Abstract
In vivo studies suggested the possibility of an IgE-dependent regulation of high-affinity (FcRI) IgE receptor expression on basophils. The current studies extend these observations to in vitro cultures of human basophils. Incubation of basophils for 3 to 4 weeks resulted in a slow dissociation of IgE antibody, during which time FcRI expression decreased, as measured by flow cytometry using the anti-FcRIα monoclonal antibody, 22E7, or by measuring FcRIα mass by Western blotting of whole-cell lysates. Culture of basophils with IgE resulted in upregulation of FcRIα expression by both flow cytometry and Western blotting of whole-cell lysates. Upregulation followed a linear time course during 2 weeks of culture. The relative increase in FcRIα density depended on the starting density; with starting densities of FcRIα of 10,000 to 170,000 per basophil, the upregulation varied 20- to 1.1-fold, respectively. Upregulation occurred in high-purity basophils, was not influenced by IgG at concentrations up to 1 mg/mL, and was inhibited by dimeric IgE. Heat-inactivated IgE was less effective and the monoclonal antibody CGP51901 that prevents IgE binding to FcRIα blocked the ability of IgE to induce upregulation. The dose-response curve for IgE-induced upregulation had an effective concentration50 of 230 ng/mL. Although the receptor through which IgE induces this upregulation is not yet known, several characteristics suggest that the upregulation is mediated by IgE interacting through FcRIα itself.
THE HIGH-AFFINITY receptor for IgE, FcεRI, is a critical component in the chain of events that lead to type I hypersensitivity reactions, as this receptor allows mast cells and basophils (as well as other recently identified cell types) to bind IgE and therefore respond to environmental antigens. Consequently, varied levels of expression of this receptor probably have an impact on the severity of hypersensitivity reactions. It has not been clear how expression of this receptor is regulated. However, in the late 1970s, it was noted that there was an excellent correlation between the density of FcεRI on circulating basophils (measured as total IgE density after saturation of the cells with IgE) and the serum IgE titer.1 At that time, two hypotheses were advanced to explain the correlation, one of which stated that IgE itself regulated FcεRI expression, while the other stated that there was an underlying linkage that determined the relative expression of IgE and FcεRI. Overnight culturing of basophils with or without IgE antibody did not show any change in FcεRI densities. Because longer cultures were not possible at that time, the question was left unresolved.
More recent experiments noted that unoccupied receptor densities were remarkably constant across the entire 100-fold range of FcεRI densities observed on basophils from a wide range of donors.2 This result suggested that the cell acted to maintain a low level of unoccupied receptors and, taken together with the studies by Malveaux et al,1 further implied that IgE levels determined the relative expression of FcεRI. This was an indirect argument and a more direct experimental protocol was necessary to support the belief that IgE itself regulated FcεRI expression. Most recently, an in vivo test of this hypothesis was made possible by monitoring basophil FcεRI expression in patients receiving humanized monoclonal anti-IgE antibody.3 This antibody resulted in 100-fold reductions in circulating free IgE concentrations and a 15- to 50-fold reduction in the expression of FcεRI followed within weeks, providing further support for the possibility that free IgE levels determine the expression of FcεRI on basophils. However, there were other explanations for the effect observed in vivo and it remained necessary to test the hypothesis using more controlled in vitro studies. Recent studies using mouse bone marrow mast cells4,5 or basophils,6 in vitro and in vivo, indicate that IgE regulates FcεRI expression in this species. Human basophils can now be cultured reasonably well for relatively long periods in the presence of interleukin-3 (IL-3).7 Thus, we examined the expression of FcεRIα on basophils cultured in the presence or absence of IgE for extended periods. These studies demonstrate that IgE itself does appear to upregulate the expression of FcεRIα on human basophils.
MATERIALS AND METHODS
Buffers.
Piperazine-N,N-bis-2-ethanesulfonic acid (PIPES; Sigma Chemical, St Louis, MO) was used as stock buffer; 25 mmol/L PIPES containing 110 mmol/L NaCl, 5 mmol/L KCl, and 40 mmol/L NaOH was adjusted to pH 7.3 and stored at 10 times the above concentration. PAG consisted of PIPES (1X) containing 0.003% human serum albumin (HSA; Miles Laboratories, Elkhart, IN) and 0.1% glucose; PAGCM was PAG with 1.0 mmol/L CaCl2 and 1.0 mmol/L MgCl2; PAG-EDTA was PAG with 1.0 mmol/L EDTA. Acetate elution buffer contained 0.05 mmol/L sodium acetate, 0.085 mol/L NaCl, 10 mmol/L EDTA and 0.03% HSA at pH 3.7.2 Borate buffered saline was 0.01 mol/L borate and 0.14 mol/L, NaCl at pH 8.4.
Reagents.
Polyclonal goat antihuman IgE was prepared as described previously8; the antibody used for these studies represented the IgG fraction of goat serum prepared by DE-52 chromatography. Penicillin (benzylpenicilloyl [BPO])-specific IgE was partially purified from the serum of a penicillin-allergic patient by affinity methods previously described in detail.9 It is a combination of both IgG and IgE specific for penicillin, and more than 95% of the IgE is specific for the penicillin hapten. BPO(11)-HSA was prepared as described previously.9 Purified IgE-PS myeloma was a gift from Dr T. Ishizaka.10 Anti-nitrophenyl (NP) IgE was obtained from Dr Robert Hamilton (Johns Hopkins) and anti-gp120 chimeric IgE obtained from Tanox Biosystems (Houston, TX) was prepared by methods previously described.11 CGP51901 was obtained from Tanox Biosystems and its preparation and properties have been previously described.12 For most of the experiments the IL-3 used for culture was a gift from Dr Stephen Gillis, formerly of Immunex (Seattle, WA). Later experiments also used IL-3 from Biosource (Camarillo, CA).
PS myeloma was heat-inactivated by treating a 10-mg/mL solution in borate-buffered saline to 56°C for 90 minutes (previous studies have used 30 minutes of heat activation, but this was found to be insufficient). The extent of heat inactivation was tested by assessing the ability of treated or untreated IgE antibody to inhibit histamine release from basophils challenged with covalent dimers of IgE antibody. Dimeric IgE-induced release requires challenge in the presence of deuterium oxide to enhance secretion13 and release is dependent on the presence of unoccupied receptors. Basophils were preincubated with concentrations of IgE antibody (±heat treatment) ranging from 10 ng/mL to 100 μg/mL for 30 minutes and then challenged with 500 ng/mL dimeric IgE (a gift from Dr Henry Metzger) without further washing. Histamine release was measured by automated spectrofluorometry as described previously.
Cell preparation.
Two types of basophil preparations were used. Most of the studies used cells obtained from leukapheresis and were prepared as previously described.14 Basophil purities in these preparations ranged from 15% to 95%, with a median of 33%. In some experiments, cells were isolated from peripheral blood using the double Percoll method.15 The blood was diluted with EDTA-saline and centrifuged at 500g for 15 minutes to obtain a buffy coat. The buffy-coat cells were diluted in saline and layered onto a two-step Percoll gradient: 1.065 g/mL/1.079 g/mL as described previously.16 After centrifugation at 450g for 15 minutes, the interface between the 1.065 Percoll/plasma upper layer and the 1.079 lower Percoll layer was harvested and washed as described earlier (basophil purities, 8% to 45%). Which cell preparations were used will be noted in the text.
Cell culture.
Enriched basophil preparations were cultured in Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Gaithersburg, MD) containing 2% fetal calf serum (FCS), 40 μg/mL gentamycin, and 10 ng/mL IL-3. The total cell density was 2 × 106/mL and culturing was performed in 96-, 24-, or six-well tissue culture-treated plates (Costar, Cornell, NY).
Unoccupied receptor densities.
In a few experiments, the cells were sensitized with BPO-specific IgE before its elution to determine unoccupied receptor densities. Briefly, unoccupied receptors were saturated with BPO-specific IgE, the cells counted, the IgE eluted with a acetate elution buffer, and the IgE quantified in a BPO-specific IgE radio allergo sorbant test (RAST).2 The combination of cell counts and the amount of IgE eluted allows a calculation of the unoccupied receptor density.2 A similar procedure is used to determine endogenous IgE density except that a total IgE radio immuno sorbant test (RIST) is used to measure the eluted IgE antibody.2From the rate that unoccupied receptors were loaded during sensitization, the on-rate constant (kon) was estimated. Short preincubations with high concentrations of IgE or longer incubations with lower concentrations of IgE were used to estimate this value. A similar protocol was used to determine the degree of FcεRI saturation following various incubations with PS myeloma IgE. Basophils that were incubated with PS myeloma IgE were then tested for the presence of remaining unoccupied receptors by loading the remaining receptors with BPO-specific IgE and eluting as described earlier. Basophils that had not been incubated with IgE myeloma provided a measure of the starting unoccupied receptor densities.
Western blotting.
High-speed cell pellets (≈14,000g for 5 to 10 seconds) were resuspended at 3 × 107 cells/mL in lysis buffer (50 mmol/L Tris-HCl, pH 7.5, 5 mmol/L EDTA, 10 mmol/L EGTA, 5 mmol/L dithiothreitol, 1% nonidet P-40, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 20 μg/mL leupeptin, 100 μg/mL aprotinine, and 10 mmol/L benzamidine). After 20 seconds of vortexing, the cell lysates were kept on ice for 20 minutes, and microfuged for 15 minutes at 4°C. Supernatant was collected as a protein extract that contained lysed cell components without nuclei.17 Previous studies have established that lysing basophils or contaminating cells with these buffers generates equivalent protein levels. Protein levels in the extracts from different days may have been different, as this was not measured, but normalizing with respect to cell number was the information desired, rather than protein levels, which might change during culture in IL-3. Extracts that contained equal basophil cell numbers (3 × 105 cell equivalents per lane) were diluted with an equal volume of 2× loading buffer (0.125 mol/L Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate [SDS], 0.005% bromophenol blue, 20% glycerol; NOVEX, San Diego, CA) containing 0.05% β-mercaptoethanol, and subjected to 4% to 20% Tris glycine density gradient gel electrophoresis (NOVEX). Gels were then transferred to pure nitrocellulose membranes (Schleicher & Schuell, Keene, NH) by Trans Blot (NOVEX).
Electrophoresis and transfers were performed according to the manufacturer's recommendations. After transfer, membranes were immersed in Tris-buffered saline with Tween 20 (TBST) that contained 5% nonfat dry skim milk (Carnation, Los Angeles, CA) overnight to block nonspecific binding. Membranes were then washed three times for 5 minutes with TBST. Immunoreactive proteins were detected using the 22E7 antibody diluted to 5 μg/mL in TBST containing 1% skim milk. After a 4-hour incubation, membranes were washed with TBST and incubated with peroxidase-labeled sheep antimouse Ig antibody (Amersham, Arlington Heights, IL) for 1 hour. After five 10-minute washes, membrane-bound antimouse Ig antibody was visualized by enhanced chemiluminescence (ECL) Western blotting detection reagents (Pierce, Rockford, IL), and Hyper-ECL luminescence detection film (Amersham). The ECL films were converted to digital format with a UVP (Upland, CA) digital camera and the images analyzed with NIH Image (Wayne Rasband).14 Pilot studies using human basophils (99.8%), mast cells, lymphocytes and monocytes demonstrated a detectable band only in the basophil preparations, an extremely weak band in the mast cells, which generally have low receptor densities,18 and no bands for the lymphocytes or monocytes.
Flow cytometry.
A flow-cytometric technique incorporating light-scatter characteristics was used to quantify cell-surface IgE and FcεRIα chain expression on basophils as described.19 Cell-surface IgE was detected using a monoclonal antihuman IgE (TES-19 or E10-10-3; Tanox Biosystems). Cell-surface expression of FcεRIα chain was detected using a mouse IgG1 antihuman FcεRIα chain monoclonal antibody (22E7; generously provided by J. Kochan, Roche Pharmaceuticals, Nutley, NJ20) and was compared to labeling with an identical concentration of irrelevant mouse IgG1 (Coulter, Hialeah, FL). The 22E7 antibody has been shown to recognize an epitope that is unaffected by FcεRIα occupancy.20 In several experiments, the monoclonal antibody 15A5 was also used. This antibody binds only to unoccupied FcεRIα.20 Aliquots of cells were labeled in phosphate-buffered saline that contained 0.2% HSA with 1 mg/mL human IgG to minimize nonspecific binding to FcγR.19 Each of the monoclonal antibodies was used at concentrations predetermined to be optimal for labeling. Binding of monoclonal antibodies was detected using saturating concentrations of R-phycoerythrin (PE) conjugated polyclonal goat antimouse IgG (Tago, Burlingame, CA). An EPICS Profile flow cytometer (Coulter) was used to analyze fluorescent signals after excitation at 488 nm. Bitmap gates, intermediate between the forward- and side-scatter characteristics of lymphocytes and monocytes, were used to select for a population of cells that were predominantly basophils. Since the cells were already enriched in basophils, these bitmaps can select a population of cells that is generally greater than 80% basophils, with the primary contaminants being lymphocytes. Data are expressed as the mean fluorescence in labeled cells minus the mean fluorescence of IgG1 controls. Day-to-day variability in the sensitivity of the flow cytometer was corrected by noting or adjusting the photomultiplier tube voltage to generate the same signal for a set of standard calibration beads (Immunochek; Coulter).
The cells used to study basophil survival and apoptosis were obtained by venipuncture and two-step Percoll gradient enrichent. When annexin-V labeling was used, the cell cultures were harvested, centrifuged once, and resuspended in buffer that contained anti-IgE antibody, TES-19. Following a 10-minute incubation, the cells were centrifuged once and resuspended for annexin-V binding according to the manufacturer's directions (ApoAlert; Clonetech, Palo Alto, CA), with the exception that antimouse PE was included in the buffer. Following an additional 10-minute incubation, the cells were not subjected to further washing, but directly examined by flow cytometry. Forward- and side-scatter bitmaps were used to select the approximate population containing basophils, and these cells were further gated for annexin-V binding by selecting those that were stained with anti-IgE antibody.
The flow-cytometric measurements were calibrated by examining the fluorescence staining of six donors' basophils that spanned a moderate range of staining intensities (7 to 120 fluorescent units or 8,000 to 140,000 FcεRI per basophil) and simultaneously assessing receptor or IgE density by the acetate elution method described earlier. 22E7 staining (ordinate) compared with total FcεRI density by acetate elution (after sensitizing with PS myeloma IgE as described earlier) was linear with a slope of 0.00084 (ie, a fluorescence measurement of 100 represents approximately 120,000 receptors) with R = .963. Likewise, total IgE density versus fluorescent staining with E10-10-3 anti-IgE antibody (ordinate) was linear with a slope of 0.00145 andR = .992.
Statistics.
Student's t-test was used for most statistical comparisons, while others were made with a nonparametric Wilcoxon signed-rank statistic or analysis of variance (ANOVA). If errors or error bars are shown, they represent the standard error of the mean unless otherwise indicated.
RESULTS
Downregulation of FcεRIα.
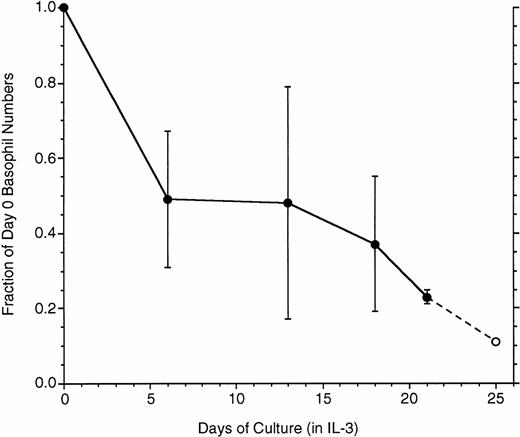
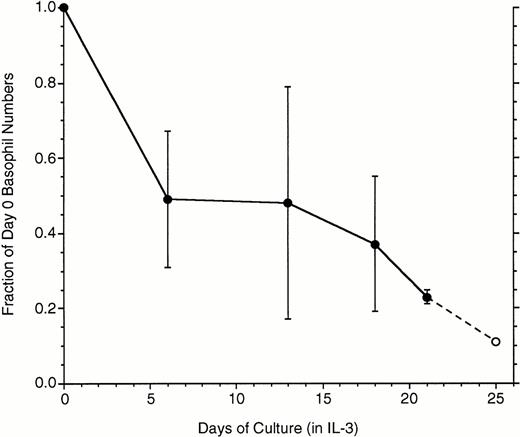
These studies required long incubations of basophils, so the survivability of basophils in these relatively long cultures (mature basophils have not been observed to proliferate) was assessed. Figure1 shows data derived from basophils isolated from whole blood and enriched by two-step Percoll gradients. Substantial variability was observed among basophil preparations during the first 2 weeks, although there was a consistent 75% loss by 3 weeks. These cells were also examined for apoptotic changes as assessed by the binding of annexin V. In these experiments, cells carefully harvested from culture (see Methods) were colabeled with anti-IgE antibody/antimouse-PE and fluorescein isothiocyanate (FITC) annexin V. For flow cytometry, cells were gated according to both forward- and side-scatter characteristics and labeling with anti-IgE antibody. It was subsequently found that about the time a small fraction of the basophils acquire a moderate labeling with FITC-annexin V, these same basophils fall into a lower region of the forward-side-scatter profile. This process occurred throughout the 3-week period, which indicates that the relatively high concentration of 10 ng/mL of IL-3 was not able to prevent basophils from entering apoptosis over this time frame. Attempts to culture enriched basophils in 20% autologous serum did not improve survival (data not shown). For the experiments that follow, the forward-side-scatter gates were set to exclude the region most associated with these apoptotic basophils.
Loss of basophils during culture in IL-3 (n = 7, except the last data point, ○, where 2 experiments were extended to 25 days). Data are plotted as the fraction of the starting basophil number, means ± SD.
Loss of basophils during culture in IL-3 (n = 7, except the last data point, ○, where 2 experiments were extended to 25 days). Data are plotted as the fraction of the starting basophil number, means ± SD.
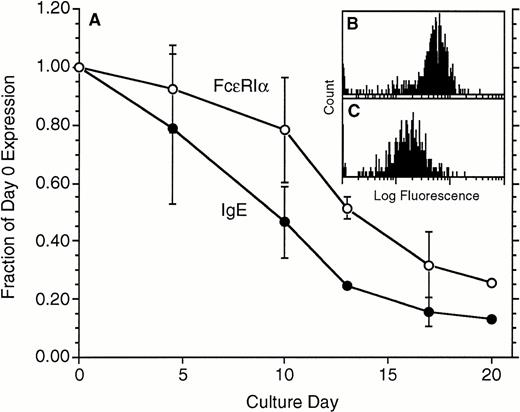
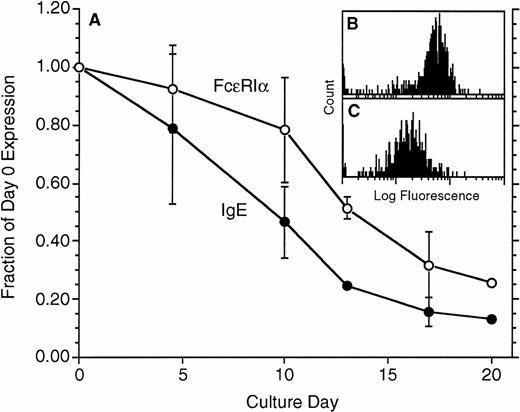
In the in vivo studies of anti-IgE treatment, the treatment led to a marked decrease in FcεRIα expression. To examine whether the same decrease would occur in vitro, basophils were cultured in the absence of IgE in the media for 14 to 21 days and were tested for FcεRIα expression using the FcεRIα-specific monoclonal antibody 22E7 and for cell-surface IgE using a mouse monoclonal anti-IgE antibody, TES-19 or E10-10-3. The data shown in Fig 2 were derived from basophils that were obtained by the double Percoll method and represent partially enriched basophil preparations ranging from 5% to 40% in purity. Figure 2 shows that basophil-bound IgE declined, with the 50% reduction point occurring only after approximately 8 days. There was a lag in the decrease in FcεRIα so that the 50% point was reached only after 13 days. Similar results have been observed in preparations of basophils obtained from leukapheresis packs, which range in purity from 15% to 95% (data not shown). While FcεRIα expression decreased, the distributions remained unimodal (one example from these four experiments, with measurements made on day 0 and day 17, is shown in Fig 2B and C, respectively).
Downregulation of both IgE and FcRIα during culture in the absence of IgE in the culture medium (n = 4). Both parameters were measured by flow cytometry, IgE with TES-19 anti-IgE monoclonal antibody (•) and FcRIα (○) with monoclonal antibody 22E7. On average, the starting levels of expression for FcRIα were 110 ± 25 flow fluorescence units, equivalent to ≈130,000 FcRIα per cell (see Materials and Methods). Data are expressed as a fraction of the day 0 level of expression. (B and C) Representative flow-cytometric profiles for 1 of the experiments; (B) day 0 distribution (mean fluorescence, 63), and (C) day 17 distribution (mean = 17) using 22E7 to detect FcRIα.
Downregulation of both IgE and FcRIα during culture in the absence of IgE in the culture medium (n = 4). Both parameters were measured by flow cytometry, IgE with TES-19 anti-IgE monoclonal antibody (•) and FcRIα (○) with monoclonal antibody 22E7. On average, the starting levels of expression for FcRIα were 110 ± 25 flow fluorescence units, equivalent to ≈130,000 FcRIα per cell (see Materials and Methods). Data are expressed as a fraction of the day 0 level of expression. (B and C) Representative flow-cytometric profiles for 1 of the experiments; (B) day 0 distribution (mean fluorescence, 63), and (C) day 17 distribution (mean = 17) using 22E7 to detect FcRIα.
The slow rate of dissociation of IgE from the basophils in these cultures was not predicted. Published accounts of the affinity of human IgE for FcεRI would predict 50% dissociation occurring in the first 12 to 48 hours.21-23 The inclusion of an anti-IgE antibody to capture dissociating IgE should eliminate the various ways that IgE may rebind. In a short series of experiments (Table1), enriched basophils were cultured without IgE and with or without CGP51901, a chimeric monoclonal antibody that binds to IgE on an epitope hidden when IgE is bound to the FcεRIα subunit. Therefore, this antibody does not bind to IgE bound to FcεRIα, but does bind to dissociated IgE with an affinity high enough12 to minimize dissociated IgE rebinding to FcεRIα. Table 1 lists the results from three experiments in which the loss of cell-surface IgE on basophils was monitored during culture for several days. The test conditions were cultured with or without CGP 51901, which was tested at either 10 or 200 μg/mL. On theoretical grounds,24 25 both concentrations of antibody should adequately remove any dissociating IgE; however, the higher concentration was included to provide a vast excess of antibody. These data indicate that inclusion of CGP51901 had no effect on the rate of IgE dissociation, which suggests that the observed rate is a true reflection of the high affinity of the receptor for IgE under these particular conditions.
In the first 10 days of culture, there is a significant loss in cell-surface IgE expression that is not accompanied by as great a loss in the receptor. The implication is that there should be a larger number of unoccupied FcεRI than found at the start of the culture. This interpretation was first supported by examining the ratio of fluorescence obtained by labeling with 22E7 versus TES-19 antibodies at various times during culture. For most donors, the ratio of total receptors to total IgE is close to 1.0 when freshly isolated,2 so the ratio of fluorescent intensities following labeling with these two primary antibodies under our standard conditions of flow cytometry is relatively constant, 0.64 ± 0.05 (22E7/TES-19) (n = 6 for the following comparisons). After 10 days of culture, the ratio becomes 1.29 ± 0.14 (different from the starting ratio, P < .01), which indicates significantly more receptors than IgE (and therefore more unoccupied receptors). If these day 10 cells were cultured with 10 μg/mL of IgE for 20 minutes or for 1 day, the ratio returned to 0.54 ± 0.12 or 0.69 ± 0.12, respectively. These indirect results were verified by examining the cells at various times during the first 2 weeks of culture with the monoclonal antibody 15A5, which binds only to unoccupied FcεRIα. 15A5 binding steadily increased and stabilized by 10 to 14 days at a value 585% of preculture levels (n = 2, data not shown). A second method used the acetate elution method of measuring unoccupied receptors. This method counts unoccupied receptors by first filling the receptors with a BPO-specific IgE, eluting the IgE, and measuring the eluted IgE in a BPO-RAST. With cell counts available, the absolute unoccupied receptor density can be calculated. In the one experiment performed this way, unoccupied receptors started at 6,300 ± 900 and increased to 26,000 ± 3,000 by day 11.
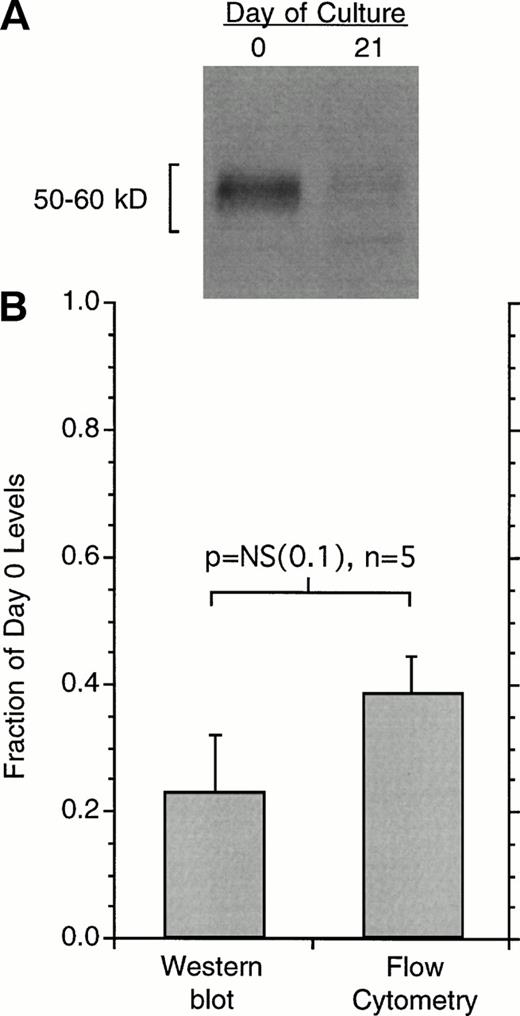
Modest IgE antibody-dependent FcεRI downregulation and upregulation has been observed in rat basophilic leukemia (RBL) cells.26For the RBL cell, IgE appears to stabilize the cell-surface expression of FcεRI by preventing its endocytosis. We therefore tested for the presence of FcεRIα in human basophils following its downregulation in culture by lysing the cells for Western blotting. Figure 3 shows the results for 21-day cultures. As found previously, the average expression of FcεRIα as determined by flow cytometry was 30% to 40% of day 0 levels; the Western blotting data reflected a similar loss, not statistically different than the data for flow cytometry.
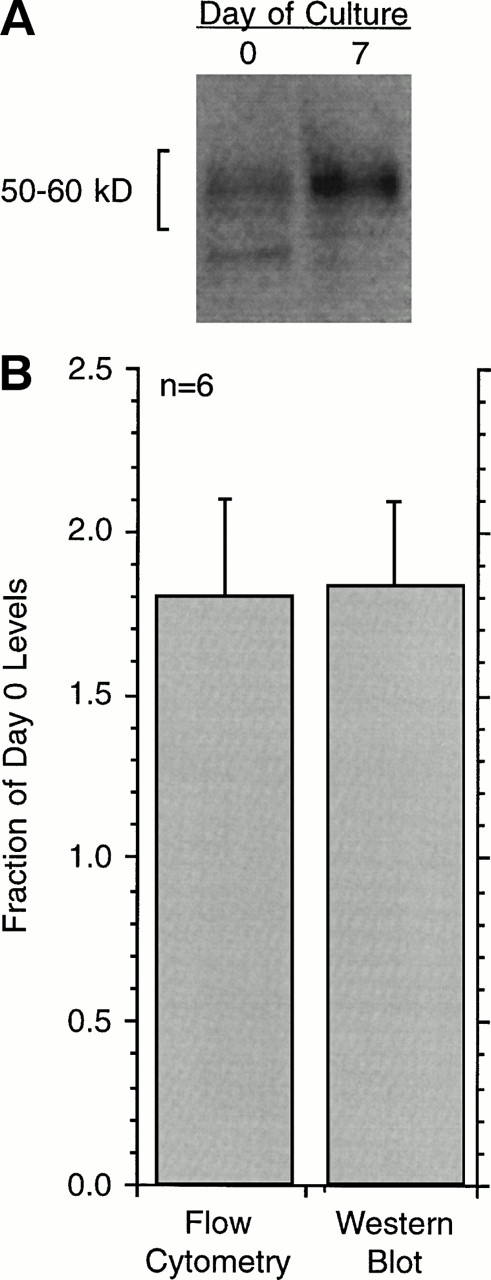
Changes in the cell-surface expression of FcRIα as measured by 22E7 binding or changes in total cell FcRIα as measured by Western blotting. (A) An example of the Western blot data for cells at day 0 or day 21 after culture. The number of total cells is held constant and the basophil purity was found to be the same for the 2 time points, averaging 41% ± 13% for the 5 experiments. (B) Average data for flow-cytometric measurements or Western blotting in terms of day 0 levels (n = 5) Western blot films were digitally imaged to determine optical densities of the bands. The difference in relative change as measured by flow cytometry or Western blotting was not statistically significant, while both were significantly less than day 0 (P < .001).
Changes in the cell-surface expression of FcRIα as measured by 22E7 binding or changes in total cell FcRIα as measured by Western blotting. (A) An example of the Western blot data for cells at day 0 or day 21 after culture. The number of total cells is held constant and the basophil purity was found to be the same for the 2 time points, averaging 41% ± 13% for the 5 experiments. (B) Average data for flow-cytometric measurements or Western blotting in terms of day 0 levels (n = 5) Western blot films were digitally imaged to determine optical densities of the bands. The difference in relative change as measured by flow cytometry or Western blotting was not statistically significant, while both were significantly less than day 0 (P < .001).
Upregulation of FcεRIα.
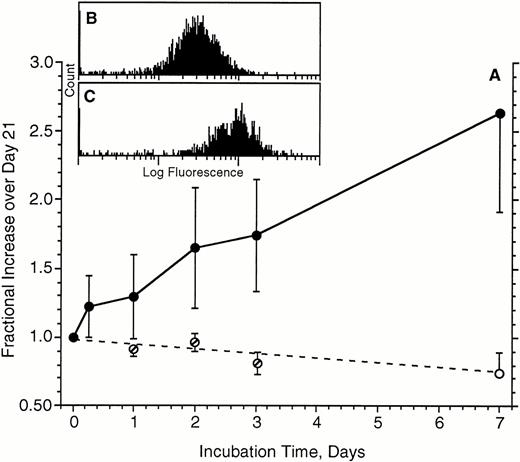
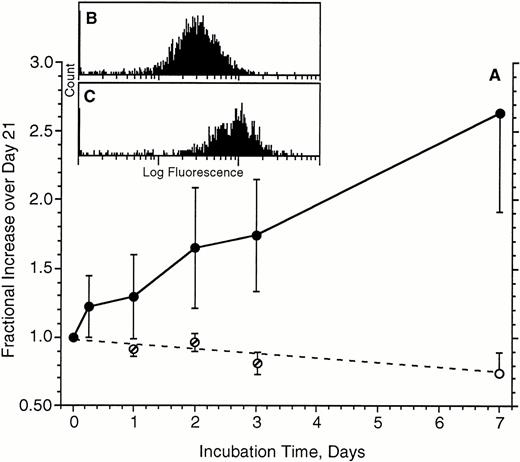
The remaining experiments focused on the upregulation of FcεRIα expression. One implication of our recent in vivo studies is that FcεRI expression is markedly upregulated during the life span of a basophil. Unpublished experiments in the early 1980s had shown that no significant upregulation of FcεRI occurred in a 24-hour culture. We anticipated that upregulation induced by IgE would be more observable if the starting receptor density was moderately low at the onset of a culture with IgE antibody. Therefore, for many preliminary experiments testing this hypothesis, the cells were first cultured for approximately 21 days to downregulate FcεRI. Figure4 shows the kinetics of upregulation in the presence (or absence) of 500 ng/mL of PS myeloma IgE in these cells that had been first cultured for 21 days. The upregulation is relatively linear for the 7-day period examined and averages an increase of 2.6 ± 0.3-fold by 7 days. The flow-cytometric distributions for FcεRIα expression remained unimodal throughout the experiments (an example from one of these five experiments is shown in Fig 4B and C for day 0 and day 7, respectively). Note that in the absence of IgE, FcεRIα expression continued to fall beyond the point labeled day 0, which is actually day 21 after the isolation of the cells (for cells cultured in the absence of IgE, the day 1, 2, and 3 measurements were derived from only three of the five experiments). In two of these experiments, the incubation in the presence of IgE was performed for 2 weeks. However, no further upregulation beyond the 1-week values was found (data not shown), although it was noted that only 5% to 10% of the basophils survived into the fifth week.
Upregulation of FcRIα expression on basophils during a 7-day culture in the absence (○) or presence (•) of 500 ng/mL of IgE (n = 5). Cells used for these experiments had already been cultured for 21 days to downregulate FcRIα. Average starting level of expression was 37 ± 13, which translates to approximately 45,000 FcRIα per basophil (see Materials and Methods). Data are expressed as the increase relative to the day 21 levels of expression (the start of the upregulation phase of the cultures). For cells cultured in the absence of IgE, measurements were made on days 1, 2, and 3 for only three of five experiments (∅); day seven measurements were made for all five experiments. (B and C) Representative flow-cytometric profiles for 1 of the experiments; (B) starting distribution (mean fluorescence, 27), (C) distribution (mean, 86) after 7 days of culture with IgE.
Upregulation of FcRIα expression on basophils during a 7-day culture in the absence (○) or presence (•) of 500 ng/mL of IgE (n = 5). Cells used for these experiments had already been cultured for 21 days to downregulate FcRIα. Average starting level of expression was 37 ± 13, which translates to approximately 45,000 FcRIα per basophil (see Materials and Methods). Data are expressed as the increase relative to the day 21 levels of expression (the start of the upregulation phase of the cultures). For cells cultured in the absence of IgE, measurements were made on days 1, 2, and 3 for only three of five experiments (∅); day seven measurements were made for all five experiments. (B and C) Representative flow-cytometric profiles for 1 of the experiments; (B) starting distribution (mean fluorescence, 27), (C) distribution (mean, 86) after 7 days of culture with IgE.
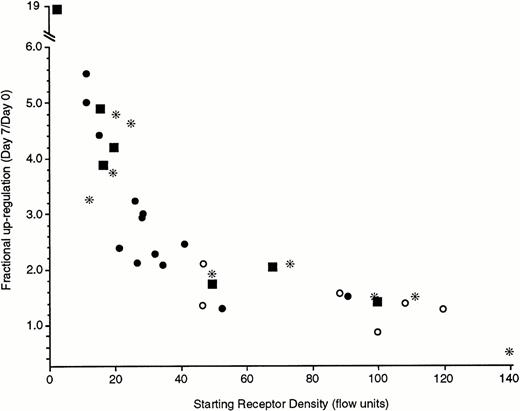
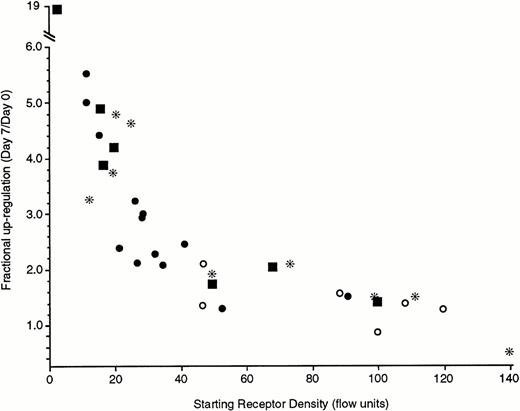
The magnitude of the upregulation was not as marked as might be anticipated. Based on in vivo studies, receptor densities could cover a 50-fold range. It seemed likely that the higher the starting density, the smaller the subsequent fold-increase (see below and Discussion). Figure 5 shows results compatible with this perspective. The data in Fig 5 are derived from a number of sources. Two types of preparations were studied using cells from leukapheresis packs: cells obtained from leukocyte packs that had been first downregulated by 21-day culture or leukapheresis pack cells in which IgE was added to the cultures immediately after isolation. Likewise, cells obtained by standard venipuncture techniques and double Percoll isolation were also used immediately for upregulation or upregulated after a 21-day culture period. The upregulation was clearly inversely related to the starting level of FcεRIα expression. With starting levels below 20 fluorescence units (which we have calibrated to be equivalent to 20 to 25,000 receptors per cell, see Materials and Methods), there is greater than fivefold upregulation over 7 days. In one extreme case of a very low starting density, a nearly 20-fold increase was observed. In six experiments in which the cells were immediately placed into culture for upregulation, a 2-week measurement was also made. In all cases (starting levels of 21 to 98 fluorescence units), the 2-week levels of FcεRIα expression were higher than the 1-week levels (1.6-fold to 2.6-fold), and in two experiments in which the starting levels averaged a moderately low 22 fluorescence units, the 1- and 2-week increases averaged 2.6- to 4.8-fold, respectively. It should also be noted that the IgE concentration in these cultures did not appreciably decrease during culture. IgE concentrations after 1 week of culture were 0.96 ± 0.05 of the starting concentration, as determined in a RIST. Furthermore, the ability of this 1-week-old IgE to sensitize basophils remained intact. This was tested by culturing a large number of basophils in the absence of IgE, as well as by culturing a smaller number of the same cells in the presence of IgE. After 1 week, the culture supernatant that contained IgE was recovered and used to make serial dilutions of IgE, which were used to sensitize the cells that had not been cultured with IgE. The dose-response curve for sensitization of the later cells with the “old” IgE was examined by testing the cells after overnight culture for an ability to further bind BPO-specific IgE. The titration curves of old versus fresh IgE at the same concentrations were the same. Taken together, these data indicate that there was no functional loss of the IgE that is present in 1-week cultures.
Upregulation of FcRIα of different types of basophil preparations under the influence of 500 ng/mL of PS myeloma IgE during a 7-day culture. The four symbols represent different methods to obtain basophils; (*) basophils obtained from leukapheresis packs and incubated for 21 days before addition of IgE, (○) basophils obtained from leukapheresis packs but cultured for 7 days without the downregulation step of 21-day culture, (•) basophils obtained from standard venipuncture and double Percoll gradient separation and incubated for 21 days before addition of IgE, (▪) basophils obtained from standard venipuncture and double Percoll gradient separation but cultured for 7 days without the downregulation step of a 21-day culture. Each symbol represents the results from a single donor. The starting receptor density (x-axis) is the 22E7 fluorescence by flow cytometry and the fractional upregulation (y-axis) is the ratio of day 7 fluorescence to day 0 fluorescence.
Upregulation of FcRIα of different types of basophil preparations under the influence of 500 ng/mL of PS myeloma IgE during a 7-day culture. The four symbols represent different methods to obtain basophils; (*) basophils obtained from leukapheresis packs and incubated for 21 days before addition of IgE, (○) basophils obtained from leukapheresis packs but cultured for 7 days without the downregulation step of 21-day culture, (•) basophils obtained from standard venipuncture and double Percoll gradient separation and incubated for 21 days before addition of IgE, (▪) basophils obtained from standard venipuncture and double Percoll gradient separation but cultured for 7 days without the downregulation step of a 21-day culture. Each symbol represents the results from a single donor. The starting receptor density (x-axis) is the 22E7 fluorescence by flow cytometry and the fractional upregulation (y-axis) is the ratio of day 7 fluorescence to day 0 fluorescence.
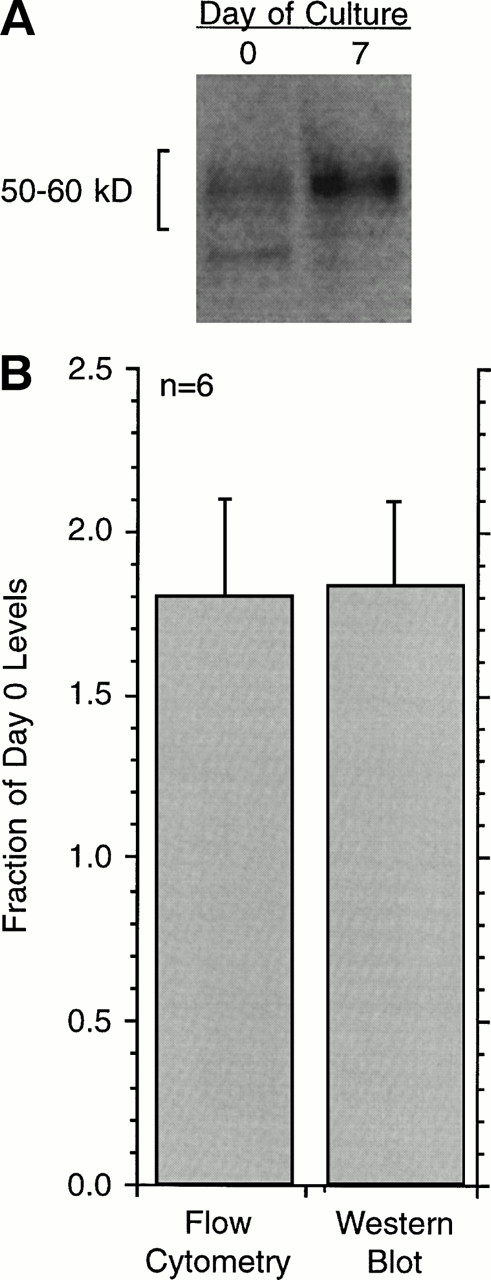
With the more typically modest upregulation, it appeared possible that upregulation only involved the cell-surface expression of cryptic receptors.26 In such a situation, lysis of the cells and analysis by Western blots should show no change in the total cellular mass of FcεRIα during the period of upregulation. However, this was not the case. As shown in Fig 6, following a 1-week culture in the presence of IgE, there was a statistically significant increase (P = .01) in the mass of FcεRIα: approximately twofold in the experiments shown. Likewise, the cell-surface expression increased approximately twofold in these particular experiments.
Upregulation of FcRIα as determined by either flow cytometry or Western blotting of lysed cells (n = 6). (A) Example of Western blot data for cells at day 0 or day 7 after culture in the presence of 500 ng/mL of PS myeloma IgE; day 0 is the day that IgE was added to the cultures, which was generally after a 21-day culture to downregulate the receptors (basophils were obtained from leukapheresis packs). The number of total cells was held constant and the basophil purity was found to be the same for the two time points, averaging 45% ± 5% for the six experiments. (B) Average data for flow-cytometric measurements or Western blotting in terms of day 0 levels (n = 6) Western blot films were digitally imaged to determine the optical densities of the bands. The difference in the relative change as measured by flow cytometry or Western blotting was not statistically significant, but both measurements for day 7 were statistically different than day 0 (P = .022 and P = .010, respectively). Average starting fluorescence of the basophils was 52 ± 15 on day 0, corresponding to ≈60,000 receptors per basophil (see Materials and Methods).
Upregulation of FcRIα as determined by either flow cytometry or Western blotting of lysed cells (n = 6). (A) Example of Western blot data for cells at day 0 or day 7 after culture in the presence of 500 ng/mL of PS myeloma IgE; day 0 is the day that IgE was added to the cultures, which was generally after a 21-day culture to downregulate the receptors (basophils were obtained from leukapheresis packs). The number of total cells was held constant and the basophil purity was found to be the same for the two time points, averaging 45% ± 5% for the six experiments. (B) Average data for flow-cytometric measurements or Western blotting in terms of day 0 levels (n = 6) Western blot films were digitally imaged to determine the optical densities of the bands. The difference in the relative change as measured by flow cytometry or Western blotting was not statistically significant, but both measurements for day 7 were statistically different than day 0 (P = .022 and P = .010, respectively). Average starting fluorescence of the basophils was 52 ± 15 on day 0, corresponding to ≈60,000 receptors per basophil (see Materials and Methods).
The ability of IgE to upregulate FcεRIα was not limited to PS myeloma; upregulation occurred with other IgE antibodies, an anti-NP IgE and an anti-gp120 IgE. IgG at similar concentrations or up to 1 mg/mL caused no upregulation of FcεRIα nor did it inhibit the upregulation by IgE (Table 2). It is possible that aggregates of IgE are required to observe upregulation; however, we have found that covalently crosslinked dimers of IgE antibody did not induce upregulation of FcεRI (however, these dimers induced histamine release when basophils were challenged in the presence of deuterium oxide, as previously found,27 see Materials and Methods). A combination of dimeric IgE and monomeric IgE resulted in less upregulation than monomeric IgE alone (Table 2). Treating the cells with a crosslinking goat polyclonal anti-IgE antibody (0.1 μg/mL) caused downregulation of the receptor. In two experiments, basophils were cultured in the presence or absence of anti-IgE antibody for 3 days. FcεRI expression remained constant for cells cultured without anti-IgE, while FcεRI decreased to 62% and 42% of day 0 levels for the two experiments for cells cultured with anti-IgE antibody. Last, in two experiments, basophils of 90% and 97% purity demonstrated upregulation that was no different that from cells of lower purity with similar starting levels of FcεRIα expression (Table 2).
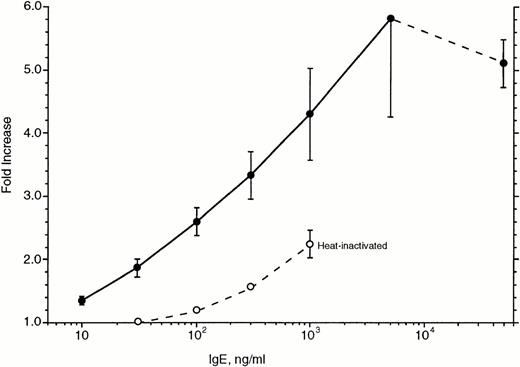
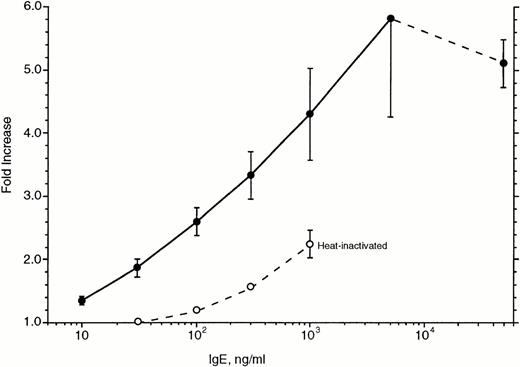
Figure 7 shows the concentration dependence of this upregulation. Basophils were cultured for 7 days in the presence of various concentrations of IgE and again assayed for the expression of FcεRIα. Surprisingly, maximal upregulation appeared to require IgE concentrations greater than 5 μg/mL; the difference between 500 and 5,000 ng/mL is statistically significant. In two experiments, we tested 50 μg/mL of IgE and found small decreases (12%) in the level of upregulation compared with that observed for 5 μg/mL. Based on the data for these six experiments and the apparent saturation at approximately 5 μg/mL, the effective concentration50(EC50) for upregulation averaged 230 ng/mL.
Concentration dependence of IgE-induced upregulation of FcRIα in basophils cultured for 7 days (n = 6). The abscissa is expressed as the ratio of 22E7 fluorescence for cells incubated with IgE at the various concentrations to cells not incubated with IgE. (•) Data for PS myeloma; (○) data for heat-inactivated PS myeloma. The average starting fluorescence of the basophils was 25 ± 4, corresponding to ≈30,000 receptors per basophil (see Materials and Methods). Basophils were obtained from leukapheresis packs and first downregulated by a 21-day culture.
Concentration dependence of IgE-induced upregulation of FcRIα in basophils cultured for 7 days (n = 6). The abscissa is expressed as the ratio of 22E7 fluorescence for cells incubated with IgE at the various concentrations to cells not incubated with IgE. (•) Data for PS myeloma; (○) data for heat-inactivated PS myeloma. The average starting fluorescence of the basophils was 25 ± 4, corresponding to ≈30,000 receptors per basophil (see Materials and Methods). Basophils were obtained from leukapheresis packs and first downregulated by a 21-day culture.
Heat-inactivated PS myeloma was examined for its ability to upregulate FcεRIα in 1-week cultures. The heat-inactivated IgE was tested in the dose-response curve experiments shown in Fig 7. In these experiments, heat-inactivated IgE was 20-fold less potent than the untreated IgE. It should be noted that if the heat-inactivated IgE was used to inhibit the ability of dimeric IgE to induce histamine release in basophils (see Materials and Methods), it was found to be approximately 100-fold less potent than untreated IgE.
In the same context, the importance of the FcεRIα binding domain of IgE (and the region surrounding this part of IgE) was examined by testing the effects of CGP51901 on upregulation. As noted earlier, CGP51901 binds to this epitope of IgE, inhibiting its ability to bind to FcεRIα and CD23. It is not known to bind to IgE already bound to FcεRIα. Basophil preparations were treated with or without IgE and with or without CGP51901 at a 20-fold excess concentration (10 μg/mL). Table 3 shows that, by itself, CGP51901 had no effect on the downregulation of FcεRIα, but did inhibit the IgE-mediated upregulation (ANOVA, P = .0001, withP < .001 for columns 2, 3, or 5 different from column 4, the treatment with IgE alone). Upregulation was modest in these experiments, because fresh cells were studied with moderately high starting FcεRIα expression.
DISCUSSION
These studies show a change in the expression of FcεRIα on human basophils when IgE antibody is excluded or included in the media used for culturing. Both downregulation and upregulation of the receptor under these culture conditions is relatively slow and appear to result from the loss or gain of FcεRIα mass. Both of these observations occur with relatively high-purity basophils (up to 97%). If a cell other than the basophil is involved in the upregulation process, very low numbers are required. IgG antibody is unable to effect an increase nor does it inhibit the upregulation by IgE antibody. Therefore, it seems unlikely that there is interaction of IgE through the IgG receptor on basophils (CD32, FcγRII). Aggregation of IgE does not appear to be necessary for upregulation, as polyclonal anti-IgE induces modest downregulation over a period of days and dimeric IgE does not appear to cause either upregulation or downregulation. Further, dimeric IgE mixed with monomeric IgE results in less upregulation than monomeric IgE alone. Taken together, these data suggest that monomeric IgE interacts with a receptor on basophils to induce upregulation of FcεRI. It is not clear why dimeric IgE does not induce some upregulation, but there may be a balance of downregulation, as a consequence of aggregation, with the upregulatory effects of monomeric binding.
Ig-dependent expression of other Fc receptors is a common theme in the immune system. In particular, IgE is known to modulate the expression of CD23 (FcεRII) on B cells.28,29 Likewise, IgA and IgG have been found to regulate the expression of their respective receptors.30-32 In the case of CD23, IgE appears to stabilize the cell-surface expression of FcεRII by preventing its proteolytic cleavage from the cell surface.29 Indeed, the lifetime of a CD23 molecule on the cell surface appears short if IgE is not bound. Unlike the CD23/lymphocyte model, the model in RBL cells appears to depend on endocytosis of FcεRIα when IgE is not bound and it appears that FcεRI is recycled so that there remains an internal pool that is constantly cycling to the surface.26The human basophil does not appear to strictly follow this model, since the model would predict no loss in the mass of FcεRIα during the loss of cell-surface expression, while in our experiments, the mass of FcεRIα was lost during the period of downregulation. However, we cannot yet distinguish between loss from the cell surface to the medium and loss by endocytosis followed by degradation. In the RBL cell, the upregulation is limited to the stabilized appearance of presynthesized FcεRI and this limits the level of upregulation to twofold to threefold. These numbers are similar to those in a typical basophil experiment and might also suggest that a similar mechanism plays a role in human basophils. However, we can also observe an increase in the mass of FcεRIα during upregulation, which did not occur in the RBL cell studies.
Our in vivo studies reported downregulation of FcεRI that averaged 26-fold, with a range from fivefold to 50-fold. The range of FcεRI receptor densities among the general population is approximately 100-fold, although those at the low end are relatively rare. Thus, one might expect at least 10-fold changes in FcεRI expression in these culture experiments. The slow loss of basophils in these relatively long cultures and the appearance of apoptotic basophils suggests that the relatively modest levels of upregulation in the presence of exogenous IgE may result from basophils cultured under suboptimal conditions (or the loss of basophils reflecting their natural lifespan, which has been estimated to be up to 2 weeks in vivo). Despite these unfavorable conditions, the increase in FcεRIα in human basophils was not limited to twofold to threefold. The starting density of FcεRI was the strong determinant of the extent of upregulation. In the few cases in which the starting density was as low as we have observed after 3 months of treatment with therapeutic anti-IgE antibody,3 and the cells were cultured with IgE the day that they were first obtained, larger increases in FcεRIα were noted and the process appeared linear for at least 2 weeks. For similar starting densities, both freshly isolated basophils and cells first cultured for 21 days showed similar upregulation, which suggests this effect was not limited to the select small percentage of basophils that survived for 3 to 4 weeks. Taken together, the in vivo studies and the current in vitro studies indicate a far more dynamic range of regulation, which is probably not simple recycling. We have observed eightfold upregulation in bone marrow–derived mast cells cultured with IgE,4 and studies by Yamaguchi et al5 and Lantz et al6 have found that upregulation of FcεRI on mouse mast cells can be greater than 10-fold and is likely to result from the synthesis of new receptors.
From these studies, only a limited conclusion can be drawn regarding the receptor involved in mediating the effects of IgE on FcεRI expression. There are a limited number of known choices; these include FcεRI, FcεRII, CD32,33 or εBP,34 and future studies will address some of these possibilities further (until recently, IgE-dependent histamine-releasing factor would have been included in this list, but it is not currently believed to bind IgE35,36). With respect to IgE interacting with FcεRI, the high concentration-dependence of the upregulation (Fig 7) is difficult to interpret at this time. The interpretation of the data is somewhat dependent on the Ka one chooses to accept for the IgE/FcεRIα interaction. The literature value of approximately 1 × 1010 translates to half-occupancy at approximately 20 ng/mL.21-23,37 Provided our IgE is fully intact and 100% is able to bind to receptor, this value is less than one tenth of our noted EC50 value. This raised the possibility that the PS myeloma being used did not have the expected affinity for FcεRIα. However, as noted earlier, other preparations of IgE resulted in similar data, which suggests the apparently lower affinity was not restricted to one IgE preparation. Although the apparent shift in the concentration dependence (for binding to FcεRI) will need further study, it may reflect the nature of the underlying mechanism for upregulation. Upregulation may be dependent not on equilibrium conditions, which are unlikely to be completely established in these cultures given the low rate of association, but on the binding dynamics. With an association rate constant ranging from 3 × 104 to 1 × 105mol/L−1 · s−1, low concentrations of IgE may require many days to reach the equilibrium point so that if the mechanism is dependent on the stability of an unoccupied versus occupied receptor, this binding dynamic becomes important. The current data do not support a role for CD23. In human lymphocytes, FcεRII affinities for IgE would suggest an EC50 more than 10 μg/mL,38 indicating that the EC50 of 230 ng/mL is a far lower concentration than expected if IgE were interacting with FcεRII. In B lymphocytes, dimeric IgE induced better upregulation of CD23,28 which suggests that if CD23 is involved in upregulating FcεRI in basophils, then dimeric IgE might be a better signal. However, the opposite was true in these studies. Finally, although flow cytometry is relatively insensitive and may not, therefore, provide a proper assessment of low-density receptors, flow-cytometric studies have not detected CD23 on human basophils. The data concerning the effects of IgG do not support a role for CD32. On balance, the data suggest that IgE exerts its influence through FcεRIα itself. Future studies will need to verify this conclusion.
The kinetics of downregulation seemed appropriate for the rate that IgE was found to dissociate from the basophils. However, the slow rate of IgE dissociation was puzzling. The literature for normal human IgE interacting with FcεRI on normal human basophils or mast cells is sparse. A variety of methodologies, some using assumptions invalidated by the current studies, have produced dissociation rates that range from 322 to 24 hours.1,39,40 On a theoretical level, there should be no significant rebinding of IgE to FcεRIα that would explain the slow dissociation in the human basophil cultures described here.24,25 Nevertheless, any type of reassociation, if it occurred, should have been eliminated by capturing the IgE with CGP51901 anti-IgE antibody, preventing its rebinding, without actually physically removing it from solution. A variety of studies have examined the dissociation of IgE from FcεRI on RBL cells and this process may be biphasic.41 The two time constants (T1/2) range 4 to 40 hours42-45 for the fast component and 150 to 250 hours for a second component. This later component, the cause of which is not known, is consistent with the long dissociation rate found in our human basophil studies. The downregulation observed in these long-term cultures is also unlikely to result only from the slow progression of basophils into apoptosis, since it was possible to hold receptor levels constant with an appropriate concentration of IgE antibody. In addition, the in vivo anti-IgE antibody treatment studies also suggest that the decrease in FcεRI expression accompanies lower free IgE titers.3
If IgE can signal through FcεRI itself to induce its expression, this represents a new role for this receptor. Future studies will be necessary to determine whether expression of this receptor and its regulation by IgE are dependent on changes in receptor synthesis and/or catabolism.
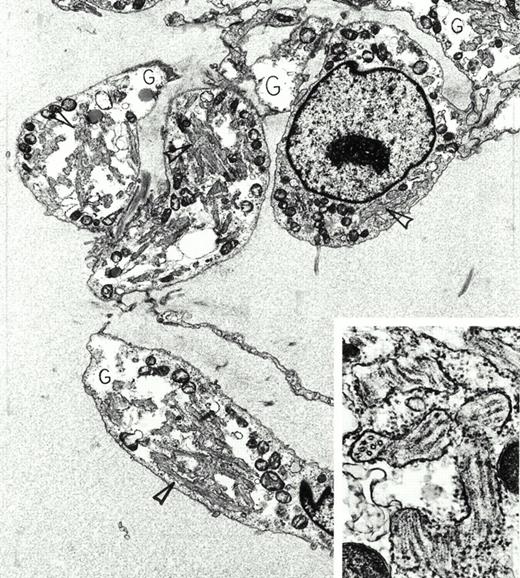
Extraskeletal myxoid chondrosarcoma. A 72-year-old asymptomatic man was found to have a large mass in the thigh by physical examination. This was removed surgically, and radiation therapy was administered to the area. Despite multiple bilateral, slowly enlarging pulmonary metastases, he remained in good health 4 years later. Immunoperoxidase stains of the tumor were positive for vimentin and negative for actin, desmin, myoglobin, S-100 protein, keratin, and epithelial membrane antigen. By electron microscopy, the tumor was composed of interconnected chains of plump polygonal and elongate spindle cells in an abundant edematous matrix. Tumor cells were focally invested in basal lamina, displayed large, electron-lucent, cytoplasmic glycogen pools (G) and extensive enlarged cisterns of rough endoplasmic reticulum packed full with tubules (arrowheads). This tubular array is seen at higher magnification and in both longitudinal and cross-sectional views in the inset. Original magnification ×8,000 (inset, ×59,000). (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215.) {/ANNT;80256n;;center;0n}
Extraskeletal myxoid chondrosarcoma. A 72-year-old asymptomatic man was found to have a large mass in the thigh by physical examination. This was removed surgically, and radiation therapy was administered to the area. Despite multiple bilateral, slowly enlarging pulmonary metastases, he remained in good health 4 years later. Immunoperoxidase stains of the tumor were positive for vimentin and negative for actin, desmin, myoglobin, S-100 protein, keratin, and epithelial membrane antigen. By electron microscopy, the tumor was composed of interconnected chains of plump polygonal and elongate spindle cells in an abundant edematous matrix. Tumor cells were focally invested in basal lamina, displayed large, electron-lucent, cytoplasmic glycogen pools (G) and extensive enlarged cisterns of rough endoplasmic reticulum packed full with tubules (arrowheads). This tubular array is seen at higher magnification and in both longitudinal and cross-sectional views in the inset. Original magnification ×8,000 (inset, ×59,000). (Courtesy of Ann M. Dvorak, MD, Department of Pathology, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215.) {/ANNT;80256n;;center;0n}
Supported in part by National Institutes of Health Grants No. AI20253 and AI07290.
Address reprint requests to Donald MacGlashan, Jr, PhD, Johns Hopkins Asthma and Allergy Center, 5501 Hopkins Bayview Cir, Baltimore, MD 21224.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.