Abstract
During inflammatory processes, inflamed tissues signal the bone marrow (BM) to produce more mature leukocytes in ways that are not yet understood. We report here that, during the development of lung allergic inflammation, the administration of neutralizing antibodies to the chemotactic cytokine, Eotaxin, prevented the increase in the number of myeloid progenitors produced in the BM, therefore reducing the output of mature myeloid cells from BM. Conversely, the in vivo administration of Eotaxin increased the number of myeloid progenitors present in the BM. Furthermore, we found that, in vitro, Eotaxin is a colony-stimulating factor for granulocytes and macrophages. Eotaxin activity synergized with stem cell factor but not with interleukin-3 or granulocyte-macrophage colony-stimulating factor and was inhibited bypertussis toxin. We report also that CCR-3, the receptor for Eotaxin, was expressed by hematopoietic progenitors (HP). Thus, during inflammation, Eotaxin acts in a paracrine way to shift the differentiation of BM HP towards the myeloid lineage.
DURING INFLAMMATION, the bone marrow (BM) produces and exports extra mature leukocytes that are required at the inflammatory site. Cytokines, such as interleukin-5 (IL-5), IL-3, and granulocyte-macrophage colony-stimulating factor (GM-CSF), produced by T lymphocytes, were thought to play a critical role in controlling the differentiation and proliferation of hematopoietic progenitors in the BM during inflammation.1-5 However, it has been recently reported that hematopoiesis can take place normally in the complete absence of signaling events mediated by these cytokines both in steady state and during hematopoietic stress.6 It was therefore suggested that early phases of hematopoiesis and rapid hematopoietic responses during inflammation must be dependent on alternative mechanisms.
It is well documented that, in addition to their function as chemoattractants, chemokines can also affect the proliferation and/or the differentiation of hemopoietic cells.7,8Macrophage inflammatory protein-1α (MIP-1α), a CC chemokine, was first shown to both inhibit or enhance the proliferation of hematopoietic progenitors (HP) in response to different cytokines.9-12 In addition to MIP-1α, transforming growth factor-β (TGF-β) and tumor necrosis factor-α (TNF-α) were also shown to both inhibit or enhance the proliferation of hemopoietic progenitors in response to other cytokines.13-17
The phenotype of mice deficient for the CXC chemokine, pre-B–cell growth-stimulating factor (PBSF), further suggested an essential role for this chemokine in the development of both B-cell lymphopoiesis and BM myelopoiesis.18-20 The phenotype of IL-8 receptor-deficient mice also suggest a role for IL-8 in controlling the production of mature hematopoietic cells in the BM.21,22The fact that chemokines can affect the proliferation and the differentiation of hematopoietic cells, in addition to their activity as chemoattractants, prompted us to study their role in controlling the proliferation and differentiation of HP during inflammation. We focused our study on Eotaxin, an eosinophil-specific chemoattractant that has been recently cloned using different rodent models of allergic inflammation.23-26 Using a mouse model of allergic inflammation, we have previously shown that Eotaxin is upregulated in the lung within 3 hours of antigen challenge.23 24 We report here that, during lung allergic inflammation, in addition to its activity as a chemoattractant, Eotaxin produced at the site of inflammation can signal to the BM to increase the production and differentiation of cells from the myeloid lineage.
MATERIALS AND METHODS
Mice and in vivo procedures.
Eight- to 10-week-old male and female C57BL/6J or Balb/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and kept in the Center for Blood Research Specific Pathogen Free Mouse Facility. Pulmonary eosinophilia in response to ovalbumin (OVA; Sigma, St Louis, MO) was generated in these mice as described.23,24 Briefly, mice were sensitized with intraperitoneal OVA (0.1 mg/mouse) on day 1 followed by exposure to aerosolized antigen (2% OVA for 5 minutes on day 8 and 1% OVA for 20 minutes on days 15 through 21) to induce the response. At different times after allergen challenge, animals were killed by barbiturate overdose and analyzed. Phosphate-buffered saline (PBS) was administered (intraperitoneally and aerosolized) to mice as a negative control. In the blocking experiments, mice were injected with neutralizing polyclonal antibody against murine Eotaxin (20 μg/mouse, intravenous)23 30 minutes before OVA administration from day 15 to day 21 and then analyzed 3 hours after allergen challenge on the days indicated. OVA-treated control mice were injected with the same amount of control antibody (Ab; Rabbit Immunoglobulin fraction; DAKO, Carpenteria, CA) at the same time points indicated during treatment. No endotoxin contamination was detected in all reagents used, as assessed by LAL assay (BioWhittaker, Walkersville, MD). White blood counts and differential counts for lymphocytes and neutrophils from treated and untreated mice were performed by Tufts Veterinary Diagnostic Laboratory (Grafton, MA).
GM colony assay.
Murine BM cells were aspirated from the tibia and femurs of BALB/c mice 3 to 4 weeks of age (Charles River Lab, Wilmington, MA), C57BL/6J mice, or GM-CSF–deficient mice (kindly given by Glenn Dranoff, Dana Farber Institute, Boston, MA). Erythrocytes were lysed in ammonium chloride lysis buffer and the cells were washed twice with PBS. The number of BM cells was counted, and the total number of BM cells was calculated assuming that the tibia and femurs contain 20% of the total body BM cells. Lin− cells (5 × 103/plate) or BM cells (105/plate) were cultured in methylcellulose (0.9%) containing Iscove's modified Dulbecco's medium (IMDM; BioWhittaker) supplemented with 20% fetal bovine serum (FBS; Intergen, Purchase, NY) and the different growth factors, and incubated at 37°C in 5% CO2 for 7 days. Cells were seeded in the concentration of between 10,000 and 5,000 cells/wells (in triplicates). In a different set of experiments, single cells were seeded (30 cells/96 wells) and 500 wells were scored. Cells from single colonies in each plate were collected at day 7 and then washed twice with PBS. The cells were counted (cells/plate), cytospun, and stained with Giemsa. The number of cells per colony was calculated by collecting all the cells from a plate and dividing the number of cells per plate by the number of colonies per plate.
Cell proliferation assays.
Lin− cells were plated into 96-well microtiter plates at a density of 104 cells/well in IMDM media supplemented with 10% FBS and 5 × 10−6 mol/L 2-β-mercaptoethanol, 1 μCi 3[H] thymidine (Amersham, Arlington Heights, IL), and the different growth factors. FDCP-1 and FDCP-MIX cells27 28 were plated into 96-well microtiter plates at a density of 5 × 103cells/well in IMDM media supplemented with 10% FBS and 5 × 10−6 mol/L 2-β-mercaptoethanol, 1 μCi3[H] thymidine (Amersham), IL-3 (1 ng/mL), and Eotaxin (10 ng/mL). Cultures were incubated for 24 hours at 37°C in 5% CO2 and were harvested with a multiple-sample harvester (Tomtec, Gaithersburg, MD), and their radioactivity was assessed by a liquid scintillation counter (Betaplate; Wallac, Turku, Finland). For long-term proliferation, Lin− cells or FDCP-1 and FDCP-MIX cells were seeded in 24-well plates and incubated at 37°C in 5% CO2 for 1 to 6 days.
Cytokines used in colony and proliferation assays.
Recombinant mouse stem cell factor (rmSCF; Genzyme, Cambridge, MA), rmIL-3 (Pepro Tech, Rocky Hill, NJ), rmGM-CSF (Immunex, Seattle, WA), rmMIP-1α (R&D Systems, Minneapolis, MN), and rmEotaxin (lots 095683, I155(D), and I155(M); Pepro Tech) were all used at a concentration of 100 ng/mL. (Eotaxin Lot 105684 had lower activity and was not used throughout this study.) rmIL-5 was used at 20 ng/mL (R&D), and rhTGF-β (British Bio-technology, Oxon, UK) and rmTNF-α (Genentech, San Francisco, CA) were used at 10 ng/mL and 50 ng/mL, respectively. Monoclonal antibodies against IL-5 (TRFK-5) were added at a concentration of 20 μg/mL (kindly provided by P.T. Bozza, Instituto Oswaldo Cruz-Rio De Janiero, Rio De Janiero, Brazil). Neutralizing polyclonal antibodies against IL-3 or IL-5 were purchased from R&D Systems and were used as recommended by the supplier. Pertussis toxin was purchased from Sigma. The concentration of growth factors used in this study (as described above) falls within the range of concentrations used by other investigators in similar studies. Thus, chemokines such as MIP-1α were generally used at 10 to 1,000 ng/mL, SCF was at 20 to 200 ng/mL, IL-3 and GM-CSF at 10 to 50 ng/mL, TGF-β at 2 to 20 ng/mL, and TNF-α at 10 to 50 ng/mL.11-16,28 29It should be noticed that the concentrations of IL-3 or GM-CSF used for the studies reported here were higher (100 mg/mL) than the concentration used by others (10 to 50 mg/mL). Maximal concentrations of the different growth factors were used throughout this study to get their maximal effect.
Purification of Lin− BM cells.
Murine BM cells were aspirated from tibia and femurs of BALB/c mice 3 to 4 weeks of age (Charles River Lab), C57BL/6J mice, or GM-CSF–deficient mice (kindly given by Glenn Dranoff, Dana Farber Cancer Institute). Erythrocytes were lysed in ammonium chloride lysis buffer and the cells were washed twice with PBS. The number of BM cells was counted, and the total number of BM cells was calculated assuming that the tibia and femurs contain 20% of the total BM cells. The Lin− cells were selected using previously reported techniques.29 30 Briefly, 0.5 μg/106 cells of rat antimouse antibodies specific for RB6-8C5 (GR-1), RA3-6B2 (B220), MAC-1, Lyt-2 (CD-8), L3T4 (CD-4), and Ter-119 (all purchased from Pharmingen, San Diego, CA) were added to BM cells and incubated for 20 minutes at 4°C in PBS supplemented with 2% FBS (PBS/FBS). The cells were washed twice, centrifuged, and resuspended in 3.5 mL PBS/FBS medium. Sheep antirat IgG immunomagnetic beads (Dynal, Oslo, Norway) were then added to the cell suspension at a bead to target-cell ratio of 40:1 and incubated for 20 minutes at 4°C with constant rotation. The cells were magnetically separated with a particle concentrator (Dynal) and Lin− cells were washed with PBS/FBS and resuspended in IMDM supplemented with 10% FBS.
Giemsa and immunoflourescence staining for cells collected from GM colonies and culture plates stimulated with SCF, Eotaxin, and GM-CSF.
To determine the cell type in the GM colonies (or tissue culture plates), cells taken separately from granulocyte and macrophage type of colonies were applied to glass slides by cytocentrifugation, air dried for 10 minutes, and then immersed in Giemsa stain (Sigma), rinsed with distilled water, air-dried, and mounted. For immunoflourescence staining, cells were stained with fluorescein isothiocyanate (FITC)/phycoerythrin (PE)-labeled monoclonal antibodies specific for MAC-1 and GR-1 (PharMingen, San Diego, CA). Briefly, 105cells were washed with staining buffer (0.1% bovine serum albumin, PBS, 0.02% sodium azide) and incubated with 10 μg/mL (1:50) of purified antimouse CD16/CD32(FcR) (PharMingen) for 20 minutes at 4°C. Cells were then washed with staining buffer and the labeled antibodies were added at a dilution of 1:50 for 20 minutes at 4°C. The stained cells were washed twice and analyzed by FACSscan flow cytometer using Cell Quest software (Becton Dickinson, Mountain View, CA).
RESULTS AND DISCUSSION
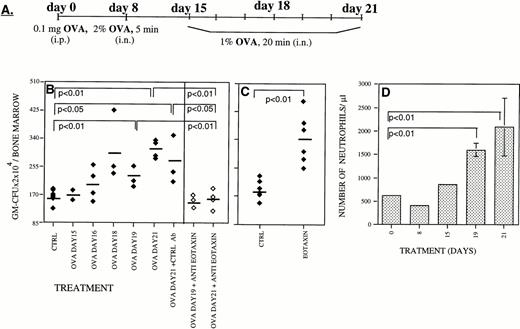
We have recently reported that Eotaxin, an eosinophil-specific chemoattractant, is highly expressed in the lung during the inflammatory phase of a mouse model of lung allergic inflammation.23,24 In this model, mice are sensitized with a single intraperitoneal injection of OVA and then OVA-challenged by repeated aerosolized exposure at day 8 and daily between days 15 and 21 (Fig 1A). When OVA-treated mice were analyzed, we found a progressive increase in both the number of eosinophils infiltrating the lung as well as in the expression of Eotaxin during the inflammatory phase between days 15 and 21.23,24 Using neutralizing antibodies to Eotaxin, we were able to partially inhibit (56%) the accumulation of eosinophils in the lung of OVA-treated mice.23 The assessment of the possible role that Eotaxin could play on the proliferation and differentiation of BM hematopoietic progenitors was studied using the same mouse model of lung inflammation.23,24 We report here that during the inflammatory phase there was an increase in the number of granulocyte-macrophage colony-forming units (GM-CFU) in the BM (Fig 1B, ⧫). Interestingly, we also found that during this period there was an increase in the percentage of neutrophils in the blood from 12% at day 1 of the treatment to 36% by day 21 (Fig 1D). Although transient elevation of eosinophils in the blood was detected after each OVA administration, we failed to detect a maintained elevation of eosinophil numbers in the blood of these OVA-treated mice. We have previously reported that, during the inflammatory phase of this lung allergic reaction, there is an eightfold induction of Eotaxin mRNA levels (relative to 28s rRNA) in the lung as judged by RNAse protection assay (Gonzalo et al23 24 and data not shown). This increase in mRNA was paralleled assay by an increase in Eotaxin protein levels in the serum of OVA treated mice (twofold to fourfold as estimated by Western blot analysis; data not shown).
During inflammatory processes Eotaxin stimulates the production of myeloid progenitors in the BM. Mice were sensitized with intraperitoneal OVA on day 0 followed by exposure to aerosolized OVA on day 8 and on days 15 through 21 (A). In a murine model of lung allergic inflammation, administration of neutralizing antibodies to Eotaxin (◊) prevented the increase in the number of granulocyte-macrophage colony-forming units (GM-CFU/BM) in the BM during the inflammatory phase between days 15 and 21 (⧫)22 (B). In vivo administration of Eotaxin increased the number of GM-CFU present in the BM (C). The increase in the number of blood neutrophils in the blood of OVA-treated mice is shown in (D). Each point is the mean of three determinations ± standard deviation (SD). For each panel the significance of differences between treated and untreated groups of mice were determined by a standard Student's t-test.
During inflammatory processes Eotaxin stimulates the production of myeloid progenitors in the BM. Mice were sensitized with intraperitoneal OVA on day 0 followed by exposure to aerosolized OVA on day 8 and on days 15 through 21 (A). In a murine model of lung allergic inflammation, administration of neutralizing antibodies to Eotaxin (◊) prevented the increase in the number of granulocyte-macrophage colony-forming units (GM-CFU/BM) in the BM during the inflammatory phase between days 15 and 21 (⧫)22 (B). In vivo administration of Eotaxin increased the number of GM-CFU present in the BM (C). The increase in the number of blood neutrophils in the blood of OVA-treated mice is shown in (D). Each point is the mean of three determinations ± standard deviation (SD). For each panel the significance of differences between treated and untreated groups of mice were determined by a standard Student's t-test.
During this inflammatory phase, Eotaxin expression in the lung increased23 24 concomitant with an increase in serum concentration (data not shown). Intravenous administration of neutralizing antibodies to Eotaxin during the inflammatory phase (day 15 to day 21) totally inhibited the increase in the number of GM-CFU in the BM (Fig 1B, ◊). This result suggests a role for Eotaxin in stimulating the production of myeloid cells in the BM during lung inflammation. Further analysis of the in vivo effects of Eotaxin was accomplished by intravenous injection of recombinant Eotaxin into BALB/c mice. Two days after the administration of Eotaxin, we detected a significant increase in the number of GM-CFU in the BM (Fig1C).
The results obtained in vivo indicate that Eotaxin might act as a proliferation and differentiation factor for hematopoietic cells. Therefore, we tested the effect of Eotaxin on the proliferation of Lin− hematopoietic progenitors in vitro. Using thymidine incorporation as a short-term proliferation assay, we found that Eotaxin could stimulate the short-term proliferation of Lin− cells, as well as SCF, GM-CSF, and IL-3 (Fig 2A1). When Eotaxin was added to cells stimulated with SCF, it induced an additive proliferative effect (Fig2A4), but it had no effect on the proliferation of cells stimulated by GM-CSF or IL-3 (Fig 2A3). As shown previously by others, MIP-1α, TGF-β, and TNF-α by themselves had no proliferative effect on Lin− cells (Fig 2A1). However, as others have shown,11-16,31 32 when any of these growth factors were added to cells stimulated by the combination of SCF and IL-3, they inhibited their proliferation (Fig 2A2). Our results suggest that Eotaxin acts on the same Lin− target cells stimulated by GM-CSF and IL-3 but not on those stimulated by SCF. Alternatively, Eotaxin may use the signal transduction pathways used by IL-3 and GM-CSF but not that used by SCF. Experiments to address these two possibilities are currently underway.
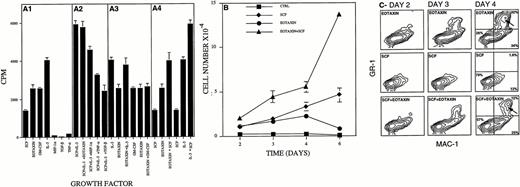
Eotaxin induced the proliferation and differentiation of Lin− hematopoietic progenitors into granulocytes and macrophages. Thymidine incorporation (see the Materials and Methods; measured in counts per minute per well) induced by Eotaxin (A1), Eotaxin plus SCF and IL-3 (A2), Eotaxin plus IL-3 or GM-CSF (A3), and Eotaxin plus SCF (A4) is shown in (A). Proliferation of Lin− cells (5 × 103) stimulated with SCF, Eotaxin, or the combination of the two is shown in (B). The expression of the granulocyte and macrophage cell surface differentiation markers GR-1 and MAC-1 is shown in (C). Granulocytes that were double-stained for GR-1 and MAC-1 are marked by an arrow. The percentage of cells either not expressing MAC-1 and GR-1 or expressing MAC-1 or MAC-1 and GR-1 is shown at day 4 (C). Three different experiments were performed; the results shown are of one representative experiment. Each point is the mean of three to six determinations ± SD.
Eotaxin induced the proliferation and differentiation of Lin− hematopoietic progenitors into granulocytes and macrophages. Thymidine incorporation (see the Materials and Methods; measured in counts per minute per well) induced by Eotaxin (A1), Eotaxin plus SCF and IL-3 (A2), Eotaxin plus IL-3 or GM-CSF (A3), and Eotaxin plus SCF (A4) is shown in (A). Proliferation of Lin− cells (5 × 103) stimulated with SCF, Eotaxin, or the combination of the two is shown in (B). The expression of the granulocyte and macrophage cell surface differentiation markers GR-1 and MAC-1 is shown in (C). Granulocytes that were double-stained for GR-1 and MAC-1 are marked by an arrow. The percentage of cells either not expressing MAC-1 and GR-1 or expressing MAC-1 or MAC-1 and GR-1 is shown at day 4 (C). Three different experiments were performed; the results shown are of one representative experiment. Each point is the mean of three to six determinations ± SD.
We further analyzed the long-term effect(s) of Eotaxin on the proliferation and differentiation of BM HP by growing the cells in the presence of Eotaxin. Eotaxin induced the proliferation of Lin− hematopoietic progenitors for up to 4 days (Fig2B). This proliferative response was coupled with terminal differentiation of the Lin− cells into macrophages that express the differentiation cell surface marker MAC-1 (34%) and granulocytes that express the differentiation cell surface markers MAC-1 and GR-1 (Fig 2C, 32%, arrow). In contrast, most of the cells proliferating in response to SCF, used as a control, maintained their blast morphology and did not express either MAC-1 or GR-1 (Fig 2B, 2C, 79%). In agreement with the short-term proliferation assay (Fig 2A), addition of Eotaxin to cells stimulated with SCF induced an additive effect on the expansion and differentiation of the MAC-1+(25%) and MAC-1+/GR-1+ (12%) hematopoietic cells that were found in the cultures after 4 to 6 days (Fig 2C, arrow). We therefore concluded that Eotaxin by itself could induce short-term proliferation and terminal differentiation of hematopoietic progenitors into macrophages and granulocytes.
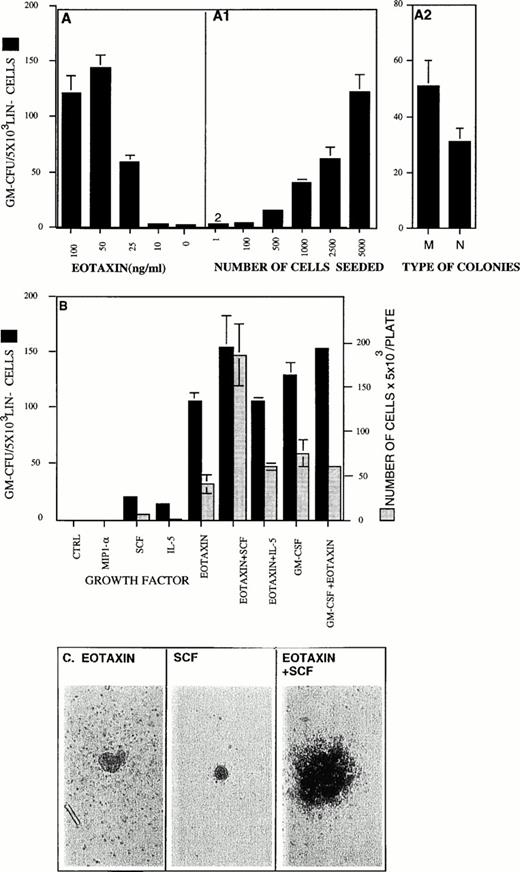
Eotaxin was also tested for its ability to stimulate the formation of GM colonies in methylcellulose. We found that Eotaxin acts as a GM-CSF for Lin− cells in the range of 10 to 100 ng/mL (Fig 3A). Eotaxin has been shown to induce chemotaxis of eosinophils in vitro at the same concentration range.26 In the absence of exogenous growth factors, few, if any (0 to 2 colonies/500 cells) colonies were observed (Fig 3A). To rule out an indirect colony-stimulating effect by Eotaxin through the activation of more mature cells, we performed dilution experiments. We found a direct correlation between the number of seeded cells and the number of colonies counted (Fig 3A1). In multiple experiments, we seeded as few as 100 cells per plate and this resulted in the generation of 2 to 3 colonies per well (at this concentration of cells, in the absence of exogenous growth factors, no colonies were detected; not shown). When we seeded 500 cells per well, we obtained approximately 20 (18 ± 2, n = 96) colonies per well in the presence of Eotaxin and 0 to 2 colonies in the absence of exogenous growth factors. These results suggest that Eotaxin acts directly on the progenitor cells. However, these results cannot exclude the possibility that Eotaxin induced secretion of an autocrine growth factor. Eotaxin stimulated the formation of both macrophages and neutrophil type of colonies (Fig 3A2). No differences were found in the colony-stimulating activity of Eotaxin when Lin cells purified from Balb/C and C57BL/6J mice were used (data not shown). Eotaxin stimulated the same number of colonies as GM-CSF, but the Eotaxin-induced colonies were smaller and the number of cells per colony was between twofold and fivefold lower (Eotaxin/GM-CSF, ∼500 to 1,000/∼2,000 to 3,000 cells per colony, Fig 3B). In control colony assays, both IL-5 and SCF stimulated few colonies with a low number of cells per colony (Fig 3B). However, when SCF or IL-5 were added with Eotaxin, only SCF had an additive effect on the number of GM colonies stimulated by Eotaxin (Fig 3B). The effect of SCF on the number of cells per colony induced by Eotaxin was synergistic (Fig 3B and C). In agreement with the short-term proliferation assay, Eotaxin did not significantly modify the number of colonies or the number of cells per colony induced by GM-CSF or IL-3 (Fig 3B, data not shown).
Eotaxin is a GM-CSF for Lin− hematopoietic progenitors and can synergize with SCF to stimulate the production of granulocytes and macrophages. The concentration-dependent colony-stimulating activity of Eotaxin is shown in (A). The ability of Eotaxin (100 ng/mL) to stimulate colony formation in Lin−cells seeded in the concentration of 1 to 5,000 cells/plate is shown in (A1; see the Materials and Methods). Macrophages (M) and granulocyte-neutrophils (N) types of colonies stimulated by Eotaxin are shown in (A2). The colony-stimulating activities of Eotaxin, IL-3, MIP-1α, GM-CSF, SCF, and IL-5 and their combinations are shown (▪). The number of cells per plate collected from the GM-CFU assay (see the Materials and Methods) is shown (▧). (C) The pictures of colonies stimulated by either SCF, Eotaxin, or both were taken at an original magnification of ×200. Five different experiments were performed. The results shown are of one representative experiment. Each point is the mean of three determinations ± SD.
Eotaxin is a GM-CSF for Lin− hematopoietic progenitors and can synergize with SCF to stimulate the production of granulocytes and macrophages. The concentration-dependent colony-stimulating activity of Eotaxin is shown in (A). The ability of Eotaxin (100 ng/mL) to stimulate colony formation in Lin−cells seeded in the concentration of 1 to 5,000 cells/plate is shown in (A1; see the Materials and Methods). Macrophages (M) and granulocyte-neutrophils (N) types of colonies stimulated by Eotaxin are shown in (A2). The colony-stimulating activities of Eotaxin, IL-3, MIP-1α, GM-CSF, SCF, and IL-5 and their combinations are shown (▪). The number of cells per plate collected from the GM-CFU assay (see the Materials and Methods) is shown (▧). (C) The pictures of colonies stimulated by either SCF, Eotaxin, or both were taken at an original magnification of ×200. Five different experiments were performed. The results shown are of one representative experiment. Each point is the mean of three determinations ± SD.
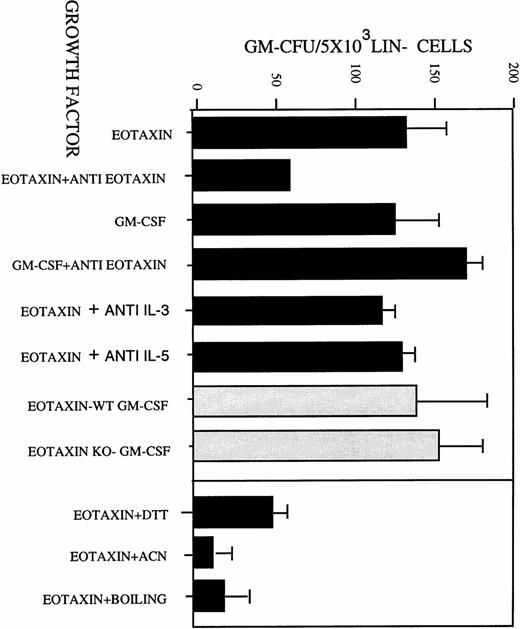
To rule out possible autocrine GM-CSF secretion by cells stimulated by Eotaxin, Lin− progenitors purified from the BM of GM-CSF–deficient mice were studied. We found no difference in the GM colony-stimulating activity of Eotaxin on both Lin−progenitors purified from wild-type or GM-CSF–deficient mice (Fig 4A, ▧). We also did not find any difference in the GM colony-stimulating activity of Eotaxin when neutralizing antibodies to IL-3 or IL-5 were used in these assays (Fig4A). We therefore concluded that neither IL-3, nor IL-5, nor GM-CSF are involved in the colony-stimulating activity of Eotaxin. However, this does not exclude the possibility that other growth factors are involved in this proliferative response. In addition, the colony-stimulating activity of Eotaxin was inhibited by (1) treating the protein with either 1 mmol/L dithiothreitol (DTT) or acetonitrile, (2) boiling, or (3) neutralizing antibodies to Eotaxin (Fig 4A).
Eotaxin induced the differentiation and proliferation of hematopoietic progenitors into metamyelocytes and macrophages in a GM-CSF–independent pathway. (A) Eotaxin was either treated with 1:100 polyclonal antibodies to Eotaxin or 50 μg/mL neutralizing polyclonal antibodies to IL-3 or to IL-5. As a control, antibodies against Eotaxin were also mixed with GM-CSF. The treated and untreated Eotaxin and GM-CSF were then used to perform a GM-CFU assay using Lin− cells that were purified from BM of wild-type (WT) BALB/c mice (▪). Eotaxin was used to stimulate GM colony formation using Lin− cells that were purified from BM of GM-CSF–deficient mice (KO)3 or wild-type (WT) BALB/c mice (▧). The activity of Eotaxin was inactivated by treatment with 1 mmol/L DTT, acetonitrile (ACN), and boiling for 5 minutes (B). The results shown in (A) are the mean of three individual experiments ± SD.
Eotaxin induced the differentiation and proliferation of hematopoietic progenitors into metamyelocytes and macrophages in a GM-CSF–independent pathway. (A) Eotaxin was either treated with 1:100 polyclonal antibodies to Eotaxin or 50 μg/mL neutralizing polyclonal antibodies to IL-3 or to IL-5. As a control, antibodies against Eotaxin were also mixed with GM-CSF. The treated and untreated Eotaxin and GM-CSF were then used to perform a GM-CFU assay using Lin− cells that were purified from BM of wild-type (WT) BALB/c mice (▪). Eotaxin was used to stimulate GM colony formation using Lin− cells that were purified from BM of GM-CSF–deficient mice (KO)3 or wild-type (WT) BALB/c mice (▧). The activity of Eotaxin was inactivated by treatment with 1 mmol/L DTT, acetonitrile (ACN), and boiling for 5 minutes (B). The results shown in (A) are the mean of three individual experiments ± SD.
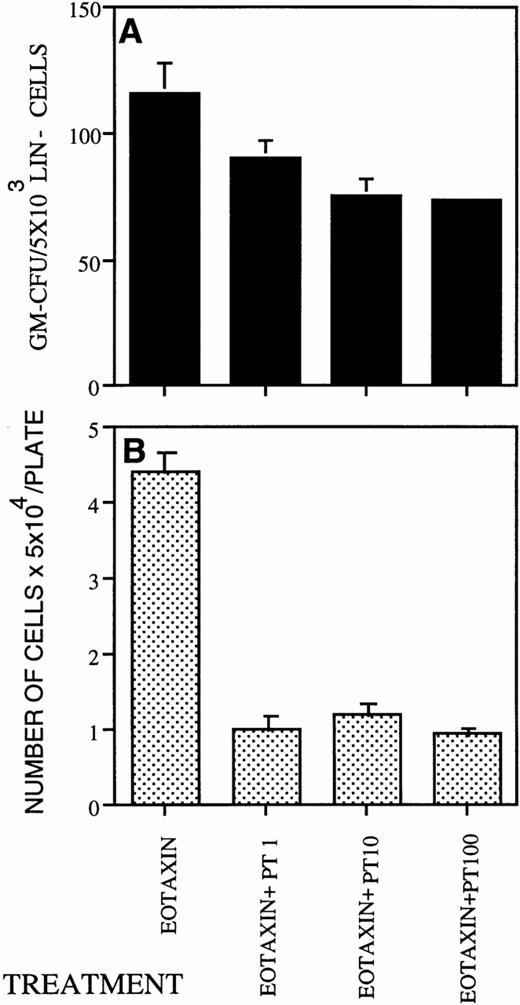
Pertussis toxin blocks cell activation induced by Gi-protein–coupled receptors. Pretreatment of eosinophils withpertussis toxin completely inhibited the Eotaxin-induced responses.33 We therefore tested the effect ofPertussis toxin on the colony-stimulating activity of Eotaxin. In the concentration of 100 ng/mL Pertussis toxin inhibited the number of colonies stimulated by Eotaxin by 40% ± 1.1% (Fig 5A) and the number of cells produced in these colonies by 75% ± 8.3% (Fig 5B). As expected, we found that Lin− cells expressed the Eotaxin receptor, CCR-3 (Rothenberg et al,34 Ponath et al,35 Daugherty et al,36 and data not shown).
Pertussis toxin inhibits the colony-stimulating activity of Eotaxin. (A) Pertussis toxin in the range of 1 to 100 ng/mL blocked the colony-stimulating activity of Eotaxin (100 ng/mL). The number of cells per plate collected from the GM-CFU assay is shown in (B). Three different experiments were performed. The results shown are of one representative experiment. Each point is the mean of three determinations ± SD.
Pertussis toxin inhibits the colony-stimulating activity of Eotaxin. (A) Pertussis toxin in the range of 1 to 100 ng/mL blocked the colony-stimulating activity of Eotaxin (100 ng/mL). The number of cells per plate collected from the GM-CFU assay is shown in (B). Three different experiments were performed. The results shown are of one representative experiment. Each point is the mean of three determinations ± SD.
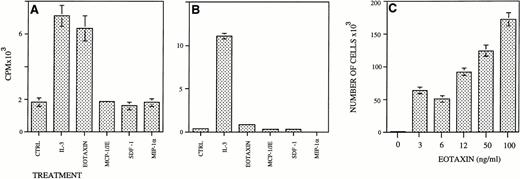
We then searched for a mouse myeloid cell line that could respond by proliferation to Eotaxin. We found that the IL-3–dependent FDCP-1 myeloid cells but not IL-3–dependent FDCP-mix myeloid cells responded to Eotaxin by proliferation (Fig 6A and B). SDF-1, MIP-1α, and MCP-1 could not support the proliferation of FDCP-1 cells. Eotaxin induced a dose-dependent proliferation response in these cells in concentrations greater than 3 ng/mL (Fig 6C). Partially purified heparin-bound Eotaxin produced in p3x63 myeloma cells24 was also able to stimulate the proliferation of FDCP-1 cells (data not shown). As shown for MIP-1α,17 the relatively high doses of Eotaxin needed for the stimulation of HP proliferation could be the result of an inbalance between the monomeric form of Eotaxin and its polymerized form.
Eotaxin induces the proliferation of FDCP-1 but not FDCP-MIX IL-3–dependent myeloid cell lines. FDCP-1 (A) or FDCP-MIX cells (B) (5,000 cells/well) were seeded in 96-well plates and supplemented with the different growth factors. Thymidine incorporation was measured after 24 hours as counts per minute (CPM)/well. FDCP-1 (5 × 103) cells were seeded in 24-well plates and stimulated with different concentrations of Eotaxin. Cells were collected and counted after 6 days (C). Five different experiments were performed. The results shown are of one representative experiment. Each point is the mean of three determinations ± SD.
Eotaxin induces the proliferation of FDCP-1 but not FDCP-MIX IL-3–dependent myeloid cell lines. FDCP-1 (A) or FDCP-MIX cells (B) (5,000 cells/well) were seeded in 96-well plates and supplemented with the different growth factors. Thymidine incorporation was measured after 24 hours as counts per minute (CPM)/well. FDCP-1 (5 × 103) cells were seeded in 24-well plates and stimulated with different concentrations of Eotaxin. Cells were collected and counted after 6 days (C). Five different experiments were performed. The results shown are of one representative experiment. Each point is the mean of three determinations ± SD.
In this study, we have compared the effects of the novel chemokine Eotaxin along with MIP-1α, TGF-β, or TNF-α on the proliferation and differentiation of Lin− cells. Our results indicate that Eotaxin by itself or in combination with SCF can stimulate the proliferation and differentiation of HP cells. Furthermore, we found that this stimulatory effect was dependent on the network of cytokines used by these cells. In fact, MIP-1α has been shown to reversibly inhibit the proliferation of murine day-12 colony-forming units-spleen (CFU-S). MIP-1α was also shown to enhance IL-3– and GM-CSF–induced colony formation of Lin− progenitors, but had no effect on G-CSF– and CSF-1–induced colony formation. However, the inhibitory effects of MIP-1α could only be detected when a combination of two or more cytokines were used. In human cells, MIP-1α inhibits the proliferation of CFU-granulocytes, erythrocytes, macrophages, megakryocytes, burst-forming unit-erythroids, and CFU-GM, whereas more mature CFU-erythrocytes, CFU-granulocytes, and CFU-GM progenitors are stimulated.9-1215,31,32Although less effective than TGF-β and TNF-α, in our experiments, MIP-1α also partially blocked the proliferative effect induced by SCF and IL-3 (Fig 2A). TGF-β was shown to inhibit the action of SCF, IL-3, and CSF-1 on mouse hematopoietic progenitors. It was also shown to inhibit primitive HP cells, whereas more mature cells were not affected. However, TGF-β was also shown to enhance the growth of BM progenitors in response to GM-CSF.15,16,28 TNF-α was shown to inhibit the proliferation of primitive Lin−and Lin− SCA-1+ cells. It was also shown to inhibit the proliferation of HP cells induced to proliferate by SCF. The stimulatory effects of TNF-α were shown to be indirect and were the result of increased production of IL-3 and GM-CSF.13-15TNF-α, TGF-β, and MIP-1α were shown by us to inhibit the proliferation of Lin− cells in response to SCF and IL-3. By themselves, none of these stem cell inhibitors could induce proliferation of Lin− cells (Fig 2A1). SCF was shown to induce the proliferation of primitive HP cells. In vivo, this stimulation was coupled with increased myeloid differentiation.28 29
During inflammation, signals produced at the site of injury enhance the production and migration of mature leukocytes from the BM. It has been believed that IL-3/GM-CSF/IL-5 produced by T cells play a major role in the expansion of hematopoietic cells in an emergencies. However, it was recently shown that the entire function of the IL-3/GM-CSF/IL-5 is dispensable for hematopoiesis in an emergencies as well as in a steady state.6 These investigators suggest the existence of an alternative mechanism to produce blood cells in both situations. Our results indicate that chemokines might be involved in such an alternative mechanism.
We have shown in this study that the chemotactic cytokine Eotaxin can act both in vivo and in vitro as a growth and differentiation factor for myeloid hematopoietic progenitors. Our results suggest that Eotaxin produced at inflammatory sites can stimulate the differentiation and proliferation of myeloid progenitors in the BM, thereby contributing to the generation of mature leukocytes needed to perform the inflammatory reaction.
ACKNOWLEDGMENT
The authors are indebted to Barrett Rollins and Frank Lee for critical reading of this manuscript. We thank G.-Q. Jia and M. Bozza for their important suggestions and continuous support of this project and C. Martinez-Alonso and J.P. Albar for the neutralizing polyclonal antibodies against murine Eotaxin. The authors thank Marci Melzer for her editorial assistance.
Supported by National Institutes of Health Grants No. HL 148675-02, CiCyT PB93-0317, HL94-10-B, and HL36028 and by the Aplastic Anemia Foundation of America grants. J.-C.G.-R. is the Amy C. Potter Fellow. A.P. is a recipient of the Dorot Fellowship of the Israeli Academy of Science and Harvard Medical School. J.A.G. is a recipient of the postdoctoral Fellowship from the Spanish Ministry of Science.
Address reprint requests to J.-C. Gutierrez-Ramos, PhD, Millennium Pharmaceuticals, Inc, 640 Memorial Dr, Cambridge, MA 02139-4815.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.