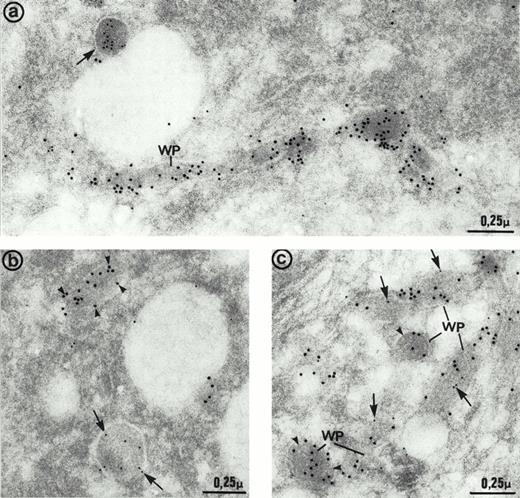
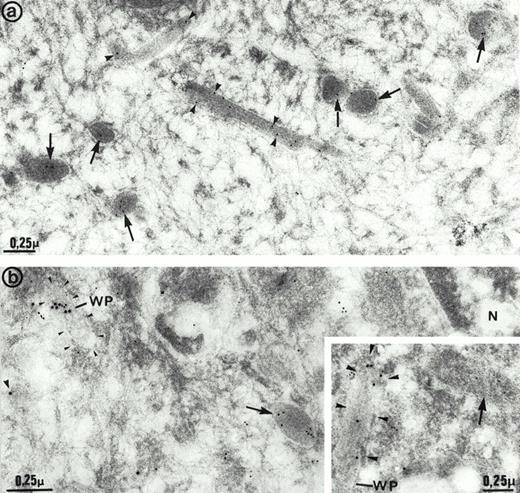
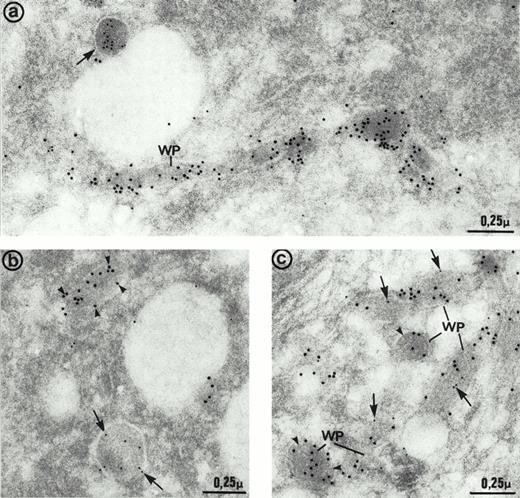
The ultrastructure of the multimerin granules in endothelial cells was investigated by using immunoelectron microscopy and polyclonal antimultimerin. No gold particles were present in the control sections labeled with nonimmune antisera. In sections labeled with antimultimerin, gold particles were identified within small vesicles and within round, dense-core granules (Fig6a) that had the appearance of regulated secretory granules. Within the dense-core granules, multimerin was often in an eccentric position, resembling its distribution within platelet α-granules.34,35,53 Multimerin was also identified within elongated structures, limited by a unit membrane and containing longitudinally aligned tubules similar to Weibel-Palade bodies16 (Fig 6a and b). Some, but not all, of the elongated granules within endothelial cells were labeled by the multimerin antibodies. The round granules containing multimerin were negative for P-selectin (Fig 6b). However, the P-selectin positive Weibel-Palade bodies contained either no detectable multimerin (Fig 6b, inset) or low levels (Fig 6b) of multimerin. In most sections examined by double immunoelectron microscopy, multimerin and von Willebrand factor were located within different membrane-bound structures (Fig 7a and b). Occasionally, multimerin and von Willebrand factor were observed within the same granule, but within these organelles the two proteins were often located in different regions, suggesting compartmentalization of proteins within these structures (Fig 7c). von Willebrand factor was the predominant labeled protein in the granules that contained multimerin and von Willebrand factor. In preparations double-labeled with antimultimerin and 10 nm gold, followed by nonimmune or irrelevant antisera and 15 nm gold, there was no labeling of granules by the 15 nm gold and only occasional background labeling that was not associated with specific structures.
Immunoelectron microscopy studies of human umbilical vein endothelial cells. (a) Cells labeled with multimerin antibodies. Gold particles were located in round dense-core granules (arrows) and less often in elongated structures (arrowheads) that closely resembled Weibel-Palade bodies. (b and inset) Cells double immunolabeled for multimerin (10 nm gold) and P-selectin (15 nm gold). Multimerin immunolabel was mainly observed in dense-core granules (arrows) that were negative for P-selectin. P-selectin– positive Weibel-Palade bodies (WP; arrowheads) displayed either low levels (b) or no detectable (inset) multimerin immunolabel. N indicates the cell nucleus.
Immunoelectron microscopy studies of human umbilical vein endothelial cells. (a) Cells labeled with multimerin antibodies. Gold particles were located in round dense-core granules (arrows) and less often in elongated structures (arrowheads) that closely resembled Weibel-Palade bodies. (b and inset) Cells double immunolabeled for multimerin (10 nm gold) and P-selectin (15 nm gold). Multimerin immunolabel was mainly observed in dense-core granules (arrows) that were negative for P-selectin. P-selectin– positive Weibel-Palade bodies (WP; arrowheads) displayed either low levels (b) or no detectable (inset) multimerin immunolabel. N indicates the cell nucleus.
Immunoelectron micrographs of human umbilical vein endothelial cells double-immunolabeled with antibodies to multimerin (10 nm gold) and von Willebrand factor (15 nm gold). (a) Most of the multimerin-positive granules were round in shape (arrows) and distinct from the von Willebrand factor-containing elongated Weibel-Palade bodies (WP). (b) Two dense-core granules of similar size with distinct appearances and contents. One is elongated and contains von Willebrand factor (arrowheads) whereas the other one is more spherical and contains multimerin (arrows). (c) Weibel-Palade (WP) bodies containing von Willebrand factor were generally devoid of multimerin (arrowheads). Occasionally, both proteins were found in their matrix, but the labeling for multimerin (arrows) was minimal compared to von Willebrand factor.
Immunoelectron micrographs of human umbilical vein endothelial cells double-immunolabeled with antibodies to multimerin (10 nm gold) and von Willebrand factor (15 nm gold). (a) Most of the multimerin-positive granules were round in shape (arrows) and distinct from the von Willebrand factor-containing elongated Weibel-Palade bodies (WP). (b) Two dense-core granules of similar size with distinct appearances and contents. One is elongated and contains von Willebrand factor (arrowheads) whereas the other one is more spherical and contains multimerin (arrows). (c) Weibel-Palade (WP) bodies containing von Willebrand factor were generally devoid of multimerin (arrowheads). Occasionally, both proteins were found in their matrix, but the labeling for multimerin (arrows) was minimal compared to von Willebrand factor.
Studies of multimerin in the extracellular matrix.
In nonpermeabilized and permeabilized preparations of primary and first-passage cultures there was intense, patchy, fibrillary extracellular matrix staining with both monoclonal (Fig 8a-d) and polyclonal antimultimerin, and double-labeling experiments indicated the same extracellular matrix structures were labeled by these antibodies (not shown). The extracellular matrix was not labeled by normal rabbit or mouse IgG, or when the polyclonal antimultimerin was preadsorbed with purified multimerin (not shown). Extensive, multimerin-labeled fibrillary structures were seen in some (Fig 8a-d) but not all regions (images in Figs 1-4) of the culture monolayers, and many contained endothelial cells enmeshed within their fibrils (Fig 8a). The number, size, and appearance of the multimerin-containing, fibrillary extracellular matrix structures were similar in quiescent and secretagogue-treated monolayers, and they were the most intensely labeled structures in nonpermeabilized (Fig 8a and c) and permeabilized (Fig 8b and d) quiescent and secretagogue-treated (not shown) endothelial cell cultures. In addition to fine fibrillary structures (Fig 8b, multimerin panel, solid arrows), intense labeling of thicker fibrils (Fig 8b, multimerin panel, open arrowheads) was observed with antimultimerin. Triton X-100 extraction of the cell monolayers resulted in loss of the intracellular, multimerin granular staining but it did not alter the appearance of the multimerin-containing extracellular matrix structures (not shown), indicating that multimerin was associated with the Triton-insoluble, extracellular matrix.
In early passage endothelial cell cultures, differences were observed in the extracellular matrix distributions of multimerin and fibronectin (Fig 8c), and the multimerin-labeled fibrillary structures contained weak to no labeling for extracellular von Willebrand factor (Fig 8b). In many regions, the von Willebrand factor–labeled Weibel-Palade bodies in the peripheral cytoplasm of endothelial cells appeared to follow the margins of the multimerin-containing fibrillary structures (Fig 8b, solid arrows), suggesting a close association between endothelial cells and the multimerin-containing fibrillary matrix.
Effect of cell passage on multimerin expression.
Variability in the multimerin staining pattern of different endothelial cell preparations led us to investigate the effect of cell passage on multimerin. Greater than 99% of the cells in primary to passage 4 cultures contained abundant von Willebrand factor storage granules. In early passage cultures (primary to passage 2), many (30% to 55%) cells contained greater than 100 multimerin granules, and most (77% to 87%) contained greater than 20 granules. However, by passage 3 and 4, most (62% to 73%) endothelial cells contained only 0 to 20 multimerin granules.
Cell passage in vitro was also associated with marked changes in the extracellular matrix multimerin. The multimerin-labeled extracellular matrix structures were larger and more numerous in primary cultures (structures/10 mm2 in primary, passage 1 and passage 4 cultures: 150, 52, and 14; Fig 8d compares typical low power fields of primary and passage 4 cultures). Although the extracellular matrix multimerin was most evident in primary cultures, the multimerin antigen levels in the culture media of primary passage cells were consistently lower than passage 1 and later cultures (multimerin antigen levels in primary and passage 1 to 4 culture media were 32, 80, 250, 330, and 270 mU/T25 flask; results of a representative experiment with different passages from the same cell harvest for all analyses). The corresponding primary, passage 1 and 4 endothelial cell lysates contained only 4.6, 4.6, and 1.5 mU of multimerin per T25 flask (equivalent to 1.5%, 1.5%, and 0.6% of the platelet multimerin content/mg of cell lysate protein), indicating that most of the synthesized multimerin was constitutively secreted.
Investigations of multimerin biosynthesis and secretion by endothelial cells.
Direct-binding experiments with radiolabeled monoclonal antimultimerin were used to study the effect of granule secretion on the amount of extracellular matrix-associated multimerin. Although secretagogue treatment increased the multimerin immunolabeling of the endothelial cell external membrane (Fig 2), Scatchard analyses indicated there were 1.6 to 1.8 × 1010 specific-binding sites for monoclonal antimultimerin per well in both control and ionophore-treated monolayers (data from two separate experiments; area/well: 1.9 cm2 containing approximately 2.5 × 105 endothelial cells). Comparisons of untreated and Triton X-100 or SDS-extracted monolayers indicated greater than 98% of the extracellular multimerin was associated with the Triton-insoluble matrix. These data, together with the immunostaining findings, suggested most of the extracellular multimerin was associated with fibrillary matrix structures that were not appreciably altered by treatment with secretagogues. Previous studies of secretagogue-treated platelets indicated that most of the released multimerin remained platelet-associated and only small multimers were found in platelet releasate.37 When washed endothelial cells were treated with secretagogues in fresh media, there was no detectable increase in the amount of multimerin in the culture media compared with control flasks, treated without secretagogues (data from 14 separate experiments, evaluated with ELISA, immunoprecipitation/immunoblot and radioimmunoprecipitation assays). However, the amount of multimerin contained within the untreated endothelial cell lysates (<5 mU/T25 flask) was close to the limit of detection of the ELISA assay.
Immunoblot and metabolic-labeling experiments were used to evaluate the biosynthesis and processing of multimerin by endothelial cells. Previous studies using Dami cells (a megakaryocytic cell line) indicated multimerin is first synthesized as a promultimerin (proM) subunit (170 kD), which undergoes extensive glycosylation, subunit proteolytic processing, and multimerization during biosynthesis to form the mature, disulfide-linked homomultimeric protein.34Pulse-chase experiments indicated a similar pattern of multimerin biosynthesis by cultured endothelial cells, and the secretion of labeled multimerin into the culture media was complete between 3 to 6 hours after synthesis (not shown). Immunoblot analyses (Fig 9A) and 18-hour metabolic labeling studies (Fig 9A, radioimmunoprecipitation panel) indicated that most of the multimerin synthesized by endothelial cells in culture was constitutively secreted into the culture media. Large amounts of multimerin were also detected in the Triton-insoluble matrix (Fig 9A).
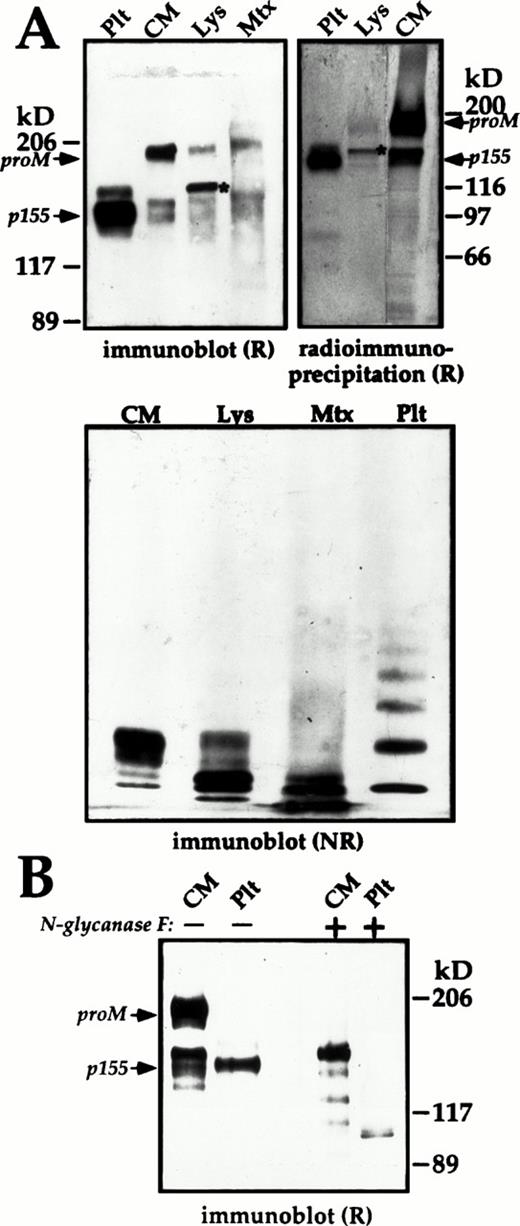
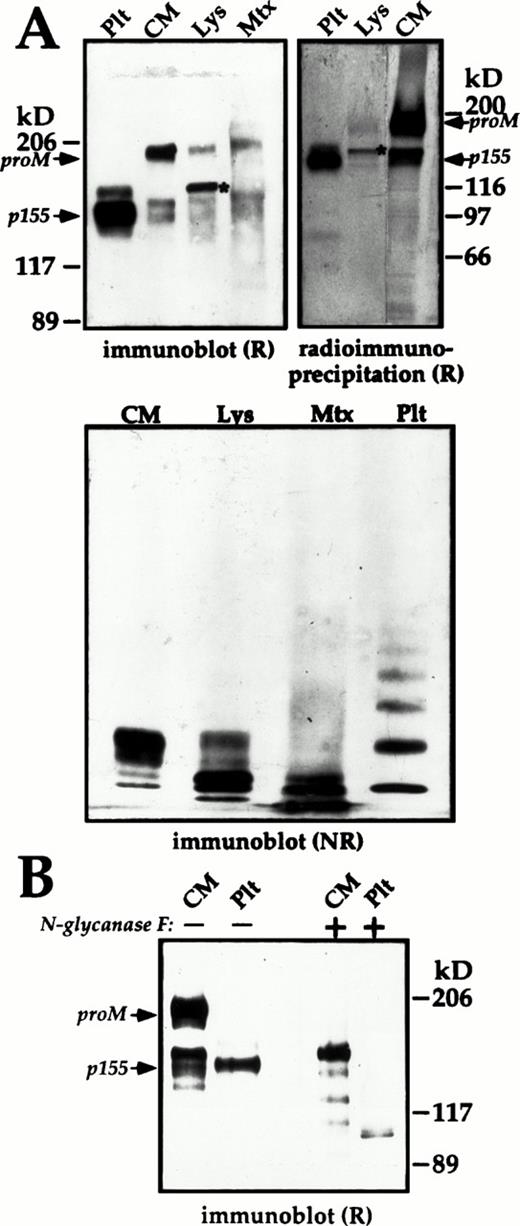
Subunit and multimer composition of endothelial cell and platelet (Plt) multimerin. The multimerin in the culture media (CM), and cell lysates (Lys) of passage-1 endothelial cells was concentrated by immunoprecipitation and analyzed by immunoblotting with polyclonal antimultimerin or by radioimmunoprecipitation. (A) Reduced (R) 4% to 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (immunoblot) and nonreduced (NR) multimer gels (origin at top) comparing the multimerin in 12%, 120%, and 24% of the culture media, cell lysate, and Triton-insoluble pellet (Mtx) from a T25 flask (material pooled from 4 flasks, harvested on day 3 at 75% confluence) with platelet multimerin. The radioimmunoprecipitation panel (18-hour labeling; 7% SDS-PAGE) compares equivalent volumes of endothelial cell culture media and cell lysates with 125I-labeled multimerin purified from platelets. (B) The mobility of reduced multimerin subunits from platelets and endothelial cell culture media, before (−) and after (+) deglycosylation with N-glycosidase F. Fully glycosylated proM, the predominant 155 kD (p155) subunit of mature platelet multimerin, and cell lysate proM containing endoglycosidase-H–sensitive forms of N-linked carbohydrate (*, panel A), are indicated in the panels showing reduced multimerin.
Subunit and multimer composition of endothelial cell and platelet (Plt) multimerin. The multimerin in the culture media (CM), and cell lysates (Lys) of passage-1 endothelial cells was concentrated by immunoprecipitation and analyzed by immunoblotting with polyclonal antimultimerin or by radioimmunoprecipitation. (A) Reduced (R) 4% to 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (immunoblot) and nonreduced (NR) multimer gels (origin at top) comparing the multimerin in 12%, 120%, and 24% of the culture media, cell lysate, and Triton-insoluble pellet (Mtx) from a T25 flask (material pooled from 4 flasks, harvested on day 3 at 75% confluence) with platelet multimerin. The radioimmunoprecipitation panel (18-hour labeling; 7% SDS-PAGE) compares equivalent volumes of endothelial cell culture media and cell lysates with 125I-labeled multimerin purified from platelets. (B) The mobility of reduced multimerin subunits from platelets and endothelial cell culture media, before (−) and after (+) deglycosylation with N-glycosidase F. Fully glycosylated proM, the predominant 155 kD (p155) subunit of mature platelet multimerin, and cell lysate proM containing endoglycosidase-H–sensitive forms of N-linked carbohydrate (*, panel A), are indicated in the panels showing reduced multimerin.
Similar to Dami cells, 34 the predominant multimerin subunit in reduced endothelial cell lysates was recently synthesized proM containing endoglycosidase H sensitive forms of N-linked carbohydrate (Fig 9A, upper panels; the bands in lanes Lys indicated by an *). Fully glycosylated forms of proM (Mr 186 kD, reduced, band indicated as proM in Fig 9) and smaller multimerin subunits were detected in the culture media (Figs 9A and B and10A), and in lesser amounts in the cell lysates (Figs 9A and 10A).
Long-term metabolic labeling studies of endothelial cell multimerin. Multimerin inmmunoprecipitates of equivalent volumes of culture media (CM; from 18 hours [18h] and 3 days [3d] of labeling in media supplemented with 40% unlabeled media; 18-hour [C1] and 3-hour [C2] chases in unlabeled media; and ionophore A23187 [+] or buffer [−] treated cells) and endothelial cell lysates (Lys; ionophore A23187 and buffer-treated cells) were compared with125 I labeled platelet multimerin (Plt), and immunoprecipitates prepared by using normal mouse IgG (mIgG). Reduced SDS-PAGE (A) and nonreduced multimer gels (B) analyses of single or double-immunoprecipitates (*) from two experiments are shown. The images represent 3-day (A, lanes 1-3), 20-day (A, lanes 4-10), and 32-day (A, lanes 11-16; B) exposures. The arrows indicate the location of the p155 and proM subunits. Small quantities of more fully proteolyzed multimerin were detected in the cell lysates after constitutive multimerin secretion was complete. Secretagogue stimulation did not release detectable amounts of multimerin into the culture media.
Long-term metabolic labeling studies of endothelial cell multimerin. Multimerin inmmunoprecipitates of equivalent volumes of culture media (CM; from 18 hours [18h] and 3 days [3d] of labeling in media supplemented with 40% unlabeled media; 18-hour [C1] and 3-hour [C2] chases in unlabeled media; and ionophore A23187 [+] or buffer [−] treated cells) and endothelial cell lysates (Lys; ionophore A23187 and buffer-treated cells) were compared with125 I labeled platelet multimerin (Plt), and immunoprecipitates prepared by using normal mouse IgG (mIgG). Reduced SDS-PAGE (A) and nonreduced multimer gels (B) analyses of single or double-immunoprecipitates (*) from two experiments are shown. The images represent 3-day (A, lanes 1-3), 20-day (A, lanes 4-10), and 32-day (A, lanes 11-16; B) exposures. The arrows indicate the location of the p155 and proM subunits. Small quantities of more fully proteolyzed multimerin were detected in the cell lysates after constitutive multimerin secretion was complete. Secretagogue stimulation did not release detectable amounts of multimerin into the culture media.
Consistently, the multimerin subunits from endothelial cells migrated with an apparent molecular mass that was 8 to 10 kD smaller than the multimerin subunits from Dami cells. Deglycosylation studies (with N-glycosidase F) indicated these differences were due to the quantity of N-linked carbohydrate (not shown). The smaller multimerin subunits in the culture media of endothelial cells did not comigrate with the platelet multimerin subunits after removal of N-linked carbohydrate with N-glycosidase F (Fig 9B), suggesting that there are additional differences in the post-translational processing of multimerin by these cells.
Nonreduced multimer gel analyses indicated that the multimerin in the endothelial cell fractions had a different mobility compared to platelet multimerin (Figs 9A and 10B), possibily due to differences in their subunit sizes. The endothelial cell lysates and culture media (Figs 9A and 10B) contained mainly small multimerin multimers. In immunoblots of the Triton-insoluble fractions of cultured endothelial cells, the multimerin in the high molecular weight portion of the gel migrated as a diffuse band rather than discrete multimers. However, nonreduced/reduced analyses confirmed the high molecular weight material in the Triton-insoluble fraction contained disulfide-linked multimerin subunits (not shown). Immunostaining experiments indicated that the multimerin within endothelial cell granules was extracted by Triton-X-100, suggesting that the high molecular weight multimerin in the Triton-insoluble fraction was associated with the extracellular matrix.
The storage and regulated secretion of multimerin was investigated with metabolic labeling (3-day labeling, consecutive 18- and 3-hour chases in cold media, followed by treatment with or without secretagogues). Because immunostaining experiments indicated that there was an almost complete loss of multimerin granules when the cells were grown for 3 days in medium containing less than 20% methionine, some studies were performed by using labeling media supplemented with 20% to 40% unlabeled methionine. In all studies, much larger quantities of radiolabeled von Willebrand factor were detected in the culture media and cell lysates compared with multimerin. Regulated secretion of labeled von Willebrand factor into the culture media was readily evident (2- to 5-day exposures), as previously reported.25Consistently, radiolabeled multimerin was found in the control cell lysates long after constitutive multimerin secretion was complete, and the stored multimerin exhibited more complete proteolytic processing than constitutively secreted multimerin (Fig 10A). However, regulated secretion of multimerin from endothelial cells into the culture media could not be confirmed (Fig 10A), possibly due to the very low quantities of labeled multimerin contained within the cells and the association of released multimerin with the endothelial cells and their matrix.
DISCUSSION
A number of parallels exist between multimerin and von Willebrand factor, including their similar massive multimeric structures, expression by megakaryocytes and endothelial cells, and storage in the Weibel-Palade body-like region of platelet α-granules.9,17,34-37,41-43 The focus of our current study was to investigate multimerin in human endothelium. We observed specific multimerin immunolabeling of the endothelium in large and small arterial and venous blood vessels in situ, confirming its distribution in endothelium in vivo. Within cultured endothelial cells, we identified multimerin in round to rod-shaped, dense core granules. However, by using light and electron microscopy, we found multimerin primarily within different structures than the Weibel-Palade body proteins von Willebrand factor and P-selectin, although the immunoelectron microscopy studies indicated some Weibel-Palade bodies contained small amounts of multimerin. These studies provide evidence for differences in the way multimerin and von Willebrand factor are compartmentalized within endothelial cells and megakaryocytes. Although multimerin is stored complexed with coagulation factor V in platelets,35 we were unable to detect factor V within cultured human umbilical vein endothelial cells, suggesting that multimerin has functions in endothelium that are independent of its putative function as an intragranular, factor V carrier protein.
Regulated secretory granules can be defined by three characteristics: (1) secretion that is dependent on external stimuli, (2) the formation of specialized, dense-core, membrane-bound structures, and (3) prolonged intracellular storage of their content proteins.1,5 Several features of the endothelial cell granules containing multimerin suggested these structures could be regulated secretory organelles. First, immunostaining experiments indicated multimerin was released from these structures onto the external membrane in response to secretagogues. Second, the dense-core appearance of the multimerin granules was consistent with a regulated, rather than a constitutive, secretory vesicle. Third, pulse-chase experiments indicated radiolabeled multimerin remained in the cell lysate long after constitutive secretion was complete. Fourth, granules containing multimerin persisted when endothelial cells were treated for 24 hours with high concentrations of a protein synthesis inhibitor. Fifth, we could not show any internalization of extracellular multimerin, excluding endocytic or phagocytic vesicles as the explanation for the multimerin granules. However, with secretagogue stimulation, we were unable to confirm multimerin secretion into the culture media or detect an increase in the extracellular matrix-associated multimerin, possibly due to the very low levels of multimerin stored in these cells, the binding of the released multimerin to the cell membrane, and the very large quantities of multimerin associated with the extracellular matrix under basal culture conditions. The detection of protein redistribution by using immunostaining may be more sensitive for evaluating regulated secretory proteins, because some proteins localized to endothelial cell secretory granules are secreted in amounts that cannot be measured by direct-binding assays.33
Multimerin is not detectable in normal plasma,38 even when tested neat in the multimerin ELISA (unpublished observations). Although we found small amounts of multimerin in intracellular granules in cultured endothelial cells, the multimerin synthesized by these cells was mainly constitutively secreted. We observed similar predominant constitutive secretion of multimerin in studies of a cultured megakaryocyte cell line.34 The presence of multimerin in vascular endothelium in situ and the lack of detectable multimerin in normal plasma suggests that it is rapidly cleared following its secretion in vivo, or perhaps it remains bound to platelets, endothelial cells, and/or the extracellular matrix. It is also possible that the predominant constitutive secretion of multimerin by cultured cells may not reflect its normal processing by vascular endothelial cells in situ.
The multimerin transcripts expressed in endothelial cells, platelets, and Dami cells (a megakaryocyte cell line) are identical in size36,43 yet differences are seen in the mobility of their multimerin subunit and multimers. The pattern of multimerin biosynthesis by endothelial cells in culture resembled Dami cells,34 with minor differences in the extent of multimerin N-glycosylation by these cells. Similar to Dami cells, the majority of the multimerin synthesized by endothelial cells in culture was constitutively secreted, with less complete proteolytic processing of the multimerin constitutively secreted by these cells, compared with platelet multimerin. The different mobilities of the N-deglycosylated multimerin subunits from platelets and cultured endothelial cells suggest that there may be additional differences in their post-translational processing of multimerin, perhaps due to different proteolytic processing. Many regulated secretory proteins undergo proteolytic processing during storage, although storage of a regulated, secretory protein may occur without proteolytic processing and some granular proteins are proteolytically modified only in specific cell types.1,15 54 After a long-term metabolic labeling in media supplemented with 20% to 40% methionine and a prolonged chase to allow complete secretion of constitutively secreted multimerin, we found small quantities of multimerin persisting in the cell lysates that were consistently more completely proteolytically processed than the multimerin constitutively secreted from endothelial cells. A number of ubiquitous and tissue-specific proprotein conversion endoproteases have been identified5; but the endoproteases responsible for processing regulated and constitutive secretory proteins, including multimerin, in megakaryocytes and endothelial cells are largely unknown.
Pathways for regulated and constitutive protein secretion exist in many cells.1-6,55 The factors that direct soluble proteins to common or distinct storage granules are largely unknown, but the ability of proteins to form homoaggregates or coaggregates may be one of the important determinants.1,5,56,57 The lack of interactions between multimerin and von Willebrand factor could account for their sorting to different endothelial cell structures and their apparent compartmentalization in some organelles that contained both proteins. In cells that contain different populations of regulated secretory granules, there is some variability in the completeness of protein sorting. For example, when von Willebrand factor is expressed in an endocrine cell line (AtT 20), von Willebrand factor is found in secretory granules that are distinct from those storing ACTH.58 In contrast, bovine somatomammotrophic cells and rat pituitary gonadotrophs contain a number of different storage granules, characterized by their different predominant protein hormone contents, but these granules also contain small amounts of the hormones stored in the other secretory granules.10,11 14 Similar incomplete sorting or “spillage” could account for the small quantities of multimerin detected by immunoelectron microscopy in some von Willebrand factor/P-selectin positive Weibel-Palade bodies.
The factors controlling the biogenesis of secretory granules in endothelial cells and platelets are largely unknown. The normal von Willebrand factor containing Weibel-Palade bodies in P-selectin–deficient mice indicate these storage granules do not require P-selectin for their formation.59 Luminal interactions influence the sorting of some proteins56,57; however, the cytoplasmic domain of P-selectin has been implicated as the important determinant of its sorting and internalization.60-63 The normal codistribution of P-selectin with von Willebrand factor,9,22,23 the recycling of secreted P-selectin back to von Willebrand factor-containing storage granules,60 and the P-selectin negative, multimerin-containing granules suggest P-selectin interacts with components that are restricted to the von Willebrand factor-containing granules. Although P-selectin sorts to several types of secretory granules when expressed in PC12 cells,63 we were unable to detect P-selectin in the multimerin dense-core granules. Recently, tissue-type plasminogen activator (t-PA) was localized to secretory granules in endothelial cells that are distinct from Weibel-Palade bodies64; these data indicate that endothelial cells contain several types of secretory granules that store different content proteins. As the appearance and ultrastructure of the t-PA storage granules are quite different from the granules containing multimerin (J. Emeis and F. Lupu, personal communication, August 1997), these data suggest further complexities in the sorting of proteins to secretory granules in endothelial cells. Although tissue factor pathway inhibitor is released from endothelial cells in response to secretagogues,65 unlike multimerin, it is localized within caveolae.66
Content proteins are known to influence the size and shape of secretory granules.1,58 Studies of porcine blood vessels have suggested that there are distinct populations of Weibel-Palade bodies (designated as “typical” and “atypical” Weibel-Palade bodies) in vascular endothelium30; the normal “atypical” Weibel-Palade bodies in severe von Willebrand factor–deficient pigs30 have suggested “typical” and “atypical” Weibel-Palade bodies contain different proteins. The small amounts of multimerin detected by immunoelectron microscopy in some von Willebrand factor/P-selectin–positive Weibel-Palade bodies could explain our observation that multimerin was present in granules with morphologic features of Weibel-Palade bodies. However, when we screened large numbers of cells by using light and confocal microscopy, we also identified elongated granules in endothelial cells that contained multimerin, without detectable von Willebrand factor and P-selectin. Organelles resembling Weibel-Palade bodies form in heterologous cells transfected with the von Willebrand factor cDNA,58,67 and platelet α-granular tubules and “typical” Weibel-Palade bodies are absent in severe von Willebrand disease.27 29-31 These data infer multimerin is associated with structures that have a different appearance or electron density than “typical” Weibel-Palade bodies.
Matrix assembly is important for tissue architecture and cell adhesion.68 Endothelial cells are known to synthesize a number of extracellular matrix proteins including fibronectin; von Willebrand factor; laminin; thrombospondin-1; collagen types I, III, IV, and V; and proteoglycans, among others.69 In cultured endothelium, multimerin is part of fibrillary, Triton-insoluble extracellular matrix structures that are closely associated with endothelial cells. An unusual feature of these structures was their prominence in primary cultures, even though the culture media of later passage cells contained more multimerin. One explanation for this discrepancy could be that more of the secreted multimerin is bound to the extracellular matrix in primary cultures. Studies comparing multimerin to fibronectin in early-passage endothelial cells indicated both proteins were associated with fibrillary structures in the extracellular matrix with different distributions, implying interactions with different matrix components. Fibrillary extracellular matrix structures containing other proteins have been shown in cultured endothelium,70-73 but some of these structures are assembled late during cell culture.73 The high molecular weight multimerin associated with the Triton-insoluble extracellular matrix suggest that the largest forms of multimerin (containing more ligand binding sites) may bind preferentially to the extracellular matrix. The release of multimerin onto the external membrane of endothelial cells in response to secretagogues has additional implications for function, and suggests this protein may play a role in the response to vascular injury. The precise function of multimerin in vascular endothelium awaits elucidation; multimerin's close association with endothelial cells and its assembly into large fibrillary structures suggest possible roles in matrix assembly or cell adhesion.
Tissue-dependent expression is observed with multimerin, von Willebrand factor, and P-selectin, leading to their localized distribution in platelets and in vascular endothelial cells. Differences in the distributions of these proteins in endothelial cells and platelets indicate that tissue-specific determinants influence their compartmentalization and suggest that there are different populations of dense-core, secretory granules in human endothelial cells.
ACKNOWLEDGMENT
We thank Drs. J.G. Kelton, D. Hayward, L. Arsenault, D. Andrews, F. Lupu, and C. Lupu for their helpful discussions; J. Ribau for assistance with the confocal microscopy studies; and Drs. S.J. Israels and M. Berndt for the generous gift of antibodies.
Supported by grants from the Medical Research Council of Canada (C.P.M.H.), the Heart and Stroke Foundation of Ontario (C.P.M.H. and T.J.P.), and by grants from l'Association pour la Recherche sur le Cancer (ARC) and Fondation pour la Recherche Medicale (FRM) (E.M.C.). C.P.M.H. is a Research Scholar of the Heart and Stroke Foundation of Ontario and the recipient of an Ortho Biotech Inc./American Society of Hematology Scholar Award. T.J.P. is a Career Investigator of the Heart and Stroke Foundation of Ontario.
Address correspondence to Catherine P. M. Hayward, MD, PhD, Room 2N32, McMaster University Medical Centre, Hamilton Health Sciences Corporation, 1200 Main St W, Hamilton, Ontario, Canada L8N 3Z5.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 10. Long-term metabolic labeling studies of endothelial cell multimerin. Multimerin inmmunoprecipitates of equivalent volumes of culture media (CM; from 18 hours [18h] and 3 days [3d] of labeling in media supplemented with 40% unlabeled media; 18-hour [C1] and 3-hour [C2] chases in unlabeled media; and ionophore A23187 [+] or buffer [−] treated cells) and endothelial cell lysates (Lys; ionophore A23187 and buffer-treated cells) were compared with125 I labeled platelet multimerin (Plt), and immunoprecipitates prepared by using normal mouse IgG (mIgG). Reduced SDS-PAGE (A) and nonreduced multimer gels (B) analyses of single or double-immunoprecipitates (*) from two experiments are shown. The images represent 3-day (A, lanes 1-3), 20-day (A, lanes 4-10), and 32-day (A, lanes 11-16; B) exposures. The arrows indicate the location of the p155 and proM subunits. Small quantities of more fully proteolyzed multimerin were detected in the cell lysates after constitutive multimerin secretion was complete. Secretagogue stimulation did not release detectable amounts of multimerin into the culture media.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/4/10.1182_blood.v91.4.1304/4/m_blod4043610.jpeg?Expires=1767703088&Signature=HXp0Vp5MLKyAsWY9DESMjuDAT4YGbBMYdORN4L2BpyaUqKE4yxYAKlcE2GD6QMnmA~xf-76tfrMkzhWPNCwVUGkY0tK75bmSZ7N7HJQKw0G7zc-z5qFB3eTUoigHSzyMIHSz5MXeIme1qReEF-gI0SKkJFLoBDw~1Cfs0y5efUEZyK7srzEtx32tt1CtBOPW1f33ey1DX6lWgPQMaqeMME-QpElEa5iMTCcqKasaNGFix-2lfb5o7Yj85CFpGjKYwaroMuAfrPVt92IWMvzo2eze0mMM64gk0Gtrgv3wbYd4aC17WNgUEVF~B~LOnMg1BjwMtw2GLs4MCawHq~vdnQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 10. Long-term metabolic labeling studies of endothelial cell multimerin. Multimerin inmmunoprecipitates of equivalent volumes of culture media (CM; from 18 hours [18h] and 3 days [3d] of labeling in media supplemented with 40% unlabeled media; 18-hour [C1] and 3-hour [C2] chases in unlabeled media; and ionophore A23187 [+] or buffer [−] treated cells) and endothelial cell lysates (Lys; ionophore A23187 and buffer-treated cells) were compared with125 I labeled platelet multimerin (Plt), and immunoprecipitates prepared by using normal mouse IgG (mIgG). Reduced SDS-PAGE (A) and nonreduced multimer gels (B) analyses of single or double-immunoprecipitates (*) from two experiments are shown. The images represent 3-day (A, lanes 1-3), 20-day (A, lanes 4-10), and 32-day (A, lanes 11-16; B) exposures. The arrows indicate the location of the p155 and proM subunits. Small quantities of more fully proteolyzed multimerin were detected in the cell lysates after constitutive multimerin secretion was complete. Secretagogue stimulation did not release detectable amounts of multimerin into the culture media.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/4/10.1182_blood.v91.4.1304/4/m_blod4043610.jpeg?Expires=1767884857&Signature=ZITbS0LueCblUYBl~jG0vr35U6mvQ6fzjr1KZIsT~XK9tnz4sUo-w1twR97774mf1TEqeIs3DY4wfaPWkJ~ztvm-bTLEzHL8LGy3KDgRa-rrHvutYOF1ttUEbEk74NOV1Indg1zr2ZXlUR79wh8txim1GZg7IqnH4m5ER7fuk98Y147xwfJPJaPyO3LhxnWmuFY~OXdjyfw2Z4yYWnrCAiITCJnCDTINI6irBLqCAKy2tClZxEuFXf0pJCkDjesg3SmD8XPV44hQP~rMO~yIlz77klUmQQ86Ujt4Km2XeqY99hRkaDuQtyweA9Q9RDAT2itM9VwcYjMfDY-6XmQVYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)