Abstract
Proteasome inhibitors, antioxidants, salicylates, or glucocorticoids block the cytokine-induced expression of the endothelial cell adhesion molecules E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1. These pharmacological agents have been assumed to inhibit the expression of adhesion molecules primarily by blocking activation of the transcription factor NF-κB. We found that the proteasome inhibitor ALLN, the antioxidant PDTC, or sodium salicylate, but not the glucocorticoid dexamethasone, inhibited both the constitutive and the interleukin-4– or oncostatin M–induced expression of the adhesion molecule P-selectin in human endothelial cells. ALLN, PDTC, or sodium salicylate decreased P-selectin expression without a detectable requirement for inhibition of NF-κB activation or for an intact κB element in the P-selectin gene. These results extend the potential anti-inflammatory utility of such drugs to inhibition of P-selectin expression and suggest that they have important actions that do not involve the NF-κB system.
DURING INFLAMMATION, specific combinations of signaling and adhesion molecules regulate leukocyte recruitment into extravascular tissues.1,2 Binding of L-, E-, or P-selectin to cell-surface glycoconjugates initiates tethering and rolling of circulating leukocytes on the vessel wall.3,4 Subsequent leukocyte activation promotes firm adhesion through engagement of β2 integrins with Ig-like counterreceptors such as intercellular adhesion molecule 1 (ICAM-1). The α4 integrins on mononuclear cells and eosinophils promote tethering, rolling, and firm adhesion through interactions with Ig counterreceptors such as vascular cell adhesion molecule 1 (VCAM-1).5 6
The inflammatory mediators tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and lipopolysaccharide (LPS) activate transcription of many immediate-early genes, including those encoding the endothelial cell adhesion molecules E-selectin, ICAM-1, and VCAM-1. Regulation of NF-κB transcription factors plays a critical role in activation of these genes.7 The prototypical NF-κB, a heterodimer of the p50 and p65 proteins, is normally sequestered in the cytoplasm by the inhibitor protein IκB-α. Signals generated by TNF-α, IL-1β, LPS, or other agonists induce degradation of IκB-α, allowing NF-κB heterodimers to migrate to the nucleus, bind to κB recognition elements, and activate gene expression in conjunction with other transcription factors.8
The use of pharmacological agents has provided insight into the mechanisms that regulate NF-κB function. Antioxidants block the ability of TNF-α or other agonists to mobilize NF-κB, suggesting that reactive oxygen intermediates promote, directly or indirectly, the degradation of IκB-α.9 Proteolysis of IκB-α requires its initial phosphorylation by specific kinases, which marks the protein for ubiquitination and then degradation in the proteasome. Protease inhibitors, including those relatively specific for the proteasome, block NF-κB activation by preventing degradation of IκB-α.10 Salicylates impair activation of NF-κB,11 at least in part by inhibiting the phosphorylation of IκB-α that is required for its degradation.12 Glucocorticoids inhibit NF-κB activation by augmenting expression of mRNA for IκB-α13,14 or by physical association of occupied glucocorticoid receptors with NF-κB.15 Antioxidants,16,17 proteasome inhibitors,18 salicylates,12,19 or glucocorticoids20 21 inhibit the ability of TNF-α, IL-1β, or LPS to induce expression of E-selectin, VCAM-1, or ICAM-1 in cultured human umbilical vein endothelial cells (HUVEC). These agents have been assumed to inhibit the expression of the adhesion molecules primarily by blocking activation of NF-κB.
Unlike E-selectin, which is synthesized in endothelial cells in response to TNF-α or related agonists, P-selectin is constitutively synthesized by endothelial cells and megakaryocytes. It is stored in the Weibel-Palade bodies of endothelial cells and the α granules of platelets.22-25 Thrombin or histamine rapidly mobilizes P-selectin to the cell surface, where it mediates adhesion of leukocytes.26-28 In endothelial cells, the protein is then internalized and either degraded or recycled to new Weibel-Palade bodies.29-31 This rapid but self-limited surface expression enables P-selectin to promote leukocyte tethering to the endothelial cell surface during the earliest stages of acute inflammation.
Persistent expression of P-selectin on the endothelial cell surface has also been observed in human tissues with chronic or allergic inflammation.32-34 This suggests that inflammatory cytokines augment synthesis of P-selectin under some conditions. TNF-α, IL-1β, or LPS do increase P-selectin mRNA and protein in murine endothelial cells,35-37 but they do not increase expression of P-selectin in human endothelial cells.38,39Indeed, the 5′ flanking region of the human P-selectin gene lacks a canonical recognition element for p65-containing NF-κB heterodimers.40 Instead, it has a novel κB element that binds only homodimers of p50 or p52 and that participates in the constitutive expression of P-selectin.41 These data suggest that mediators other than TNF-α, IL-1β, or LPS induce synthesis of P-selectin in endothelial cells at sites of chronic or allergic inflammation in humans. Consistent with this notion, low concentrations of IL-4 or oncostatin M (OSM) induce a delayed and prolonged accumulation of P-selectin mRNA in HUVEC. These cytokines augment transcription of P-selectin mRNA through a mechanism that requires new protein synthesis.39
To obtain further insight into the regulation of P-selectin synthesis, we examined the effects of antioxidants, proteasome inhibitors, salicylates, and glucocorticoids on the expression of P-selectin in HUVEC. We found that the first three of these agents potently inhibited both constitutive and inducible expression of P-selectin. These results extend the potential anti-inflammatory utility of these drugs to inhibition of P-selectin expression. The data also suggest that these agents have important actions that are distinct from the inhibition of NF-κB activation.
MATERIALS AND METHODS
Reagents and cells.
Recombinant human IL-4 and OSM were purchased from R & D Systems, Inc (Minneapolis, MN). Recombinant human TNF-α, N-acetyl-leucinyl-leucinyl-norleucinal-H (ALLN, Calpain inhibitor I), and N-acetyl-leucinyl-leucinyl-methional (ALLM, Calpain inhibitor II) were obtained from Boehringer Mannheim Biochemicals (Indianapolis, IN). Pyrrolidine dithiocarbamate (PDTC), sodium salicylate (NaSal), indomethacin (Indo), and dexamethasone were from Sigma Chemical Co (St Louis, MO). Stock solutions of ALLN and ALLM were made in dimethylsulfoxide (DMSO, from American Type Culture Collection) at a concentration of 50 mmol/L. A stock solution of dexamethasone was made in ethyl alcohol (EtOH, Quantum Chemical Co, Tuscola, IL) at a concentration of 1 mmol/L.
HUVEC and bovine aortic endothelial cells (BAEC) were cultured as described.40 42 Passage 1 or 2 HUVEC were used in all experiments. The viability of cells after treatment with pharmacological agents was verified with a CellTiter 96AQueous kit (Promega, Madison, WI), according to instructions from the manufacturer.
Northern blot analysis.
HUVEC were preincubated for 1 hour at 37°C with pharmacological agents dissolved in fresh culture medium. As controls for agents dissolved in DMSO or ethyl alcohol, equivalent amounts of DMSO or ethyl alcohol were added to separate groups of cells. The cells were then incubated at 37°C for 20 hours with or without IL-4 (10 ng/mL) or OSM (10 ng/mL) in the continued presence of the pharmacological agent. The cells were then lysed, and total RNA was extracted.43Northern blot analysis with 32P-labeled probes for P-selectin, VCAM-1, or CHO-B was performed as described.39,43 Quantification of RNA was performed by densitometry using a Model GS-670 imaging densitometer (Biorad Laboratories, Hercules, CA). The scans were normalized according to the levels of CHO-B transcripts,44 which did not change when endothelial cells were treated with cytokines.39
Quantification of P-selectin by enzyme-linked immunosolvent assay (ELISA).
Gel mobility shift assay.
Gel mobility shift assays with nuclear extracts were performed using a radiolabeled duplex oligonucleotide containing the κB recognition element in the murine H-2Kb gene.40,41 This element binds specifically to inducible NF-κB p50/p65 heterodimers and to constitutively expressed p50 or p52 homodimers.41 46-48
Reporter gene assay.
Two previously described reporter gene constructs containing portions of the 5′ flanking region of the human P-selectin gene fused to luciferase were used.41 Both constructs contained the 5′ flanking sequence from −309 to −13 relative to the translational start site. The first construct contained the wild-type P-selectin sequence. The second contained guanine-to-cytosine substitutions at positions −217 and −216 in the core region of the κB element; these substitutions eliminated binding of the element to p50 and p52 homodimers.41 BAEC were transfected with each reporter gene as described previously.41 After 36 hours, the cells were treated for 1 hour at 37°C with pharmacological agents. The cells were then incubated at 37°C with medium containing the indicated agent in the presence or absence of OSM (for 16 hours) or TNF-α (for 4 hours). Luciferase activity in cytosolic extracts was measured as described.41
Statistical analysis.
Statistical differences were analyzed by Student's t-test.
RESULTS
The proteasome inhibitor ALLN, the antioxidant PDTC, or sodium salicylate, but not dexamethasone, decreases constitutive and IL-4–induced expression of mRNA for P-selectin and VCAM-1 in HUVEC.
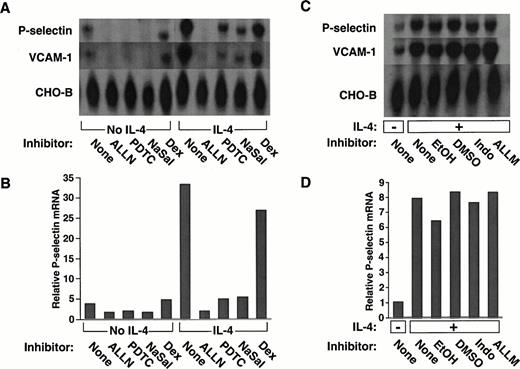
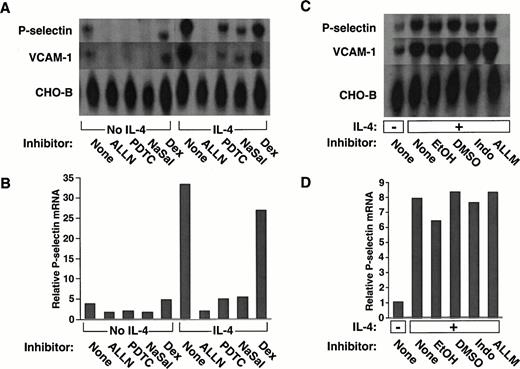
We examined whether a panel of pharmacological agents, used in concentrations that inhibit NF-κB–dependent pathways, affected constitutive or inducible expression of human P-selectin in HUVEC. Cells pretreated with each agent were incubated in the presence or absence of IL-4 for 20 hours. Steady-state levels of P-selectin mRNA were measured by Northern blot analysis of equivalent amounts of RNA from each group of cells. As observed previously,39unstimulated HUVEC expressed mRNA for P-selectin, and IL-4 markedly increased these levels (Fig 1A).Pretreatment of the cells with the proteasome inhibitor ALLN, the antioxidant PDTC, or the anti-inflammatory agent NaSal significantly decreased P-selectin mRNA levels in both unstimulated and IL-4–stimulated HUVEC (Fig 1A). Densitometric measurement of P-selectin mRNA, normalized for the amount of CHO-B, a transcript that does not change its levels when cells are stimulated,39,44confirmed that these agents reduced P-selectin mRNA levels (Fig 1B). The effect of ALLN was specific, because pretreatment with ALLM, an analogue of ALLN with little or no inhibitory activity to the proteasome,49 or with the diluent DMSO did not prevent the IL-4–induced increase in P-selectin mRNA (Fig 1C and D). The effect of sodium salicylate did not require inhibition of cyclooxygenase, because indomethacin did not prevent the IL-4–induced increase in P-selectin mRNA (Fig 1C and D).
The proteasome inhibitor ALLN, the antioxidant PDTC, or NaSal, but not dexamethasone, decreases constitutive and IL-4–induced expression of mRNA for P-selectin and VCAM-1 in HUVEC. (A) Confluent HUVEC in 25-cm2 flasks were preincubated for 1 hour at 37°C in the presence or absence of 25 μmol/L ALLN, 50 μmol/L PDTC, 10 mmol/L NaSal, or 1 μmol/L dexamethasone (Dex). The cells were then incubated for 20 hours at 37°C with or without 10 ng/mL of IL-4 in the continued presence of the respective pharmacological agent. Total RNA was then isolated, and Northern blot analysis was performed with sequential hybridization of the membrane with cDNA probes for P-selectin, VCAM-1, or CHO-B. (B) The relative levels of P-selectin mRNA were quantified by densitometric scanning, normalized according to the level of CHO-B mRNA, which was not affected by IL-4. (C and D) The experiment was performed exactly as in panels A and B, except that cells were incubated with the following controls: EtOH used as the diluent for dexamethasone, DMSO used as the diluent for ALLN, 25 μmol/L Indo, or 25 μmol/L of ALLM, a protease inhibitor with relatively little activity against the proteasome. The data in panels A and B are representative of five experiments. The data in panels C and D are representative of two experiments. The data for IL-4–stimulated cells in panel B were also pooled with the analogous results from the other four experiments. Statistical analysis of the pooled results revealed that ALLN, PDTC, or NaSal significantly decreased IL-4–induced P-selectin mRNA levels (P ≤ .01).
The proteasome inhibitor ALLN, the antioxidant PDTC, or NaSal, but not dexamethasone, decreases constitutive and IL-4–induced expression of mRNA for P-selectin and VCAM-1 in HUVEC. (A) Confluent HUVEC in 25-cm2 flasks were preincubated for 1 hour at 37°C in the presence or absence of 25 μmol/L ALLN, 50 μmol/L PDTC, 10 mmol/L NaSal, or 1 μmol/L dexamethasone (Dex). The cells were then incubated for 20 hours at 37°C with or without 10 ng/mL of IL-4 in the continued presence of the respective pharmacological agent. Total RNA was then isolated, and Northern blot analysis was performed with sequential hybridization of the membrane with cDNA probes for P-selectin, VCAM-1, or CHO-B. (B) The relative levels of P-selectin mRNA were quantified by densitometric scanning, normalized according to the level of CHO-B mRNA, which was not affected by IL-4. (C and D) The experiment was performed exactly as in panels A and B, except that cells were incubated with the following controls: EtOH used as the diluent for dexamethasone, DMSO used as the diluent for ALLN, 25 μmol/L Indo, or 25 μmol/L of ALLM, a protease inhibitor with relatively little activity against the proteasome. The data in panels A and B are representative of five experiments. The data in panels C and D are representative of two experiments. The data for IL-4–stimulated cells in panel B were also pooled with the analogous results from the other four experiments. Statistical analysis of the pooled results revealed that ALLN, PDTC, or NaSal significantly decreased IL-4–induced P-selectin mRNA levels (P ≤ .01).
The glucocorticoid dexamethasone only modestly decreased the constitutive or IL-4–induced levels of P-selectin mRNA (Fig 1A and B). Ethyl alcohol, the diluent used for the dexamethasone, elicited the same modest decrease in transcripts (Fig 1C and D). Thus, in marked contrast to the other agents, dexamethasone did not specifically affect constitutive or inducible P-selectin mRNA levels.
IL-4 is known to increase the steady-state levels of VCAM-1 in HUVEC, primarily by increasing the half-life of transcripts rather than by directly augmenting transcription.50 We found that ALLN, PDTC, or NaSal also reduced the levels of VCAM-1 mRNA in both unstimulated and IL-4–stimulated HUVEC. In contrast, dexamethasone had no specific effect on VCAM-1 mRNA levels (Fig 1A). No reduction in VCAM-1 mRNA was observed with control pretreatments (Fig 1C).
ALLN, PDTC, or NaSal, but not dexamethasone, decreases OSM-induced expression of P-selectin mRNA in HUVEC.
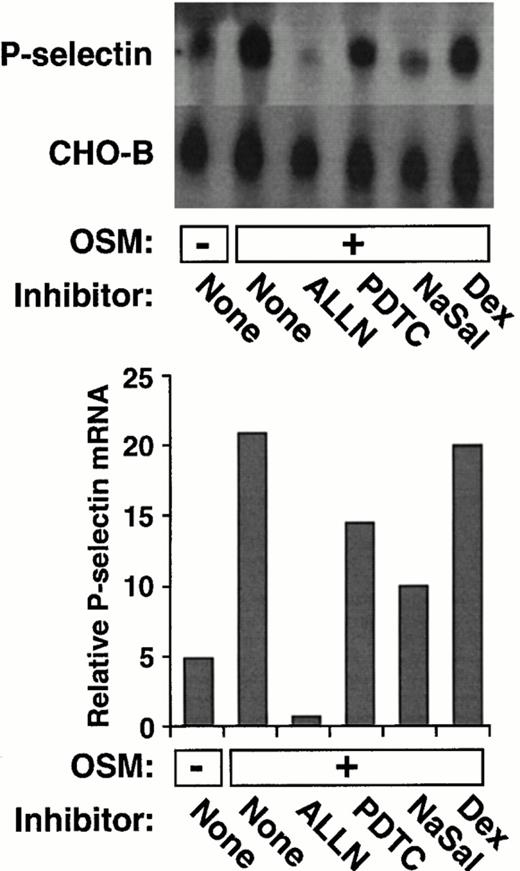
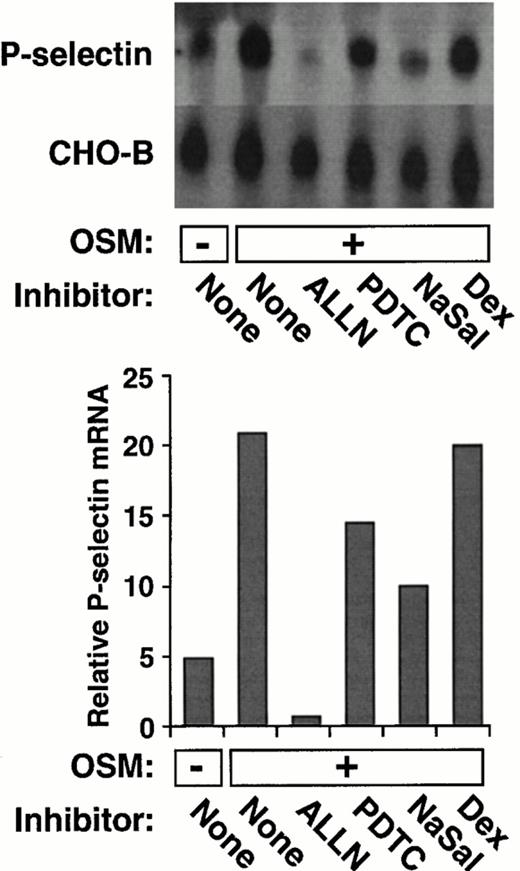
Like IL-4, the cytokine OSM induces a delayed and prolonged accumulation of P-selectin mRNA in HUVEC.39 As shown in Fig2, ALLN, PDTC, or NaSal also impaired the ability of OSM to increase P-selectin mRNA levels. In contrast, dexamethasone had no effect on OSM induction of P-selectin mRNA. These data show that the same group of pharmacological agents inhibits both IL-4– and OSM-induced accumulation of P-selectin mRNA.
ALLN, PDTC, or NaSal, but not dexamethasone, decreases OSM-induced expression of P-selectin mRNA in HUVEC. The experiment was performed exactly as in Fig 1A and B, except that the cells were incubated for 20 hours in the presence or absence of 10 ng/mL of OSM. The data are representative of two experiments. The data in panel B were also pooled with the results from the other experiment. Statistical analysis of the pooled results revealed that ALLN, PDTC, or NaSal significantly decreased OSM-induced P-selectin mRNA levels (P ≤ .05).
ALLN, PDTC, or NaSal, but not dexamethasone, decreases OSM-induced expression of P-selectin mRNA in HUVEC. The experiment was performed exactly as in Fig 1A and B, except that the cells were incubated for 20 hours in the presence or absence of 10 ng/mL of OSM. The data are representative of two experiments. The data in panel B were also pooled with the results from the other experiment. Statistical analysis of the pooled results revealed that ALLN, PDTC, or NaSal significantly decreased OSM-induced P-selectin mRNA levels (P ≤ .05).
ALLN, PDTC, or NaSal, but not dexamethasone, prevents the IL-4–induced increase in P-selectin protein in HUVEC.
We used an ELISA to determine whether the pharmacological agents affected the steady-state levels of P-selectin protein in HUVEC. As shown previously,39 treatment with IL-4 for 20 hours increased the level of P-selectin protein by approximately twofold (Fig3). ALLN, PDTC, or NaSal prevented this increase. In contrast, dexamethasone did not prevent the IL-4–induced elevation in P-selectin protein (Fig 3). ALLN, PDTC, or NaSal, but not dexamethasone, also decreased the amount of P-selectin protein in unstimulated endothelial cells (data not shown). Thus, the same agents that reduced levels of P-selectin mRNA also reduced levels of P-selectin protein.
ALLN, PDTC, or NaSal, but not dexamethasone, prevents the IL-4–induced increase in P-selectin protein in HUVEC. The cells were pretreated with the indicated pharmacological agent and then incubated in the presence or absence of IL-4 as in Fig 1A. The cells were then lysed in buffer containing Triton X-100, and the amount of P-selectin in the lysate was measured by ELISA. The data represent the mean ± SD of triplicate determinations and are representative of three experiments. *, Significantly different from the IL-4–treated group (P < .01).
ALLN, PDTC, or NaSal, but not dexamethasone, prevents the IL-4–induced increase in P-selectin protein in HUVEC. The cells were pretreated with the indicated pharmacological agent and then incubated in the presence or absence of IL-4 as in Fig 1A. The cells were then lysed in buffer containing Triton X-100, and the amount of P-selectin in the lysate was measured by ELISA. The data represent the mean ± SD of triplicate determinations and are representative of three experiments. *, Significantly different from the IL-4–treated group (P < .01).
Pharmacological inhibition of P-selectin expression does not require blockade of NF-κB activation or the presence of an intact κB element in the human P-selectin promoter.
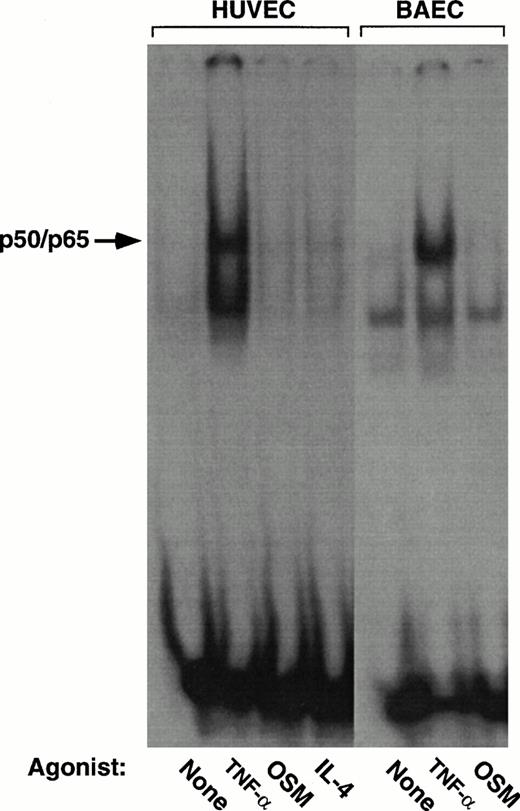
Most studies of the anti-inflammatory effects of proteasome inhibitors, antioxidants, and salicylates have emphasized the ability of these agents to inhibit activation of p65-containing NF-κB proteins.11,12,16-19 However, the only observed κB element in the human P-selectin gene (−218GGGGGTGACCCC−207) binds p50 and p52 homodimers, but not inducible p65-containing homodimers or heterodimers.41 We performed gel mobility shift studies with endothelial cell nuclear extracts that were incubated with a well-characterized κB element from the murine H-2Kb gene; this element binds inducible p50/p65 heterodimers as well as p50 or p52 homodimers.41 46-48 Stimulation of HUVEC or BAEC with TNF-α for 4 hours induced binding of p50/p65 complexes to the labeled oligonucleotide.
In contrast, stimulation of the cells with OSM or IL-4 for 4 hours did not induce binding of p50/p65 to the oligonucleotide (Fig4). Thus, constitutive and IL-4– or OSM-induced expression of P-selectin occurred in the absence of detectable activated NF-κB in the nucleus.
Stimulation of endothelial cells with IL-4 or OSM does not activate NF-κB. Nuclear extracts from HUVEC or BAEC incubated for 4 hours in the presence or absence of 100 U/mL TNF-α, 10 ng/mL IL-4, or 10 ng/mL OSM were incubated with a radiolabeled oligonucleotide containing the κB element from the murine H-2Kb gene. DNA-protein complexes were analyzed by a gel mobility shift assay, followed by autoradiography. The arrow marks the inducible complex containing the p50/p65 heterodimeric NF-κB protein. Complex formation was inhibited by an excess of unlabeled oligonucleotide, confirming its specificity (data not shown).
Stimulation of endothelial cells with IL-4 or OSM does not activate NF-κB. Nuclear extracts from HUVEC or BAEC incubated for 4 hours in the presence or absence of 100 U/mL TNF-α, 10 ng/mL IL-4, or 10 ng/mL OSM were incubated with a radiolabeled oligonucleotide containing the κB element from the murine H-2Kb gene. DNA-protein complexes were analyzed by a gel mobility shift assay, followed by autoradiography. The arrow marks the inducible complex containing the p50/p65 heterodimeric NF-κB protein. Complex formation was inhibited by an excess of unlabeled oligonucleotide, confirming its specificity (data not shown).
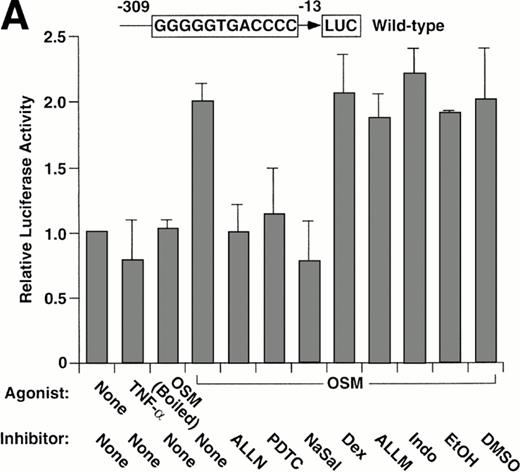
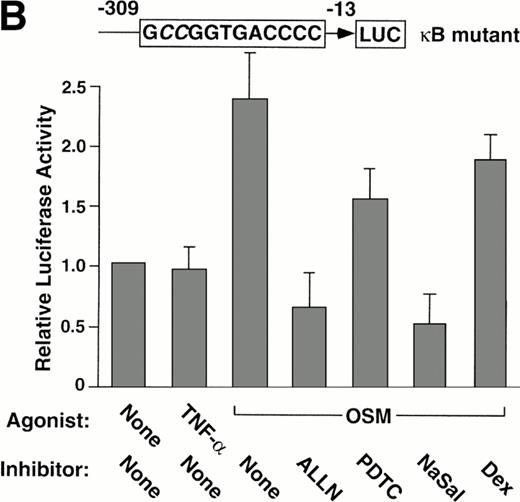
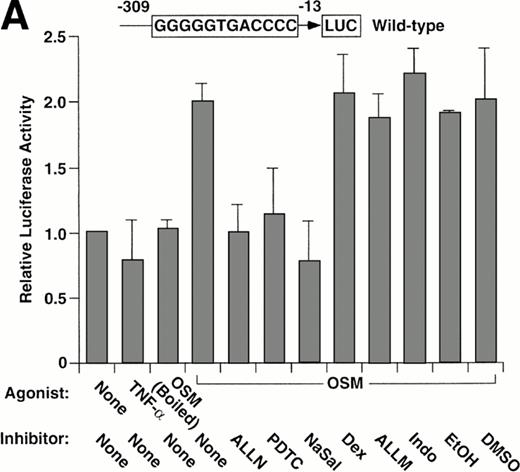
We used a reporter gene expression system to further explore whether the pharmacological agents inhibited P-selectin expression by interfering with the function of the κB element in the P-selectin gene. BAEC were transfected with either of two luciferase reporter genes driven by approximately the first 300 bp of the 5′ flanking sequence of the human P-selectin gene. One construct contained the wild-type sequence, and the other construct contained a 2-bp mutation in the κB element that abolished binding to p50 or p52 homodimers. Both constructs were previously shown to drive constitutive expression of luciferase in transfected BAEC, although expression driven by the construct with the mutated κB element is approximately 40% less than that of the wild-type construct in parallel transfections.
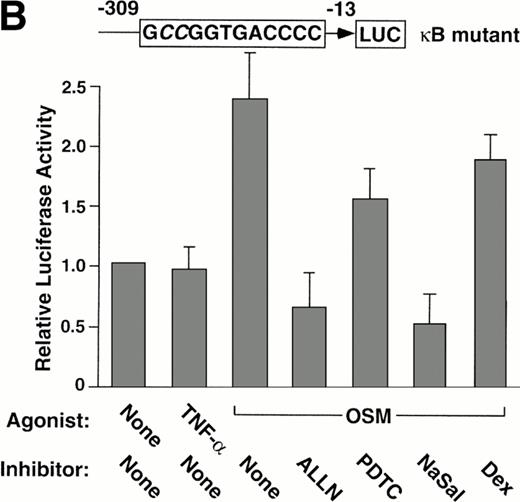
Stimulation of BAEC with TNF-α for 4 hours did not increase expression of the wild-type or mutant P-selectin reporter gene over basal levels (Fig 5). This finding is consistent with the failure of TNF-α to augment expression of endogeneous human P-selectin in HUVEC.41 In contrast, stimulation of BAEC with OSM for 12 hours elicited a twofold increase in expression of either construct (Fig 5). The effect of OSM was not caused by contamination with LPS, because boiled OSM failed to induce reporter gene expression (Fig 5A). These data show that OSM increases expression of the reporter gene, even if the κB site is disrupted. ALLN, PDTC, or NaSal prevented or decreased the OSM-induced increase expression of both reporter genes (Fig 5). Control incubations did not suppress the OSM-induced increase in expression (Fig 5A). Furthermore, dexamethasone had no effect on OSM-induced expression (Fig 5). These results show that ALLN, PDTC, or salicylates inhibits OSM-induced expression of a human P-selectin reporter gene, even if the κB element is mutated.
Pharmacological inhibition of P-selectin expression does not require the presence of an intact κB element in the human P-selectin promoter. BAEC were transfected with a luciferase reporter gene driven by the human P-selectin 5′ flanking sequence from −309 to −13 (A) or with a reporter gene driven by the same 5′ flanking sequence with the exception of a 2-nucleotide mutation in the κB element (B). After 36 hours, the cells were pretreated for 1 hour at 37°C in the presence or absence of 25 μmol/L ALLN, 100 μmol/L PDTC, 5 mmol/L NaSal, 1 μmol/L dexamethasone (Dex), 25 μmol/L ALLM, 25 μmol/L Indo, or the diluents EtOH or DMSO used for dexamethasone and ALLN, respectively. The cells were then incubated without cytokine for 12 hours, with 100 U/mL TNF-α for 4 hours, or with 10 ng/mL of boiled or active OSM for 12 hours. The luciferase activities in cell lysates were then measured; the values were normalized, with a value of 1.0 assigned to unstimulated cells incubated in the absence of pharmacological agent. The data represent the mean ± SD of triplicate transfections from each of two independent experiments.
Pharmacological inhibition of P-selectin expression does not require the presence of an intact κB element in the human P-selectin promoter. BAEC were transfected with a luciferase reporter gene driven by the human P-selectin 5′ flanking sequence from −309 to −13 (A) or with a reporter gene driven by the same 5′ flanking sequence with the exception of a 2-nucleotide mutation in the κB element (B). After 36 hours, the cells were pretreated for 1 hour at 37°C in the presence or absence of 25 μmol/L ALLN, 100 μmol/L PDTC, 5 mmol/L NaSal, 1 μmol/L dexamethasone (Dex), 25 μmol/L ALLM, 25 μmol/L Indo, or the diluents EtOH or DMSO used for dexamethasone and ALLN, respectively. The cells were then incubated without cytokine for 12 hours, with 100 U/mL TNF-α for 4 hours, or with 10 ng/mL of boiled or active OSM for 12 hours. The luciferase activities in cell lysates were then measured; the values were normalized, with a value of 1.0 assigned to unstimulated cells incubated in the absence of pharmacological agent. The data represent the mean ± SD of triplicate transfections from each of two independent experiments.
DISCUSSION
Pharmacological agents have proven useful for studying the pathways that inflammatory mediators such as TNF-α or LPS use to activate NF-κB in cells. Activated NF-κB induces the expression of many immediate-early genes, including the endothelial cell adhesion molecules E-selectin, VCAM-1, and ICAM-1.7 Proteasome inhibitors, antioxidants, salicylates, or glucocorticoids impair the activation and/or function of NF-κB.9-11,13-15These agents partially or completely block TNF-α or LPS-induced expression of E-selectin, VCAM-1, and ICAM-1, and the effects were assumed to result from inhibition of the NF-κB pathway.12 16-21 In this study, we show that proteasome inhibitors, antioxidants, or salicylates, but not glucocorticoids, inhibited the constitutive and the IL-4– or OSM-induced expression of P-selectin in human endothelial cells. The agents were effective without a detectable requirement for inhibition of NF-κB activation or for an intact κB element in the P-selectin gene. These results extend the potential anti-inflammatory utility of such drugs to inhibition of P-selectin expression and suggest that they have important actions that do not involve the NF-κB system.
Unlike many endothelial cell adhesion proteins, P-selectin is constitutively synthesized.24 TNF-α or LPS does not further increase synthesis of P-selectin in HUVEC.38,39However, IL-4 or OSM causes a sustained increase in P-selectin mRNA.39 Thus, the synthesis of P-selectin can be dissociated from the activation of p65-containing NF-κB dimers, which enter the nuclei of HUVEC stimulated with TNF-α, but not with IL-4 or OSM51 (Fig 4). IL-4 also significantly increased VCAM-1 mRNA in HUVEC in a manner that did not obviously require activation of NF-κB50,51 (Fig 1). ALLN, PDTC, or NaSal inhibited constitutive expression of P-selectin and OSM- or IL-4–induced expression of P-selectin or VCAM-1. In contrast, high concentrations of dexamethasone that disrupt NF-κB pathways failed to inhibit expression of P-selectin or VCAM-1 (Figs 1 and 2). These data suggest that ALLN, PDTC, and salicylates impair expression of both proteins by means other than blockade of NF-κB activation or function.
The promoter/enhancer of the human P-selectin gene has only one κB element, a novel sequence that binds constitutively expressed p50 or p52 homodimers but not inducible p50/p65 heterodimers.41ALLN, PDTC, or salicylates, but not dexamethasone, blocked OSM-induced expression of a human P-selectin reporter gene in transfected endothelial cells. This inhibition was observed even when the κB site in the P-selectin sequence was disrupted (Fig 5). Together, these results provide strong evidence that ALLN, PDTC, or salicylates impair P-selectin expression through an NF-κB–independent pathway.
Recent studies also suggest that these pharmacological agents affect pathways that do not involve NF-κB. Proteasomal inhibitors block IL-1β–induced expression of VCAM-1 in HUVEC under conditions that do not affect activation and nuclear translocation of NF-κB.52 Proteasomal inhibitors also block TNF-α–induced expression of VCAM-1 and ICAM-1 in HUVEC under conditions in which NF-κB activation and nuclear translocation are only partially inhibited.18 Although these results could mean that subtle reductions in nuclear levels of NF-κB prevent transcriptional activation of genes that have κB elements of relatively low affinity, they might also indicate that proteasomal inhibitors act through other mechanisms. As an example, proteasomal inhibitors enhance interferon-γ or IL-2 signaling through the JAK/STAT pathway. The inhibitors block proteasomal degradation of a ubiquitinated STAT protein53 or affect upstream events, for example, by stabilizing inhibitors of tyrosine phosphatases that otherwise dephosphorylate activated JAKs or STATs.54,55IL-4 and OSM augment P-selectin expression, in part, through tyrosine phosphorylation,39 and the JAK/STAT pathways may be important for signaling. Whether proteasomal inhibitors might inhibit rather than enhance JAK/STAT pathways in endothelial cells is unknown. It is noteworthy that serine protease inhibitors inhibit a signaling pathway that requires the mitogen-regulated serine/threonine kinase pp70s6k.56 Many cellular systems may be regulated at least partially by protein turnover. Thus, general protease inhibitors or even relatively specific proteasomal inhibitors may affect several signaling pathways.
The redox state of the cell may affect many protein-protein interactions. With regard to gene transcription, the effects of oxidants and antioxidants on the NF-κB and AP-1 pathways have been most intensively studied.57 Recently, it was shown that oxidation of the GA-binding protein-α subunit destroys its DNA-binding activity.58 Oxidants also inhibit one or more tyrosine phosphatases that normally downregulate the function of activated receptor tyrosine kinases.59 Thus, antioxidants may affect the function of diverse signaling components and transcription factors, of which only some may have been characterized. The targets for antioxidants that regulate expression of P-selectin remain unknown.
The eicosanoid-independent effects of salicylates have not been fully characterized. There is some evidence that they have antioxidant properties,60-62 which might allow them to target many intracellular systems. At the high concentrations used in these experiments, salicylates may also affect kinases or phosphatases that regulate proteins other than IκB-α.63
Glucocorticoids inhibit TNF-α– or LPS-induced expression of E-selectin in human or porcine endothelial cells.20,21 In endothelial cells, one major effect of glucocorticoids is to block the function of NF-κB, perhaps by direct physical association with the transcription factor.21,64 Glucocorticoids may have many other effects. For example, dexamethasone enhances Stat5-depending signaling in COS cells by virtue of the occupied glucocorticoid receptor serving as a transcriptional coactivator for Stat5.65 Glucocorticoid receptors may also interfere with the AP-1 and NF-AT transcription factors.66 Even at high concentrations, however, dexamethasone did not affect constitutive or IL-4– or OSM-induced expression of P-selectin in HUVEC.
In summary, we found that a proteasome inhibitor, an antioxidant, and a salicylate significantly inhibited both constitutive and cytokine-inducible expression of P-selectin in human endothelial cells. These results extend the potential anti-inflammatory effects of these agents to inhibition of P-selectin function. However, they suggest that caution should be used in assigning the mechanism of action of a pharmacological agent to a single pathway such as NF-κB activation.
ACKNOWLEDGMENT
We thank Ginger Hampton and Chris Titsworth for technical assistance and Kelsey Kennedy for preparing the figures.
Supported by National Institutes of Health Grant No. P50 HL 54502.
Address reprint requests to Rodger P. McEver, MD, W.K. Warren Medical Research Institute, University of Oklahoma Health Sciences Center, 825 NE 13th St, Oklahoma City, OK 73104.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.