Abstract
The formation and deposition of monosodium urate (MSU) microcrystals in articular and periarticular tissues is the causative agent of acute or chronic inflammatory responses known as gouty arthritis. Mononuclear phagocyte activation is involved in early triggering events of gout attacks. Because stimulated mononuclear phagocytes can constitute an important source of the inducible isoform of cyclooxygenase (COX-2), we evaluated the effects that proinflammatory microcrystals might have on COX-2 protein expression in crystal-stimulated monocytes. We found that MSU crystals, but not calcium pyrophosphate dihydrate (CPPD) crystals, induced COX-2, which correlated with the synthesis of prostaglandin E2 (PGE2) and thromboxane A2(TXA2). Crystal-induced de novo synthesis of COX-2 was dependent on transcriptional and translational events. Inhibition of tyrosine phosphorylation, by herbimycin A, blocked crystal-induced COX-2. Similarly, an inhibitor of the p38 mitogen-activated protein kinase, SB 203580, inhibited the stimulation of COX-2. Colchicine inhibited crystal-induced COX-2. In all cases, prostanoid synthesis was concomitantly inhibited. Taken together, these results implicate COX-2 in the development of MSU-induced inflammation.
DEPOSITION OF monosodium urate (MSU) and calcium pyrophosphate dihydrate (CPPD) microcrystals in articular and periarticular tissues is the cause of acute or chronic inflammatory responses known as gouty arthritis and chondrocalcinosis, respectively.1-3 Clinical symptoms of these inflammatory responses are characterized by severe pain, edema, and erythema in the joint. Cell activation by MSU microcrystals is a central feature of acute gouty arthritis4 and proinflammatory microcrystals can interact with all of the major synovial cell types, including neutrophils, monocytes/macrophages, and fibroblast-like (type B) synoviocytes.3 In monocytes, for example, microcrystals stimulate the synthesis of a number of proinflammatory cytokines, such as interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor alpha (TNFα).4-8 Monocytes-macrophages exposed to MSU crystals in vitro also release prostaglandins. Prostanoids are found in the synovial fluid of gouty arthritis patients and prostaglandin E2 (PGE2) has been shown to be involved in crystal-induced inflammation.9 Early vasodilation, enhanced vascular permeability, and pain in gouty arthritis are likely to be mediated, at least in part, by vasoactive prostaglandins, including PGE2.10 Similarly, rats deficient in essential fatty acids, biologic precursors of prostaglandins, develop less footpad swelling than do normal rats following injection of MSU microcrystals,11 which suggests an involvement of prostanoids in crystal-induced inflammation.
Cyclooxygenase (COX) catalyzes the conversion of arachidonic acid to prostaglandin H2, the first committed step in the biosynthesis of prostanoids.12,13 To date, two COX isoforms, each encoded by distinct genes,14 have been described in mammalian cells.15 The COX-1 isozyme is expressed constitutively in most tissue types, often at low levels, and appears to be a “housekeeping” enzyme that supports the levels of prostanoid biosynthesis required for maintaining organ and tissue homeostasis.13,15 The second isoform, COX-2, is only expressed in a limited number of cells, including monocytes/macrophages, synovial cells, and fibroblasts.16COX-2 can be induced by various proinflammatory agents, including endotoxin, cytokines, mitogens, and lipid mediators,17 and is thought to be the predominant COX isoform involved in the inflammatory response.18 19
Stimulated human monocytes constitute an important source of COX-2–derived prostanoids, including PGE2 and thromboxane A2 (TXA2).20 Since a phlogistic role has been established for prostaglandins in gouty arthritis, and since monocyte activation appears to be involved in triggering events in gout attacks,21 we evaluated the effects that proinflammatory microcrystals might have on COX-2 protein expression in human monocytes. We now report that MSU microcrystals induce COX-2, as well as the production of prostanoids in these cells. The results of this study indicate that MSU crystals upregulate COX-2 levels in monocytes and potentially identifies a novel site of action of MSU crystals in the pathogenesis of gouty arthritis.
MATERIALS AND METHODS
Reagents.
Materials were obtained from the following sources: Pyrogen-free Lymphoprep, Nycomed, Oslo, Norway; RPMI culture medium and fetal calf serum (FCS), Biosciences, Sydney, Australia; Minisorp polyethylene tubes, Nunc, Roskilde, Denmark; Rabbit PGE2 antiserum, actinomycin D (AD), cycloheximide (CHX), E-Toxa-Clean, and herbimycin A, Sigma Chemical, St Louis, MO; Trans-Blot transfer membranes, Bio-Rad, North Ryde, Australia; rabbit polyclonal PGHS-2 (human) antiserum raised to a synthetic human PGHS-2 peptide,22 and PGE2, Cayman Chemical, Ann Arbor, MI. TXB2antiserum was prepared from a rabbit immunized with thromboxane conjugated to thyroglobulin and has been used in previous studies.23 Peroxidase-labeled donkey antirabbit and goat antimouse antibodies, and the enhanced chemoluminescence immunoblotting analysis system were obtained from Amersham International, Little Chalfont, England. [4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)imidazole] (SB 203580), and [2-(4-Methylsulfinyl)-3-[4-(2-methylpyridyl)]-6,7-dihydro [5H] pyrrolo [1, 2-a] imidazole] (SK&F 106978) were generously supplied by Dr John C. Lee (SmithKline Beecham, King of Prussia, PA).
Preparation of microcrystals.
MSU and CPPD microcrystals were prepared using previously described methods1,24 25 with modifications and were generously provided by Dr R. De Médicis (Unité des maladies rhumatismales, CUSE, Sherbrooke, Québec, Canada). Briefly, a boiling MSU solution (0.03 mol/L pH 7.5) was prepared by dissolution of equimolar quantities of uric acid and sodium hydroxide (3 μmol/L) and filtered on an Acropor membrane filter (AN-3000; Gelman Sciences, Ann Arbor, MI). Sodium chloride (0.1 mol/L final concentration) was added to speed up and improve the uniformity of the crystallization. CPPD was obtained by mixing a calcium nitrate solution (0.1 mol/L final concentration) with an acidic solution of sodium pyrophosphate (final concentration, 0.025 mol/L of Na2P2O7 and 0.03 mol/L HNO3). The milky-white precipitate formed CPPD crystals after a 1-day incubation at 50 to 60°C. The crystals were characterized by x-ray diffraction (Rigaku Geigerflex D/max), by examination under phase and polarizing microscopy and by scanning electron microscopy. The MSU and CPPD crystals showed triclinic morphologic characteristics. Their dimensions as determined by scanning microscopy were 10 × 1 × 1 μm to 25 × 1.5 × 1.5 μm and 12 × 1.4 × 1.4 μm to 25 × 1.7 × 1.7 μm for MSU and CPPD, respectively. Crystal preparations were free of endotoxins (as assessed by by the Limulus Assay; Whittaker, Walkerville, MD) and used without opsonization. Before use, crystals were resuspended in endotoxin-free Hanks' balanced salt solution (HBSS).
Monocyte isolation.
Packed-cell preparations were obtained fresh from the Red Cross Blood Center, Adelaide, South Australia. Mononuclear cells were collected following centrifugation (800g, 30 minutes) of the packed-cell fractions on pyrogen-free Lymphoprep. After multiple washings, the mononuclear cells were suspended in 10 mL running buffer (HBSS 1×, 0.01% EDTA, 0.1% glucose, 0.1% low-lipopolysaccharide FCS). In this solution, the cells underwent counter-current centrifugal elutriation (J-6M/E Elutriation System; Beckman, Palo Alto, CA) with constant rotor speed (2,050 rpm) and with a constant flow rate of 11 mL/min during 30 minutes. Purity of the obtained monocyte fraction (>85%) was assessed either by Giemsa staining of cytocentrifuged smears, or by FACS analysis using an anti-CD14 monoclonal antibody (clone FMC 32; Serotec, Adelaide, South Australia). Contaminating cells were essentially all lymphocytes; the relative absence of contaminating platelets in such preparations, as assessed by immunoblots for the detection of COX-1, was demonstrated previously.26Viability of the cells was consistently greater than 95% as determined by Trypan blue exclusion. For the maintenance of minimal lipopolysaccharide contamination, the mononuclear-cell isolation procedure was performed under sterile conditions; all glassware, plasticware, and elutriator tubing were treated with E-Toxa-Clean before each elutriation.
Cell stimulation.
Elutriated monocytes were resuspended (2 × 106cells/mL) in RPMI 1640 supplemented with 10% low-lipopolysaccharide FCS and penicillin/streptomycin. Cells were distributed in 1-mL aliquots, in Minisorp tubes (Nunc, Roskilde, Denmark) to minimize adhesion, and incubated at 37°C with 5% CO2 in a humidified atmosphere. Where mentioned, cells were preincubated for 10 minutes with the appropriate pharmacologic agent prepared in either ethanol or dimethylsulfoxide, or with an equal volume of diluent before stimulation. Concentration of organic solvent never exceeded 0.1%. For prostanoid (PGE2 and TXA2) measurements, cell suspensions were centrifuged and cell-free supernatants were stored at −20°C.
TXA2 and PGE2 measurement.
TXA2 has a half-life of approximately 30 seconds under physiologic conditions and is readily converted to the stable metabolite TXB2, which was measured. TXB2 and PGE2 levels were determined by radioimmunoassay (RIA), as previously described.23 Cross-reactivities in the TXB2 RIA were 0.06% for PGE2, 0.05% for 6-keto PGF1α, and less than 0.05% for PGF2α. Cross-reactivities in the PGE2 RIA were less than 0.001% for TXB2, 4.6% for 6-keto PGF1α, and 3.8% for PGF2α. Internal controls were performed that confirmed that both PGE2 and TXB2 are metabolites that are readily released from the cells following their synthesis. Moreover, the presence of crystals, up to a concentration of 3 mg/mL, did not interfere with the detection of these prostanoids (data not shown).
Immunoblots.
Following the desired treatment, cell pellets were processed for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblots as described previously,27 with the following modifications. Cell pellets (5 × 106 cells) were resuspended in 75 μL of ice-cold lysis buffer (HEPES-buffered HBSS pH 7.4, 0.5% Triton X-100, 10 μg/mL phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL leupeptin, 10 μg/mL aprotinin). Seventy-five microliters of 2× sample buffer (0.125 mol/L Trizma base, pH 6.8, 20% glycerol, 4% SDS, 10% β-mercaptoethanol) were then added and the samples were boiled for 5 minutes. Samples were loaded on a 9% acrylamide gel. Typically, the equivalent of 1.7 × 106cells were loaded in each well. Proteins were transferred at 4°C for 16 hours, at 300 mA current setting onto a Trans-Blot membrane. Equal protein loading and transfer efficiency were visualized by Ponceau red staining. The membranes were soaked for 30 minutes at 25°C in Tris-buffered saline (TBS; 25 mmol/L Tris-HCl pH 7.6, 0.2 mol/L NaCl, 0.15% Tween 20) containing 5% dried milk (wt/vol), and subsequently exposed to antibodies reacting with either human COX-1 or COX-2. The membranes were then washed twice with TBS, and incubated either with a horseradish peroxidase–linked donkey antirabbit, or goat antimouse antibody. Bound antibodies were revealed with the enhanced chemoluminescence reagent, following the manufacturer's protocol (Amersham).
Statistical analysis.
Statistical analysis was performed by Student's paired t-test (two-tailed), and significance was considered to be attained whenP was less than .05.
RESULTS
Effect of microcrystals on prostanoid production in human monocytes.
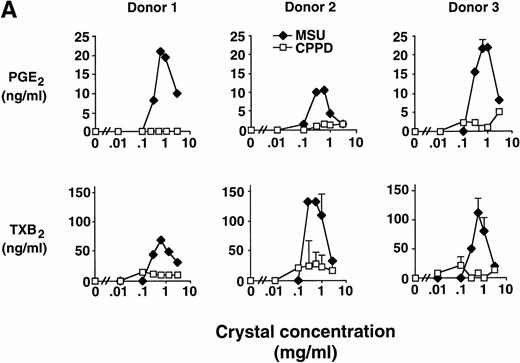
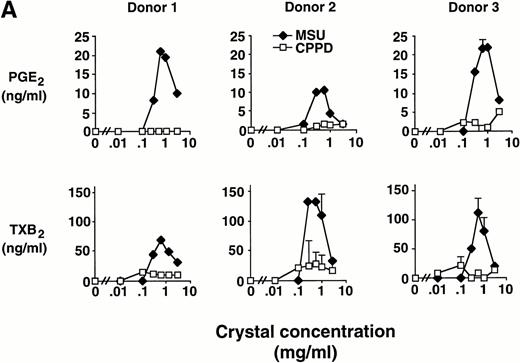
Elutriated human monocytes were incubated with increasing concentrations of inflammatory microcrystals for 24 hours, and cell-free supernatants were analyzed for prostanoid (PGE2and TXA2) synthesis. Prostanoid synthesis increased with increasing concentrations of MSU crystals, up to 1 mg/mL (Fig1A). Typically, prostanoid synthesis was first observed at a concentration of 0.3 mg/mL and was maximal at concentrations between 0.6 mg/mL and 1 mg/mL. Higher MSU crystal concentration (3 mg/mL) was associated with a diminution in prostanoid synthesis. Monocytes incubated with CPPD crystals up to a concentration of 3 mg/mL failed to consistently produce PGE2 or TXA2.
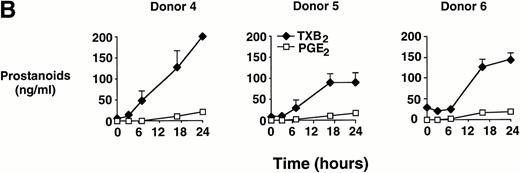
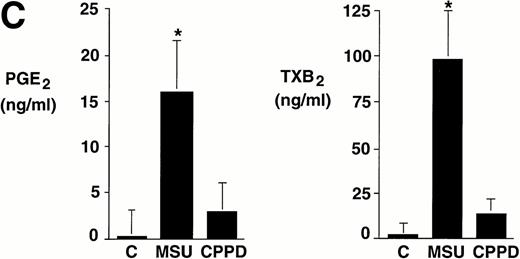
Effect of inflammatory microcrystals on prostanoid synthesis by human monocytes. (A) Monocytes were incubated for 24 hours with either MSU or CPPD crystals at the indicated concentrations and cell-free supernatants were analyzed for prostanoid (PGE2and TXB2) synthesis. Data obtained from 3 donors are presented, and values are the mean ± SD of duplicate determinations. (B) Monocytes were incubated with MSU crystals (0.6 mg/mL) for the indicated times and prostanoid synthesis was measured. Data obtained from 3 donors are presented, and values are the mean ± SD of duplicate determinations. (C) Monocytes were stimulated in the absence (C), or presence of either MSU or CPPD crystals (0.6 mg/mL) for 24 hours and prostanoid synthesis was measured. When compared with control cells, MSU microcrystals induced significant prostanoid synthesis, *P < .01 (by t test). Data presented are the mean ± SD from at least seven experiments.
Effect of inflammatory microcrystals on prostanoid synthesis by human monocytes. (A) Monocytes were incubated for 24 hours with either MSU or CPPD crystals at the indicated concentrations and cell-free supernatants were analyzed for prostanoid (PGE2and TXB2) synthesis. Data obtained from 3 donors are presented, and values are the mean ± SD of duplicate determinations. (B) Monocytes were incubated with MSU crystals (0.6 mg/mL) for the indicated times and prostanoid synthesis was measured. Data obtained from 3 donors are presented, and values are the mean ± SD of duplicate determinations. (C) Monocytes were stimulated in the absence (C), or presence of either MSU or CPPD crystals (0.6 mg/mL) for 24 hours and prostanoid synthesis was measured. When compared with control cells, MSU microcrystals induced significant prostanoid synthesis, *P < .01 (by t test). Data presented are the mean ± SD from at least seven experiments.
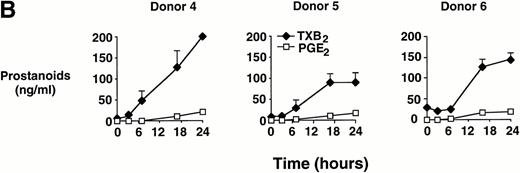
In time-course experiments, cells were incubated with 0.6 mg/mL MSU crystals for up to 24 hours. After an observed lag period of 3 to 6 hours, prostanoid synthesis was detected in MSU crystal-stimulated monocytes, and this synthesis increased with incubation time, up to 24 hours (Fig 1B). Monocytes incubated with CPPD crystals under similar conditions failed to consistently produce PGE2 or TXA2 (data not shown).
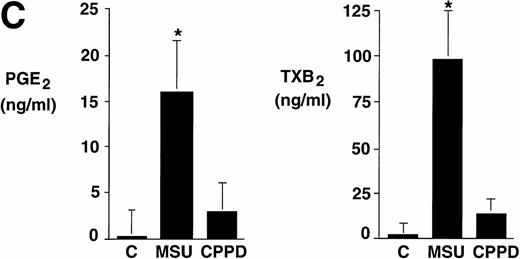
Integrated data from seven experiments in which cells were incubated with crystals at a concentration of 0.6 mg/mL for 24 hours are summarized in Fig 1C. Whereas levels of prostanoids synthesized by nonstimulated monocytes were often under the limit of detection, MSU crystal-stimulated monocytes produced more than 15 ng/mL of PGE2 and approximately 100 ng/mL TXA2. In some experiments, CPPD crystal-stimulated monocytes produced relatively low levels of prostanoids. However, results did not reach statistical significance (P > .05) when compared with nonstimulated monocytes.
Effect of microcrystals on COX-2 protein expression in human monocytes.
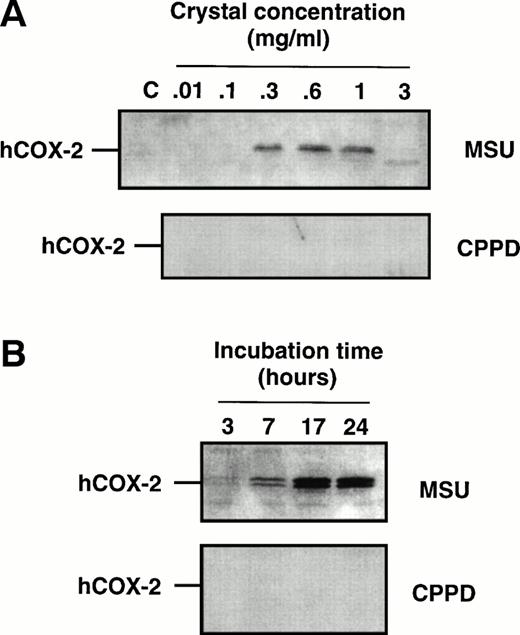
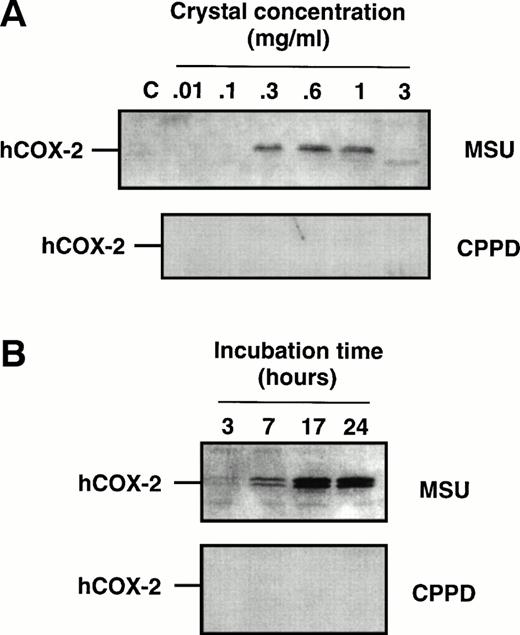
Cell samples from the aforementioned dose-response experiments were processed for immunoblots to evaluate COX-2 protein expression. When using an antibody that specifically recognizes the COX-2 isoform22,26 a characteristic doublet that likely represents differentially glycosylated forms of the enzyme,54 with a relative molecular mass of 72 to 74 kD, was detected in samples from MSU crystal-stimulated monocytes (Fig2), but not from CPPD crystal-stimulated monocytes. The intensity of the COX-2 doublet increased with increasing concentrations of MSU crystals (Fig 2A) within the range 0.1 to 1 mg/mL, and consistently decreased at the highest concentration used (3 mg/mL) in approximate correlation with the level of prostanoid production. In time-course experiments in which monocytes were incubated with MSU crystals, COX-2 protein could be observed after 3 to 7 hours of incubation with MSU crystals, and the intensity of the two bands was maximal after 17 hours (Fig 2B). Barely detectable levels of COX-1 protein were present in elutriated monocytes, and no significant change in immunoreactivity could be detected following crystal stimulation (data not shown).
Effect of inflammatory microcrystals on the protein expression of COX-2 in human monocytes. Monocytes were incubated for (A) 24 hours with either MSU or CPPD crystals at the indicated concentrations, or (B) with crystals (0.6 mg/mL) for the indicated times and centrifuged. The cell pellets were then processed for evaluation of COX-2 protein expression by immunoblotting as described in Materials and Methods. A representative immunoblot is shown.
Effect of inflammatory microcrystals on the protein expression of COX-2 in human monocytes. Monocytes were incubated for (A) 24 hours with either MSU or CPPD crystals at the indicated concentrations, or (B) with crystals (0.6 mg/mL) for the indicated times and centrifuged. The cell pellets were then processed for evaluation of COX-2 protein expression by immunoblotting as described in Materials and Methods. A representative immunoblot is shown.
RNA extraction from crystal-stimulated monocytes presented serious technical problems and did not enable evaluation of the potential effects of these crystals on the expression of COX-2 mRNA in monocytes (data not shown).
Effect of metabolic inhibitors on prostanoid synthesis and COX-2 protein expression in MSU crystal-stimulated human monocytes.
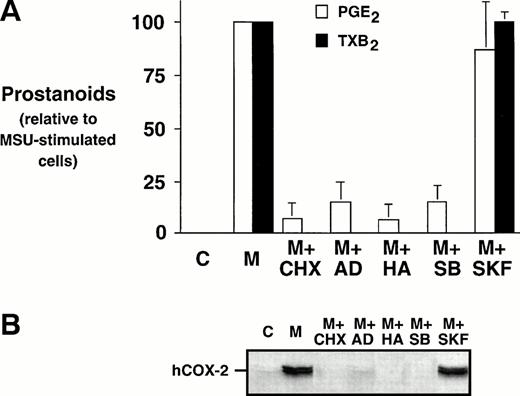
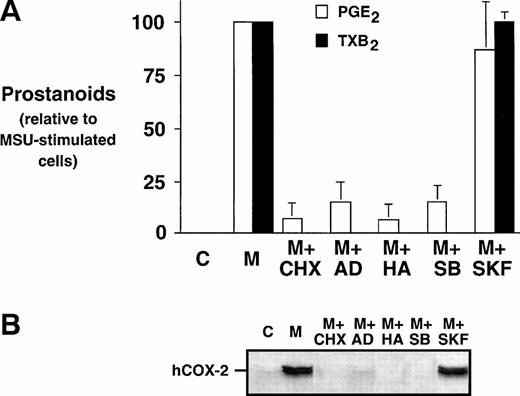
Monocytes were incubated with MSU crystals, alone or in the presence of an inhibitor of protein synthesis, CHX, an inhibitor of transcription, AD, or an inhibitor of tyrosine kinases, herbimycin A. Both CHX and AD inhibited prostanoid synthesis by at least 80% when compared with MSU crystal-stimulated monocytes (Fig 3A).These inhibitors also decreased the COX-2 protein expression under the limit of detection, which suggests that both translational and transcriptional events are required for the stimulation of COX-2 protein expression (Fig 3B). Herbimycin A decreased MSU crystal-stimulated prostanoid synthesis by more than 90%, an event that was also paralleled by an inhibition of COX-2 protein expression. SB 203580, a specific inhibitor of p38 mitogen-activated protein kinase, was also assessed. The presence of SB 203580 decreased the production of PGE2 by approximately 80% and production of TXB2 by 100%. SB 203580 decreased COX-2 protein expression below the limits of detection. In contrast, an inactive structural analog of SB 203580, SK&F 106978, had little effect on MSU crystal-stimulated prostanoid synthesis or COX-2 protein expression.
Effect of metabolic inhibitors on the MSU crystal-induced prostanoid synthesis and protein expression of COX-2 in human monocytes. Monocytes were incubated with diluent (C), or MSU crystals (M; 0.3 mg/mL) for 24 hours alone or in combination with a protein synthesis inhibitor, cycloheximide (CHX; 10 μg/mL); an inhibitor of transcription, actinomycin D (AD; 5 μg/mL), or a specific inhibitor of tyrosine kinases, herbimycin A (HA; 100 nmol/L). A p38 mitogen-activated protein kinase inhibitor, SB 203580 (SB; 10 μmol/L), as well as an inactive structural analog, SK&F 106978 (SKF; 10 μmol/L) were also used under similar conditions. (A) Cell-free supernatants were collected and analyzed for prostanoid synthesis as indicated in the Materials and Methods. Results are the mean ± SD from 3 separate experiments and are expressed as percent of MSU crystal-stimulated monocytes in the absence of metabolic inhibitor. (B) Corresponding cell samples were processed for evaluation of immunoreactive COX-2 by immunoblot as described in Materials and Methods. A representative immunoblot is shown.
Effect of metabolic inhibitors on the MSU crystal-induced prostanoid synthesis and protein expression of COX-2 in human monocytes. Monocytes were incubated with diluent (C), or MSU crystals (M; 0.3 mg/mL) for 24 hours alone or in combination with a protein synthesis inhibitor, cycloheximide (CHX; 10 μg/mL); an inhibitor of transcription, actinomycin D (AD; 5 μg/mL), or a specific inhibitor of tyrosine kinases, herbimycin A (HA; 100 nmol/L). A p38 mitogen-activated protein kinase inhibitor, SB 203580 (SB; 10 μmol/L), as well as an inactive structural analog, SK&F 106978 (SKF; 10 μmol/L) were also used under similar conditions. (A) Cell-free supernatants were collected and analyzed for prostanoid synthesis as indicated in the Materials and Methods. Results are the mean ± SD from 3 separate experiments and are expressed as percent of MSU crystal-stimulated monocytes in the absence of metabolic inhibitor. (B) Corresponding cell samples were processed for evaluation of immunoreactive COX-2 by immunoblot as described in Materials and Methods. A representative immunoblot is shown.
Effect of colchicine on prostanoid synthesis and COX-2 protein expression in human monocytes.
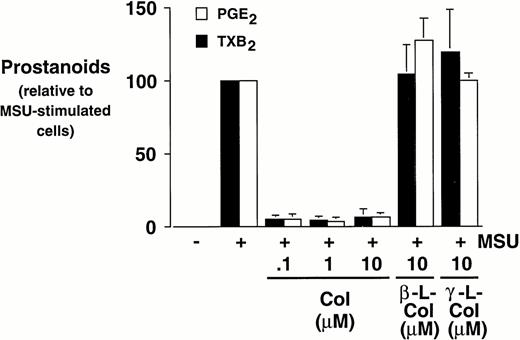
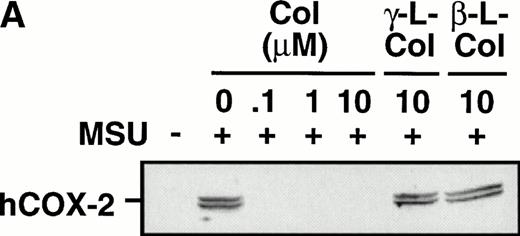
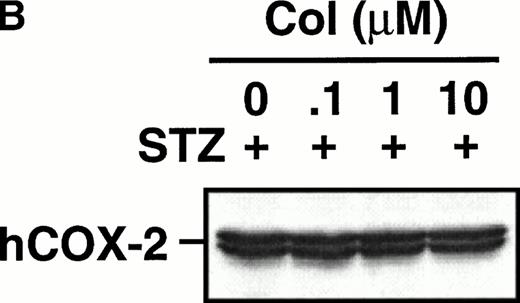
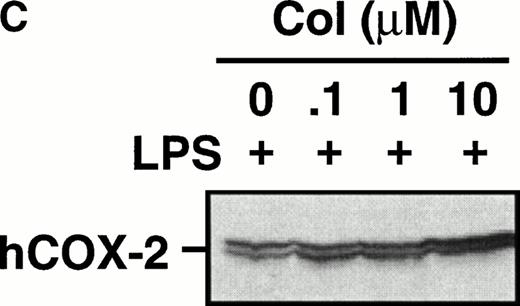
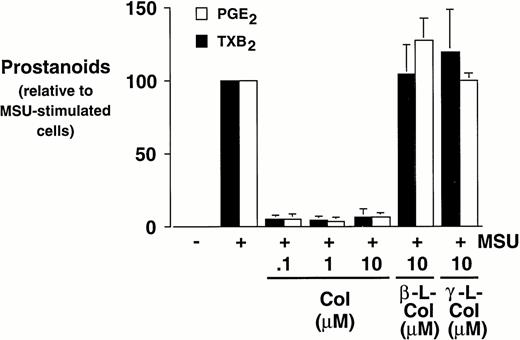
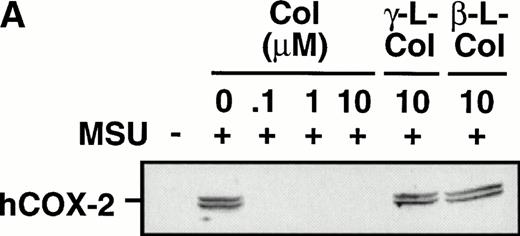
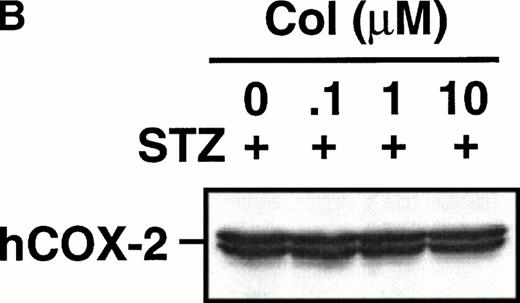
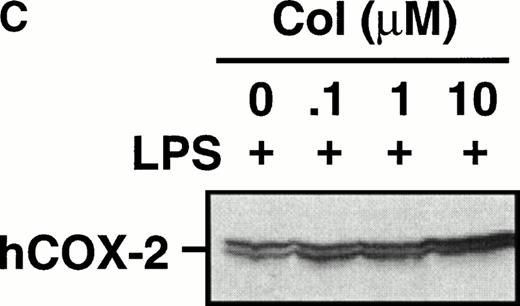
Monocytes were incubated with MSU crystals in the presence or absence of different concentrations of colchicine. This antiphlogistic agent effectively blocked prostanoid production (both PGE2 and TXA2) by approximately 90% and inhibited MSU crystal-induced COX-2 protein expression at 100 nmol/L, the lowest concentration used (Figs 4 and5). In contrast, two inactive structural analogs of colchicine, β- and γ-lumicolchicine, had little effect either on the protein expression of COX-2 or on the production of prostanoids, stimulated by MSU crystals, up to a concentration of 10 μmol/L (Figs 4 and 5). Colchicine did not inhibit monocyte COX-2 protein expression, stimulated by either serum-treated zymosan or lipopolysaccharide (Fig5B and C).
Effect of colchicine on microcrystal-induced prostanoid synthesis by human monocytes. Monocytes were incubated with diluent, MSU crystals (0.6 mg/mL), or CPPD (0.6 mg/mL) for 24 hours, alone or in combination with various concentrations of colchicine (col), or with its inactive analogs, β- and γ-lumicolchicine (L-col), both at a concentration of 10 μmol/L. Cell-free supernatants were collected and analyzed for prostanoid synthesis. The results are the mean ± SD from three separate experiments and are expressed as percent of MSU crystal-stimulated monocytes in the absence of metabolic inhibitor.
Effect of colchicine on microcrystal-induced prostanoid synthesis by human monocytes. Monocytes were incubated with diluent, MSU crystals (0.6 mg/mL), or CPPD (0.6 mg/mL) for 24 hours, alone or in combination with various concentrations of colchicine (col), or with its inactive analogs, β- and γ-lumicolchicine (L-col), both at a concentration of 10 μmol/L. Cell-free supernatants were collected and analyzed for prostanoid synthesis. The results are the mean ± SD from three separate experiments and are expressed as percent of MSU crystal-stimulated monocytes in the absence of metabolic inhibitor.
Effect of colchicine on microcrystal-induced COX-2 protein expression in human monocytes. Monocytes were incubated without or with MSU crystals (0.6 mg/mL) for 24 hours alone or in combination with various concentrations of colchicine (col) or with its inactive analogs, β- and γ-lumicolchicine (L-col), both at a concentration of 10 μmol/L. Cell samples were then processed for evaluation of immunoreactive COX-2 by immunoblot as described in Materials and Methods. (A) MSU crystal-stimulated cells. (B) Serum-treated zymosan (STZ; 100 μg/mL; 24 hours)-stimulated cells, in the presence of various concentrations (μmol/L) of colchicine. (C) Lipopolysaccharide (LPS; 2 μg/mL; 24 hours)-stimulated cells, in the presence of various concentrations (μmol/L) of colchicine. In each case, an immunoblot representative of at least three separate experiments is shown.
Effect of colchicine on microcrystal-induced COX-2 protein expression in human monocytes. Monocytes were incubated without or with MSU crystals (0.6 mg/mL) for 24 hours alone or in combination with various concentrations of colchicine (col) or with its inactive analogs, β- and γ-lumicolchicine (L-col), both at a concentration of 10 μmol/L. Cell samples were then processed for evaluation of immunoreactive COX-2 by immunoblot as described in Materials and Methods. (A) MSU crystal-stimulated cells. (B) Serum-treated zymosan (STZ; 100 μg/mL; 24 hours)-stimulated cells, in the presence of various concentrations (μmol/L) of colchicine. (C) Lipopolysaccharide (LPS; 2 μg/mL; 24 hours)-stimulated cells, in the presence of various concentrations (μmol/L) of colchicine. In each case, an immunoblot representative of at least three separate experiments is shown.
DISCUSSION
The formation and/or deposition of MSU microcrystals in articular and periarticular tissues is the cause of gouty arthritis. These microcrystals interact with all of the major synovial cell types, including neutrophils, fibroblasts, and monocytes/macrophages to produce a variety of inflammatory mediators.2 3 Clinical hallmarks of this inflammatory condition include severe pain, edema, and erythema in the joint, all of which are biologic actions of, among other agents, prostanoids such as PGE2, with its well-known role in pain. The results of the present study show that inflammatory MSU crystals, but not CPPD crystals, stimulate COX-2 protein expression in human monocytes in a dose- and time-dependent manner and that this is associated with the production of PGE2 and TXA2. Studies using various metabolic inhibitors indicate that both transcriptional and translational processes, as well as activity of tyrosine kinases, are necessary for this stimulation to occur. At supraoptimal concentrations of MSU crystals, induction of COX-2 and prostanoid synthesis were reduced, an effect that is likely to be related to the known cytolytic effects of these crystals.
Stimulated human blood monocytes constitute an important source of COX-2–derived prostanoids, including PGE2 and TXA2.20 The use of elutriation to isolate human monocytes allows the purification of a cell population essentially free of platelets, a major source of COX-1 derived prostanoids. In fact, in resting or crystal-stimulated monocytes isolated by elutriation, COX-1 was only weakly if at all detected when assessed by immunoblotting (Pouliot, unpublished observations, April 1996), supporting the relative absence of platelets in these monocyte preparations. To confirm the involvement of COX-2 in our system, signal transduction pathways that are critically involved in the expression of COX-2, but not of COX-1,26 28-35 were blocked with the use of specific inhibitors. The tyrosine kinase inhibitor, herbimycin A, as well as the p38 mitogen-activated protein kinase inhibitor, SB 203580, both decreased this stimulated production of PGE2 and essentially eliminated all detectable TXB2 production, in parallel to preventing the induction of COX-2. Moreover, MSU-induced prostanoid synthesis was blocked by inhibitors of transcription and of translation, revealing the requirement of de novo protein synthesis for increased prostanoid production to occur in this system. Collectively, these data demonstrate the preeminent role of a monocyte-derived and inducible COX isoform over the constitutive isoform in MSU-stimulated monocytes. In allowing discrimination between COX-1– and COX-2–mediated prostanoid production in human monocytes, elutriation represents a significant improvement over the adhesion technique, which produces samples that contain platelets as the primary contaminant.
The stimulation of cytokine production by monocytes is not universal to all types of inflammatory crystals. For example, it has been demonstrated that MSU-stimulated human monocytes and, to a lesser extent, cells treated with hydroxyapatite crystals, released significant amounts of TNFα, whereas cells incubated with CPPD crystals failed to produce TNFα.8 Similarly, MSU, but not CPPD crystals, induced the production of IL-1 by human monocytes.36 Another study showed that MSU crystals were approximately 50 times more potent than CPPD crystals for optimal IL-6 production by human monocytes. However, in that same study, CPPD were as potent as MSU crystals for the induction of IL-6 production by human synoviocytes.7 This indicates a cell-specific type of response to inflammatory crystals. These observations also suggest that MSU crystals may be more potent for the induction of early-response genes in human monocytes than CPPD crystals, an hypothesis that our current results further support.
A class of pyridinyl imidazole compounds that inhibit cytokine production has been developed37 and SB 203580 is a recent representative. These compounds have been found to inhibit IL-1 and TNFα production in human monocytes. They also have antiinflammatory effects in animal models such as arachidonic acid-induced edema and collagen-induced arthritis in mice, and they inhibit inflammatory cell infiltration induced in mice by MSU crystals or carrageenan.38,39 Recently, we have shown that these compounds specifically inhibit the expression of COX-2 in human monocytes stimulated with different stimuli, including serum-treated zymosan, TNF-α, IL-1, phorbol myristate acetate, and lipopolysaccharide.26 In the present study, SB 203580 also inhibited COX-2 protein expression induced by MSU crystals, which further indicates an important role for p38 mitogen-activated protein kinase in the induction of the enzyme. This result also suggests that SB 203580 affects events in the signal transduction pathways that lead to COX-2 expression and are common for different classes of monocyte-stimulating agents.
Although the effectiveness of colchicine in crystal-associated rheumatic diseases40 has been reported as early as the sixth century,41 the basis of its antiphlogistic action remains unclear. The major accepted effect of colchicine on cells is inhibition of microtubule assembly.42 However, it is uncertain whether this site of action is responsible for the in vivo antiinflammatory effects of colchicine, since concentrations greater than those achieved during therapy (1 μmol/L v 100 nmol/L) are required to inhibit microtubule-dependent functions in vitro.43-46 On the other hand, concentrations of colchicine required to inhibit outside-in-signaling through the microtubular system can occur at lower concentrations than required to inhibit some effector functions.47
In a number of different cell types, including human monocytes, tyrosine phosphorylation is central to COX-2 induction.28-35 This was further confirmed in the present study, where herbimycin A effectively blocked the protein expression of COX-2 and synthesis of prostanoids. MSU crystals induce a specific pattern of tyrosine phosphorylation in neutrophils which is inhibited by colchicine.48,49 In our study, specific inhibition of monocyte COX-2 protein expression and prostanoid synthesis by colchicine has been observed at similar concentrations (submicromolar) to those which inhibit tyrosine phosphorylation, concentrations which may be achieved therapeutically. Also, the inhibitory effect of colchicine on COX-2 was specific to MSU crystals, in that it did not inhibit lipopolysaccharide- or zymosan-stimulated COX-2 protein expression even at the highest concentration tested. This result is in good accordance with recent findings in human neutrophils, in which colchicine blocked tyrosine phosphorylation stimulated by MSU crystals, but not by chemotactic agents or by zymosan.49 In addition, recent data indicate that colchicine inhibits MSU- and CPPD-induced IL-8 production by human neutrophils, but not that induced by granulocyte-macrophage colony-stimulating factor (GM-CSF) or TNFα (McColl & Naccache, unpublished data, June 1996). Taken together, these results provide further evidence for a proposed antiprostaglandin action of colchicine in crystal-induced inflammation,50 and give further support to the concept that beneficial therapeutic effects of colchicine are considered to be relatively limited to inflammatory conditions involving inflammatory microcrystals.50 51
In addition to its pain-triggering role, PGE2 may participate in several ways to the symptoms that are marks of gouty arthritic flares, such as early vasodilation, edema, and leukocyte migration. In an in vivo model of inflammation, PGE2prevented leukotriene (LT)C4-induced vasoconstriction and cause a marked potentiation of LTC4-induced plasma extravasation in Syrian hamsters. PGE2 also potentiated histamine-induced leakage, as well as LTB4-induced leukocyte-associated leakage, from both postcapillary and larger venules.52 It appears therefore that effects of PGE2 can be potentiated by the presence of other factors including lipoxygenase-derived metabolites such as the LTs. Interestingly, MSU crystals can also trigger the formation of LTs and of hydroxy acids by human neutrophils and platelets.53However, it must be stressed that PGE2 can exert both proinflammatory and antiinflammatory properties52; this may help explain conflicting effects of nonsteroidal antiinflammatory drugs in different models. The study of other factors, such as the site of synthesis, and how the balance of synthesized lipid mediators can influence the final response, may increase our understanding of the underlying mechanisms that govern gouty arthritis episodes.
In the present study, we have demonstrated that MSU microcrystals can specifically induce COX-2 in human monocytes. These results identify COX-2 expression as a new factor involved in MSU-induced inflammation. The observed inhibition by colchicine of COX-2 protein expression and of prostanoid synthesis in MSU crystal-stimulated cells provides a possible mechanism for the antiphlogistic action of this therapeutic agent. Taken together, these results provide greater understanding of the mechanisms involved in early events that trigger gout attacks. Further studies are in progress to identify which signal transduction pathways are specifically involved in the interaction of monocytes with MSU microcrystals.
ACKNOWLEDGMENT
We thank Dr John C. Lee (SmithKline Beecham, King of Prussia, PA) for generously supplying the p38 mitogen-activated protein kinase inhibitor.
Supported by project grants from the National Health & Medical Research Council of Australia and by a grant from the Arthritis Society of Canada. M.P. is the recipient of a fellowship from the Medical Research Council of Canada.
Address reprint requests to Marc Pouliot, PhD, Centre de Recherche en Rhumatologie et Immunologie, Centre de Recherche du CHUL, Room T-1-49, 2705 Boulevard Laurier, Ste-Foy, Québec, Canada, G1V 4G2.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.