Abstract
The Philadelphia (Ph1) chromosome can be detected in chronic myelogenous leukemia (CML) and a significant number of acute lymphoblastic leukemia (ALL) cases. Generation of p210bcr/abl, a chimeric protein with enhanced kinase activity, is thought to be involved in the pathogenesis of these diseases. To elucidate the biological properties of p210bcr/abl and to create an animal model for human Ph1-positive leukemias, we generated transgenic mice expressing p210bcr/abl driven by the promoter of the tec gene, a cytoplasmic tyrosine-kinase preferentially expressed in the hematopoietic lineage. The founder mice showed excessive proliferation of lymphoblasts shortly after birth and were diagnosed as suffering from ALL based on surface marker and Southern blot analyses. Expression and enhanced kinase activity of the p210bcr/abl transgene product were detected in the leukemic tissues. In contrast, transgenic progeny exhibited marked granulocyte hyperplasia with thrombocytosis after a long latent period and developed myeloproliferative disorders (MPDs) closely resembling human CML. Expression of p210bcr/abl mRNA in the proliferating granulocytes was detected by RT-PCR. In particular, one MPD mouse showed remarkable proliferation of blast cells in the lung, which might represent an extramedullar blast crisis. The results demonstrate that the expression of p210bcr/abl in hematopoietic progenitor cells in transgenic mice can contribute to two clinically distinct hematopoietic malignancies, CML and ALL, indicating that this transgenic system provides a novel transgenic model for human Ph1-positive leukemias.
CHRONIC MYELOGENOUS leukemia (CML) is a hematopoietic disorder of pluripotent stem cells characterized by uncontrolled proliferation of the myeloid series in peripheral blood and bone marrow.1,2 In the initial stage of the disease, chronic phase, the leukemic cells proliferate but have the ability to differentiate into mature granulocytes. However, the disease eventually accelerates after several years' duration and ultimately progresses to the terminal phase, blast crisis, which involves the accumulation of monoclonal immature hematopoietic cells arrested at an early stage of differentiation.3
The cytogenetic hallmark of the disease is the Philadelphia chromosome (Ph1), a shortened chromosome 22.4,5 It is generated by a reciprocal translocation between chromosome 9 and 22, t(9;22)(q34;q11), where the c-abl proto-oncogene on chromosome 9 is translocated into a 5.8 kilobase (kb) region on chromosome 22, denoted the major breakpoint cluster region (M-bcr).6 This translocation fuses the 5′ exons of the bcr gene to most of the 3′ exons of the c-abl gene in a head-to-tail manner, thereby producing a novel 8.5kb chimericbcr/abl mRNA encoding a 210 kD protein (p210bcr/abl).
The Ph1 chromosome is also observed in 10% to 20% of acute lymphoblastic leukemia (ALL) patients.2 In approximately half of the cases, the translocation occurs at the same breakpoint as in CML patients and creates the p210bcr/abl hybrid protein. However, in the remaining Ph1-positive ALL patients, the breakpoint exists within the first intron of the BCR gene, designated the minor breakpoint cluster region (m-bcr). This event generates a 7.0 kb mRNA, which is transcribed to a 190 kD chimeric protein (p190bcr/abl) with a smaller bcr moiety.7-11 Both of the chimeric proteins (p210bcr/abl and p190bcr/abl) possess enhanced kinase activity in comparison with the normal 145 kD c-abl product and both have been considered to be implicated in the pathogenesis of Ph1-positive human leukemias.12
To investigate the biological function of the p210bcr/abl in vivo, efforts have been made to create a mouse model that mimics human Ph1-positive leukemias. For this purpose, two different approaches have been used, namely, virus-mediated gene transfer and generation of transgenic mice.
Daley et al performed bone marrow transplantation (BMT) in mice. They reconstituted lethally irradiated mice with bone marrow cells infected with p210bcr/abl-expressing retroviruses and showed that some of the recipients developed hematologic malignancies including granulocytic hyperplasias resembling human CML, ALLs, and tumors of macrophage cell type.13 DNA analysis revealed that a limited number of the infected cells had differentiated and reconstituted the hematopoietic cell series. Other studies also using syngeneic mice lethally irradiated and transplanted with p210bcr/abl-expressing bone marrow cells demonstrated development of various types of hematologic disorders such as granulocytic leukemias, myelomonocytic leukemias, pre-B and T-cell lymphomas, reticulum cell sarcomas, and erythroid tumors.14,15 As an alternative approach of virus-mediated gene transfer, Clark et al injected p190bcr/abl- or p210bcr/abl-expressing retroviruses directly into thymi of mice.16 The recipients expressing both types of chimeric proteins developed thymomas after a relatively long latent period. These results imply that hematopoietic progenitor cells infected with p210bcr/abl-containing retroviruses acquire a proliferative advantage and cause various types of hematologic malignancies.
Generating transgenic mice is another attractive approach for investigating the biological properties of p210bcr/abl. Hariharan et al microinjected abcr and v-abl fusion gene coupled with either the immunoglobulin heavy-chain enhancer (Eμ) or the part of the long terminal repeat (LTR) of the myeloproliferative sarcoma virus (MPSV) and generated transgenic mice expressing p210bcr/v-abl.17 Pre-B or T-cell lymphomas developed in some of the animals bearing either of the constructs after a variable latent period. These findings indicate thatbcr/v-abl has oncogenic activity in vivo. However,bcr/v-abl differs from bcr/abl in that it lacks parts of the bcr- and abl-derived regions and it has several amino acid substitutions in the latter.17,18 Thus, the results obtained with p210bcr/v-abl transgenic mice may not accurately reflect the biological properties of the original hybrid protein. To overcome this issue, we have generated transgenic mice expressing p210bcr/abl using the metallothionein (MT) enhancer/promoter element (MT/p210bcr/abl).19 We found two of six founder mice and transgenic progeny to develop ALLs, classified as being of T-cell origin by flow cytometric and Southern blot analyses. Expression and enhanced kinase activity of the p210bcr/abl transgene product in the leukemic tissues were detected. In addition, the tissue distribution ofbcr/abl mRNA expression indicated that the oncogenecity of p210bcr/abl is restricted to hematopoietic organs. Our results thus demonstrated that the p210bcr/ablchimeric protein contributed a proliferative advantage selectively to hematopoietic precursor cells and induced T-cell leukemia in transgenic mice.19 Subsequently, Voncken et al also generated mice transgenic for p210bcr/abl using two variations of the MT promoter and described acute hematopoietic malignancies mainly of lymphoid origin and rarely of myeloid origin.20
As described above, p210bcr/abl transgenic mice provide good models for human Ph1-positive leukemias. However, they develop exclusively ALLs, in contrast to mice reconstituted with p210bcr/abl-expressing bone marrow cells which exhibit myeloproliferative disorders (MPDs) resembling human CML. To date, no transgenic system exists that models human CML, raising the question of whether p210bcr/abl transgenic mice are capable of exhibiting CML-like disease. To address this issue, we cloned and analyzed the promoter region of mouse tecgene,21,22 a cytoplasmic kinase preferentially expressed in hematopoietic precursor cells23 and generated p210bcr/abl transgenic mice using the tecpromoter as a regulatory sequence. While the founder mice developed ALLs, the transgenic progeny indeed demonstrated MPDs closely resembling human CML.
MATERIALS AND METHODS
Construction of the transgene and generation of transgenic mice.
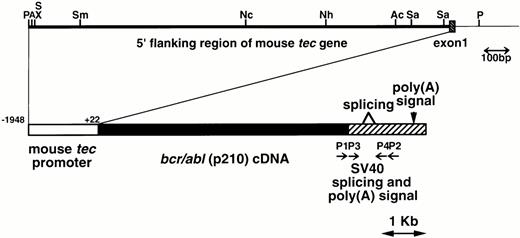
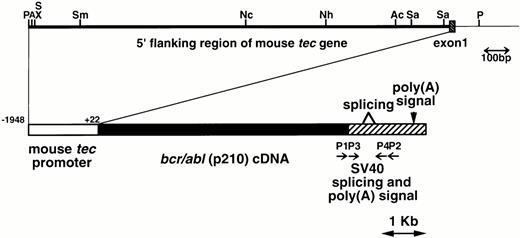
The metallothionein-I enhancer/promoter of pMT/bcr/abl19 was replaced by the mouse tecpromoter (−1948 to +22, designated the major transcription initiation site defined by RACE-PCR as +121,22). A DNA fragment containing the mouse tec promoter, the bcr/abl (p210) cDNA (b3a2 type), and the SV40 early splicing and polyadenylation signals was microinjected into pronuclei of eggs from C57BL/6XDBA/2 F2 mice essentially as described earlier.24 The schematic structure of the transgene is shown in Fig1. The transgenic mice were identified by hybridizing tail DNA with the injected fragment as described earlier.19
Schematic model of the injection fragment for generating transgenic mice. The upper part of the figure shows the genomic structure of the mouse tec gene. The 5′ flanking region and the first intron are represented by thick and thin bars, respectively and the first exon is shown as a shaded box. Restriction enzyme sites are P; PstI, A; ApaI, S; SalI, X; XhoI, Sm;SmaI, Nc; NcoI, Nh; NheI, Ac; AccIII, and Sa; SacI. The lower part illustrates the structure of the injection fragment. The tec promoter (−1948 to +22), thebcr/abl (p210) cDNA (b3a2 type), and the SV40 early splicing and poly(A) signals are shown as white, black, and shaded boxes, respectively. The primers used for RT-PCR (P1-P4) are also indicated.
Schematic model of the injection fragment for generating transgenic mice. The upper part of the figure shows the genomic structure of the mouse tec gene. The 5′ flanking region and the first intron are represented by thick and thin bars, respectively and the first exon is shown as a shaded box. Restriction enzyme sites are P; PstI, A; ApaI, S; SalI, X; XhoI, Sm;SmaI, Nc; NcoI, Nh; NheI, Ac; AccIII, and Sa; SacI. The lower part illustrates the structure of the injection fragment. The tec promoter (−1948 to +22), thebcr/abl (p210) cDNA (b3a2 type), and the SV40 early splicing and poly(A) signals are shown as white, black, and shaded boxes, respectively. The primers used for RT-PCR (P1-P4) are also indicated.
Histopathologic examination.
Autopsies were performed on dead or moribund animals. Smears of peripheral blood and stamp specimens of tissues were stained with Wright-Giemsa (WG). Tissues were also fixed in 10% neutral buffered formaldehyde for routine light microscopy. All the organs were grossly examined and representative slices were prepared for hematoxylin-eosin (HE) staining.
Western blotting, immunoprecipitation, and in vitro kinase assays.
Tissues were homogenized in RIPA lysis buffer (150 mmol/L NaCl, 50 mmol/L Tris-Cl (pH 7.4), 1% Triton X-100, 0.05% sodium dodecyl sulfate [SDS], 1% sodium deoxycholate) with 50 U/mL aprotinin. For detecting the p210bcr/abl transgene product, 100 μg aliquots of total proteins were separated by 6% SDS-polyacrilamide gel electrophoresis (PAGE), transferred to polyvinylidene membranes (Immobilon; Millipore, Yonezawa, Japan), and probed with 1:200 diluted anti–c-Abl monoclonal antibody (MoAb), AB3 (Oncogene Science, Manhasset, NY). For in vitro kinase assays, 1 mg aliquots of total proteins were incubated with 1:200 diluted AB3 and antimouse rat IgG (Sigma, St Louis, MO) coupled with protein A (Sigma). The immunoprecipitated proteins were washed five times with the lysis buffer, followed by five times with the kinase buffer (50 mmol/L Tris-Cl (pH 7.4), 10 mmol/L MgCl2, and 10 mmol/L MnCl2), and incubated with 10 μCi of γ32P-ATP (Amersham, Arlington Heights, IL) at room temperature for 15 minutes. The phosphorylated proteins were separated by 6% SDS-PAGE, dried, and autoradiographed.
Flowcytometric analysis.
Leukemic cells were stained with fluorescein isothiocyanate (FITC)-conjugated commercial MoAbs including Thy-1.2, B220, Mac-1, or Gr-1 (Fujisawa, Osaka, Japan) according to the manufacturer's instructions. The stained cells were washed three times with PBS and analysed on a FACScan (Becton Dickinson, Sunnyvale, CA).
DNA analysis of leukemic cells.
High molecular weight DNAs were extracted from thymi of leukemic mice or nontransgenic controls as previously described.25 Ten microgram aliquots of DNA were digested with appropriate restriction enzymes (Takara, Kyoto, Japan), separated in 0.7% agarose gels, transferred to Nylon membranes (Hybond-N; Amersham International plc, UK), and hybridized with a mouse TCRγ probe. Hybridization and washing conditions were as described earlier.19 The signals were detected using a Fuji image analyser (Fujix bas 2000; Fujifilm, Kanagawa, Japan).
RNA extraction and PCR amplification.
RESULTS
ALLs and myeloproliferative disorders in transgenic mice expressing p210bcr/abl.
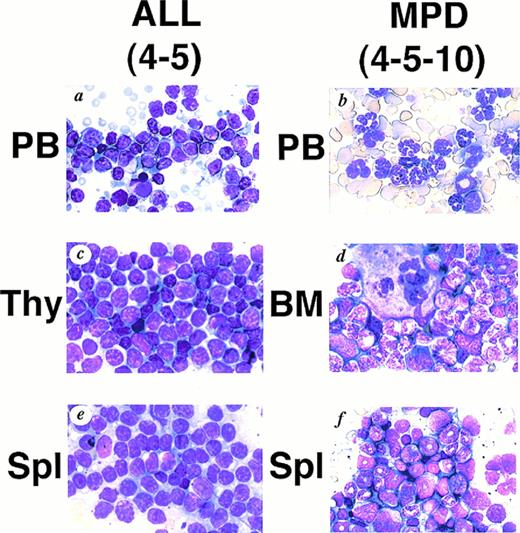
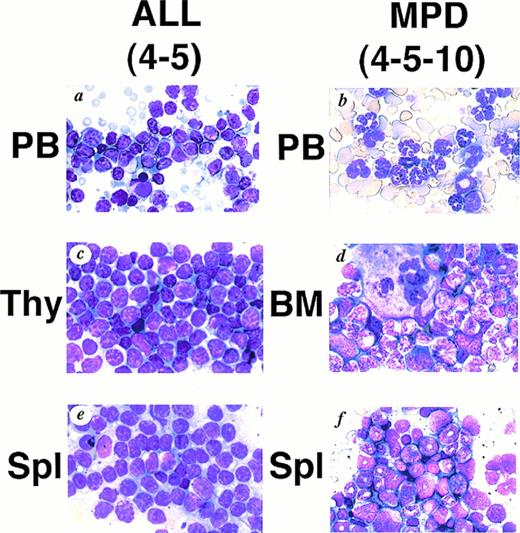
We generated five founder mice carrying five to ten copies of the transgene. Among them, two mice, numbers 4-3 and 4-5, died of leukemias 3 and 4 months after birth, respectively (Table1). Macroscopically, both mice showed thymic enlargement, marked splenomegaly, and lymphnode swelling as observed in MT/p210bcr/abl leukemic mice.19 Blood parameters for 4-5 were: WBC 30 × 103/μL (>90% blast cells), Hb 14.0 g/dL, and Plt 50 × 104/μL (Table 1). Smears of peripheral blood showed massive proliferation of blast cells with no granules, having the appearance of lymphoblasts (Fig 2A).Histopathologic examination revealed marked proliferation of blast cells in the thymus (Fig 2C), spleen (Fig 2E) and all other tissues sampled (data not shown). According to the clinical course and the results of surface marker and DNA analyses (see below), ALLs were diagnosed.
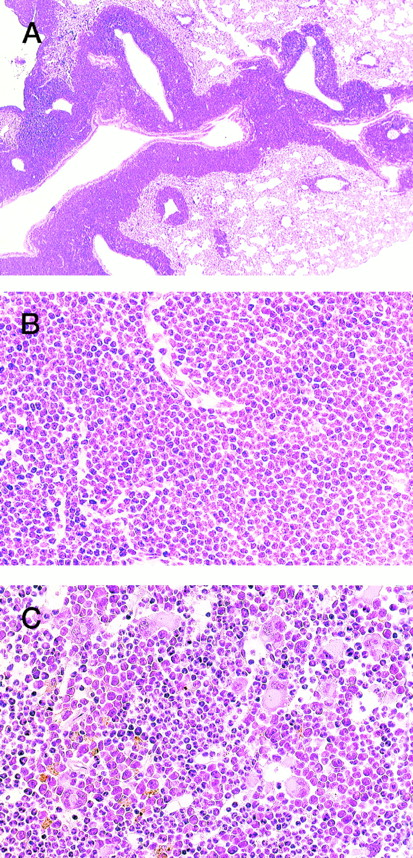
WG staining of tissues from 4-5 (A, C, E) and 4-5-10 (B, D, F) that developed ALL and MPD, respectively. (A) Peripheral blood of 4-5. Massive proliferation of lymphoblasts is apparent. (B) Peripheral blood of 4-5-10. Granulocyte hyperplasia is evident. (C) Thymus of 4-5. Note the marked infiltration of lymphoblasts. (D) Bone marrow of 4-5-10. The bone marrow is hyperplastic and contains myeloid cells at various stages of differentiation. (E) Spleen of 4-5. Note infiltrating lymphoblasts. (F) Spleen of 4-5-10. Extensive infiltration of granulocytes and immature myeloid cells is apparent.
WG staining of tissues from 4-5 (A, C, E) and 4-5-10 (B, D, F) that developed ALL and MPD, respectively. (A) Peripheral blood of 4-5. Massive proliferation of lymphoblasts is apparent. (B) Peripheral blood of 4-5-10. Granulocyte hyperplasia is evident. (C) Thymus of 4-5. Note the marked infiltration of lymphoblasts. (D) Bone marrow of 4-5-10. The bone marrow is hyperplastic and contains myeloid cells at various stages of differentiation. (E) Spleen of 4-5. Note infiltrating lymphoblasts. (F) Spleen of 4-5-10. Extensive infiltration of granulocytes and immature myeloid cells is apparent.
The transgenic progeny of 4-5, one of the two founder mice that developed ALLs, were maintained under continual observation. No obvious signs of illness were observed until approximately 1 year after birth, when one transgenic mouse, number 4-5-4, was found dead. Autopsy revealed splenomegaly without swelling of the thymus, in contrast to the marked thymic enlargement found in the parental founder mouse. Several weeks after the death of 4-5-4, two transgenic littermates, 4-5-2 and 4-5-10, became moribund. They demonstrated emaciation, unsteady gait, and necrotic areas on the skin. Peripheral blood parameter for 4-5-2 and 4-5-10 were: WBC 45 × 103/μL (>90% granulocytes), Hb 3.1 g/dL, and Plt >400 × 104/μL and WBC 15 × 103/μL (>90% granulocytes), Hb 4.1 g/dL, and Plt >400 × 104/μL, respectively (Table 1). Smears showed remarkable hyperplasia of granulocytes (Fig 2B). Pathologic analysis after death showed bone marrow to be hypercellular with a predominance of myeloid cells at various stages of differentiation and also with proliferation of megakaryocytes (Fig 2D and Fig 3C).Extensive infiltration of granulocytes and immature myeloid cells was observed in the spleen (Fig 2F) and in other organs including mesenteric lymph nodes (data not shown). Thus, the transgenic progeny of 4-5 developed myeloproliferative disorders having cardinal features of human CML.
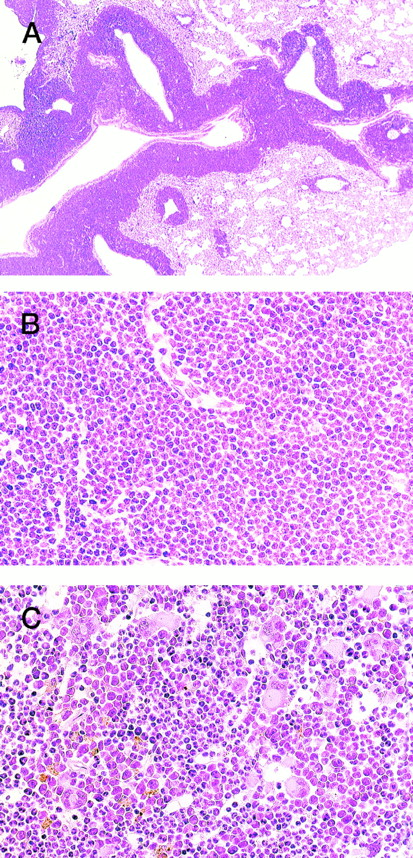
HE staining of 4-5-2 that developed extramedullar crisis in the lung. (A) Lung. Massive proliferation of blast cells is apparent around the vessels. (B) Higher magnification of (A). (C) Bone marrow. In contrast to the findings for the lung, bone marrow shows a predominance of myeloid cells with megakaryocyte proliferation.
HE staining of 4-5-2 that developed extramedullar crisis in the lung. (A) Lung. Massive proliferation of blast cells is apparent around the vessels. (B) Higher magnification of (A). (C) Bone marrow. In contrast to the findings for the lung, bone marrow shows a predominance of myeloid cells with megakaryocyte proliferation.
In addition, an aggressive proliferation of immature blast cells was found around the vessels in the lung of 4-5-2 (Fig 3, A and B) with a predominance of myeloid cells and megakaryocytes in the bone marrow (Fig 3C), indicative of extramedullar blast crisis. Another transgenic littermate, 4-5-6, also showed a hematologic picture of MPD (WBC 10 × 103/μL, (>90% granulocytes), Hb 8.0 g/dL, and Plt 200 × 104/μL, see Table 1) but was healthy in general appearance and survived for more than 2 months after the diagnosis.
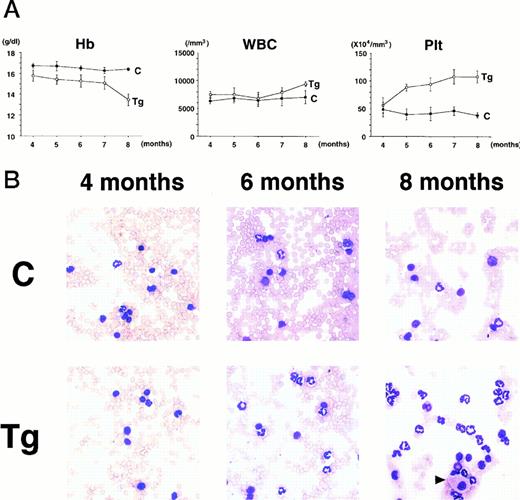
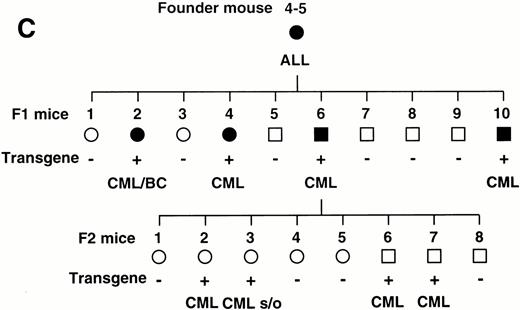
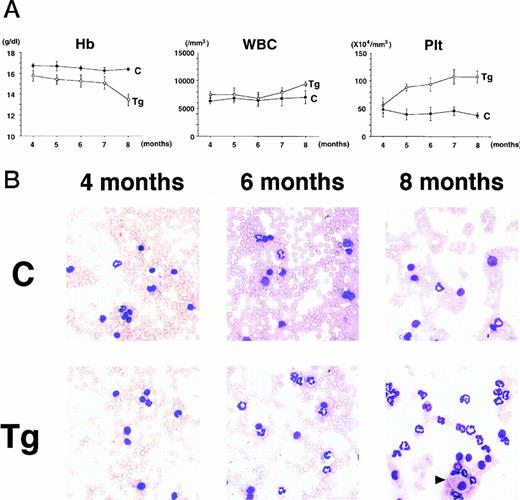
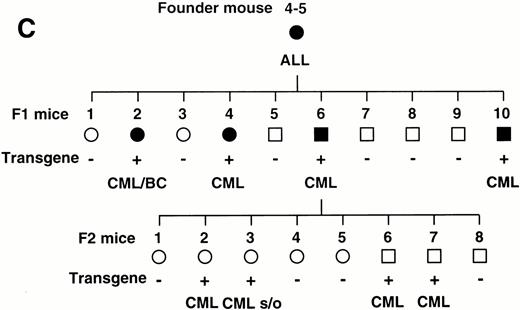
To confirm the reproducible development of the myeloproliferative disorders and to further analyze the alteration in the hematologic parameters in the time course, the peripheral blood samples of the progeny of 4-5-6 were subjected to hematologic examination. The changes of Hb, WBC, and Plt in transgenic and nontransgenic littermates from 4 to 8 months after birth are shown in Fig 4A. The most remarkable hematologic change observed in the initial phase of the transgenic group was the elevation of Plt count. In contrast to the stable Plt count in the nontransgenic group (40-50 × 104/μL), that of the transgenic group began to increase at 5 months after birth and reached approximately 100 × 104/μL (right panel in Fig 4A). Although the WBC count of the transgenic group was almost similar to that of the nontransgenic group until 6 months after birth, it seemed to be gradually increasing thereafter (middle panel in Fig 4A). In addition, a significant and progressive change in WBC was detected in the peripheral blood smears of the transgenic group (Fig 4B). At 4 months after birth, the differential count of leukocytes was almost the same between the transgenic and nontransgenic groups. It showed a slight predominance in lymphoid lineage (left panel in Fig 4B). However, as the mice grew up, proliferation of myeloid cells became apparent in the transgenic group (middle panel in Fig 4B). Moreover, at 8 months after birth, the major population of the WBC in the peripheral blood of the transgenic group was found to be mature granulocytes (right panel in Fig 4B). In addition, the appearance of large cells morphologically resembling megakaryocytes was frequently observed (shown by an arrowhead in the right panel in Fig 4B). At this point, the transgenic progeny, numbered 4-5-6-2, 4-5-6-6, and 4-5-6-7 were diagnosed as CML based on the proliferation of granulocytes in the peripheral blood (>80%) and significantly increased Plt number (>100 × 104/μL, see Table 1). Another transgenic mouse, 4-5-6-3, was strongly suggested as suffering from CML, since the granulocyte ratio in the peripheral WBC was over 60% and Plt number was up to 80 × 104/μL (Table 1). Along with the changes in WBC and Plt counts, progressing anemia was observed in the transgenic group (left panel in Fig 4A), probably due to an excessive proliferation of myeloid and megakaryocytic cells in the hematopoietic tissues such as bone marrow and spleen. In spite of these hematologic abnormalities, the transgenic mice looked healthy in general appearances. These results demonstrate that the initial hematologic abnormality occurred at an early age and that the disease has a long duration of chronic phase with no obvious phenotypical changes. Therefore, we consider these transgenic mice to be a model for human CML. The hematologic parameters observed in the descendants of 4-5 at the diagnosis are summarized in Table 1 and the family tree of 4-5 is shown in Fig 4C.
(A) Comparison of hematologic parameters in the transgenic mice (Tg, ○) and nontransgenic controls (C, •) from 4 months to 8 months after birth. The data of mean values and standard deviations for groups of four mice are shown. (B) WG-stained peripheral blood smears of a transgenic mouse (Tg) and a nontransgenic control (C) at 4, 6, and 8 months after birth. A cell morphologically resembling megakaryocyte is indicated by an arrowhead. (C) Family tree derived from a founder mouse, 4-5. Circles and squares indicate female and male mice, respectively. Transgenic or nontransgenic mice are shown as transgene + or −. Mice that died of leukemia are shown as • or ▪.
(A) Comparison of hematologic parameters in the transgenic mice (Tg, ○) and nontransgenic controls (C, •) from 4 months to 8 months after birth. The data of mean values and standard deviations for groups of four mice are shown. (B) WG-stained peripheral blood smears of a transgenic mouse (Tg) and a nontransgenic control (C) at 4, 6, and 8 months after birth. A cell morphologically resembling megakaryocyte is indicated by an arrowhead. (C) Family tree derived from a founder mouse, 4-5. Circles and squares indicate female and male mice, respectively. Transgenic or nontransgenic mice are shown as transgene + or −. Mice that died of leukemia are shown as • or ▪.
Expression of the p210bcr/abltransgene in the leukemic tissues.
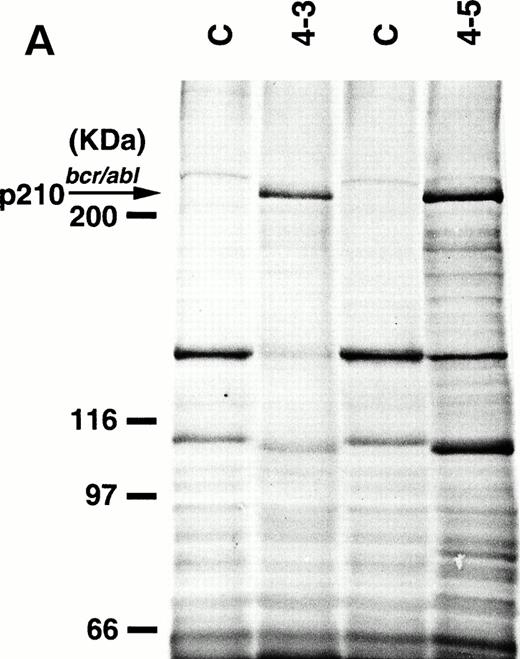
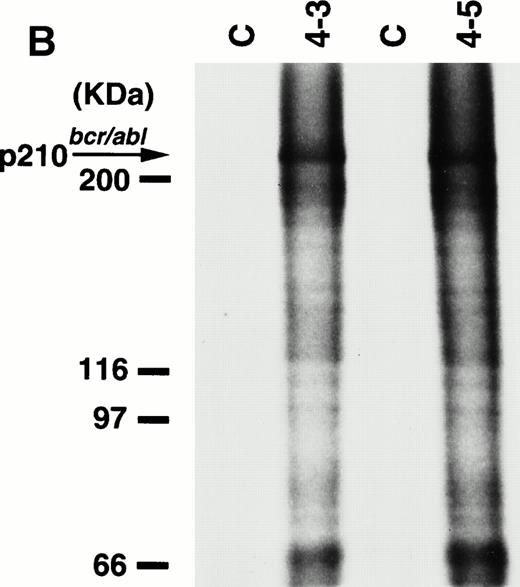
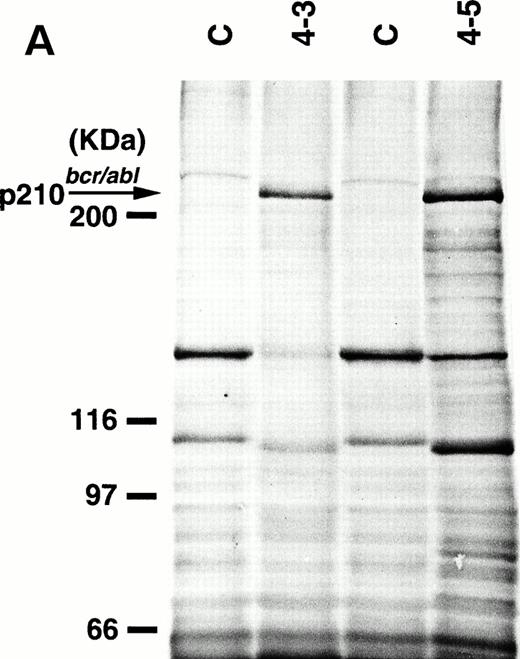
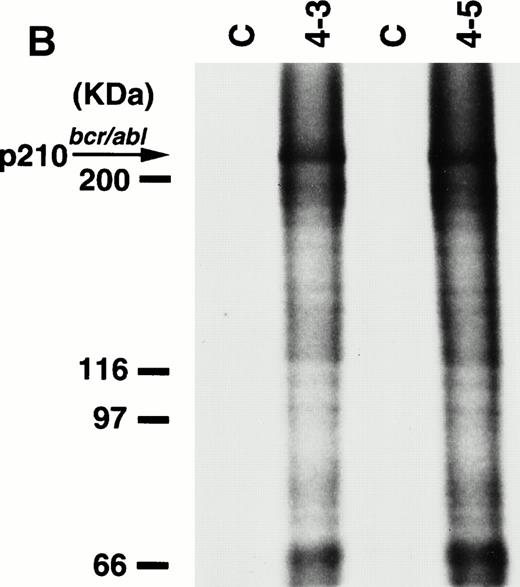
To detect the p210bcr/abl transgene product in two leukemic founder mice, 4-3 and 4-5, that developed ALLs, proteins extracted from enlarged thymi, including massive leukemic cell infiltration, were probed with anti–c-Abl MoAb, AB3. As shown in Fig5A, the p210bcr/ablprotein product could be clearly detected. The expressed p210bcr/abl was also proved to be highly tyrosine-phosphorylated (data not shown). Furthermore, in vitro kinase assays demonstrated that the p210bcr/ablpossessed enhanced kinase activity (Fig 5B). These results are similar to those observed for MT/p210bcr/abl leukemic mice.19
Expression (A) and enhanced kinase activity (B) of the p210bcr/abl transgene product in the thymi of the two founder mice, 4-3 and 4-5 that developed ALLs. The expressed and phosphorylated p210bcr/abl transgene products are indicated by arrows and the positions of protein markers are shown on the left. The thymi of nontransgenic mice were used as negative controls (C).
Expression (A) and enhanced kinase activity (B) of the p210bcr/abl transgene product in the thymi of the two founder mice, 4-3 and 4-5 that developed ALLs. The expressed and phosphorylated p210bcr/abl transgene products are indicated by arrows and the positions of protein markers are shown on the left. The thymi of nontransgenic mice were used as negative controls (C).
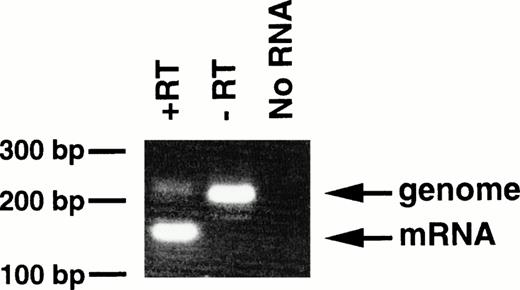
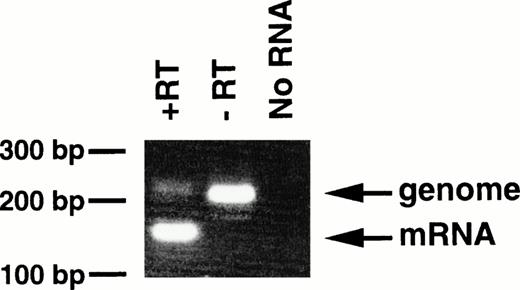
With regard to the transgenic offspring exhibiting MPDs, repeated attempts to detect or immunoprecipitate the p210bcr/abl protein in the spleen, containing mature and immature myeloid cells, were unsuccessful, probably due to the high protease activity existing in granulocytes.13 As an alternative approach, RT-PCR was carried out using mRNA extracted from peripheral blood cells of 4-5-6, in which >90% of the WBC cells were mature granulocytes. The product of genomic amplification (230 bp) can be distinguished from that of mRNA amplification (160 bp) since 70 bp should be removed by the splicing (Fig 1). As shown in Fig6, without RT (-RT), the PCR product was 230 bp; this was reduced to 160 bp when RT was added (+RT). Thus, the granulocytes in the peripheral blood of the MPD mouse were shown to express transgene-derived mRNA.
Expression of bcr/abl mRNA in peripheral granulocytes of 4-5-6. The RT-PCR products generated with RT (+RT) or without RT (−RT) were electrophoresed in a 3% agarose gel and stained with ethidium bromide. Products of genomic and mRNA amplification are indicated by arrows. RT-PCR without RNA (No RNA) was also performed as a negative control. The positions of DNA markers are shown on the left.
Expression of bcr/abl mRNA in peripheral granulocytes of 4-5-6. The RT-PCR products generated with RT (+RT) or without RT (−RT) were electrophoresed in a 3% agarose gel and stained with ethidium bromide. Products of genomic and mRNA amplification are indicated by arrows. RT-PCR without RNA (No RNA) was also performed as a negative control. The positions of DNA markers are shown on the left.
Cell lineage of the leukemic cells.
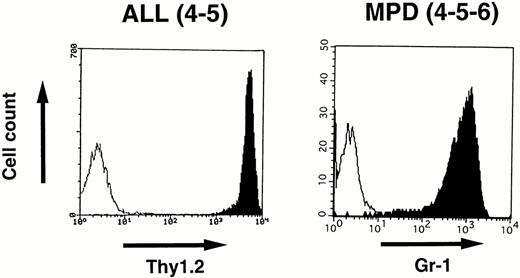
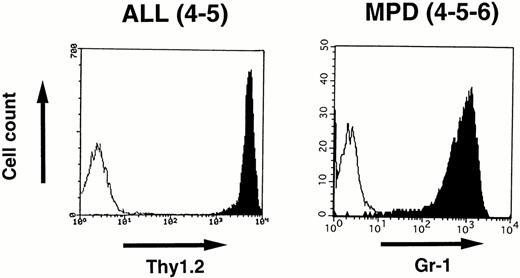
To identify the cell lineage of the leukemic cells, surface markers were analyzed by flowcytometry. Blast cells disaggregated from the enlarged thymus of 4-5 that developed ALL and white blood cells prepared from the peripheral blood of 4-5-6 that developed MPD were stained with MoAbs and analyzed by FACScan as described in the Materials and Methods. As shown in Fig 7,the lymphoblasts in 4-5 expressed a T-cell antigen, Thy1.2, at high intensity, whereas the proliferated granulocytes in 4-5-6 were positive for a myeloid antigen, Gr-1, indicating commitment to the T-cell and myeloid lineages, respectively.
Surface marker analysis of leukemic cells in 4-5 and 4-5-6 that developed ALL and MPD, respectively. Open histograms are for a negative control and marker staining is indicated by closed histograms. Lymphoblasts in the thymus of 4-5 expressed Thy1.2 at a high intensity (left), whereas proliferating granulocytes in the peripheral blood of 4-5-6 are positive for Gr-1 (right).
Surface marker analysis of leukemic cells in 4-5 and 4-5-6 that developed ALL and MPD, respectively. Open histograms are for a negative control and marker staining is indicated by closed histograms. Lymphoblasts in the thymus of 4-5 expressed Thy1.2 at a high intensity (left), whereas proliferating granulocytes in the peripheral blood of 4-5-6 are positive for Gr-1 (right).
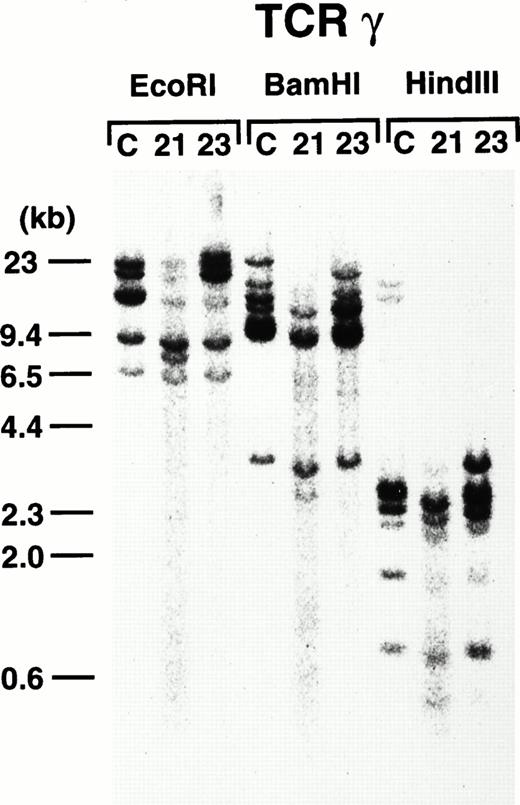
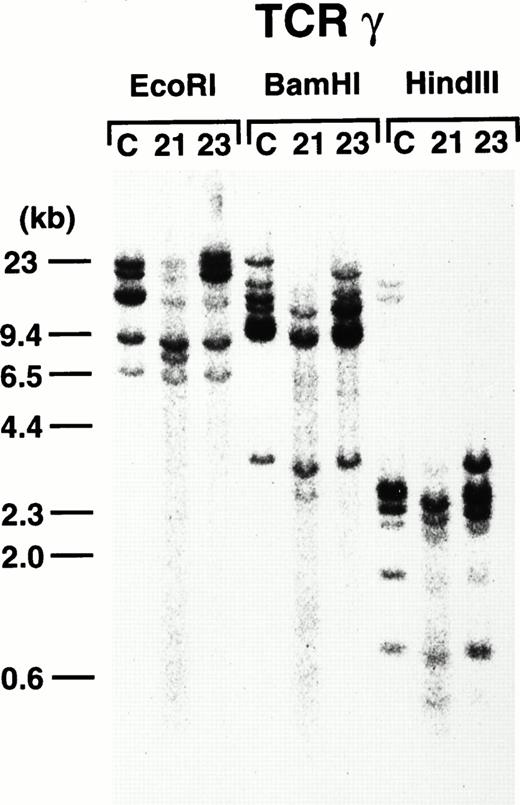
To further demonstrate the clonality of the leukemic cells in the founder mice, DNAs extracted from thymi of 4-3 and 4-5 were subjected to Southern blot analysis. As shown in Fig8, leukemic cells from both founder mice carried rearrangements in the TCR-γ loci, confirming the T-cell lineage and clonality in origin.
TCR-γ rearrangement of the leukemic cells of 4-3 and 4-5. Rearranged bands are evident in both cases after each enzyme digestion. Molecular markers are shown on the left.
TCR-γ rearrangement of the leukemic cells of 4-3 and 4-5. Rearranged bands are evident in both cases after each enzyme digestion. Molecular markers are shown on the left.
DISCUSSION
To clarify the biological properties of p210bcr/ablin vivo and to analyze the disease process caused by the chimeric protein, it is necessary to establish a model animal that expresses p210bcr/abl and eventually develops hematopoietic disorders resembling human Ph1-positive leukemias.
Earlier works focused attention on the BMT approach. Mice lethally irradiated and reconstituted with bone marrow cells infected with p210bcr/abl-expressing retroviruses exhibited various hematologic disorders including granulocytic hyperplasias, myelomonocytic leukemias, ALLs, macrophage cell tumors, reticulum cell sarcomas, and erythroid tumors.13-15 This provided evidence that p210bcr/abl indeed confers an oncogenic potential on hematopoietic cells and that the precursors infected with p210bcr/abl-expressing retroviruses acquire an ability to form various hematologic malignancies. However, the outcome of the experiments was markedly influenced by the infection conditions, the structure of the viral construct, genetic background of the donor and recipient mice, and the cell lineage and differentiation stage of the targeted cells.28,29 In addition, since the integrated sites of the retroviruses differed among the recipient mice, the results lacked reproducibility. Furthermore, the possibility that cytokines secreted with BMT might affect the reconstituted bone marrow cells cannot be excluded. Thus, it has been emphasized that transgenic models with stable transmission of the p210bcr/abltransgene to the progeny and reproducible development of Ph1-positive type leukemias are required.30
In human disease, p210bcr/abl is expressed under the control of the bcr promoter. However, expression of abcr/abl construct driven by the bcr promoter was found to result in a lethality during embryogenesis in transgenic mice.31 Therefore, we and others have used alternative promoters such as MT, Eμ, and LTR of MPSV.17,19,20Although the resultant transgenic mice expressing p210bcr/abl by these promoters developed hematologic malignancies, they were diagnosed exclusively as ALLs and no granulocyte hyperplasia resembling human CML was observed even when the LTR of MPSV, which allowed exhibition of CML-like disease in BMT experiments, was used as the regulatory element.13,17 Thus, the question of whether p210bcr/abl transgenic mice could exhibit CML-like disease and whether the transcriptional unit regulating the bcr gene expression might be required for causing CML-like disease in human and mice have remained to be clarified.20
Although the MT promoter we earlier used to create MT/p210bcr/abl transgenic mice has been proven to work efficiently in the transgenic system,19,20,32,33 the expression level is relatively low,34 the tissue distribution is ubiquitous,35 and it is not clear what lineage and developmental stage of hematopoietic cells are targeted. Thus, to create a transgenic model for human CML, a promoter that drives p210bcr/abl expression in the hematopoietic progenitor cells at a high level is necessary. With this aim, we cloned and analyzed the promoter of mouse tec gene, a cytoplasmic protein-tyrosine kinase predominantly expressed in hematopoietic precursor cells.21,22 We identified and sequenced the 5′ flanking region of mouse tec gene, determined the transcriptional initiation site by RACE-PCR, and confirmed the promoter activity using tec-expressing hematopoietic cell lines.21 Subsequently, we cloned and sequenced mousetec promoter up to approximately −2 kb from the transcription initiation site, identified regions critical for the transcriptional activity, and demonstrated nuclear proteins including PU.1 and Sp1 selectively to bind to the promoter through the regions essential for the promoter activity.22 These results suggest that the promoter we cloned will be useful and applicable for expressing a gene of interest preferentially in hematopoietic progenitor cells in vivo. Use of the −1948 to +22 region to express p210bcr/abl in transgenic mice allowed generation of five founders. Two of them developed ALLs shortly after birth, while the transgenic progeny exhibited MPDs closely resembling human CML after a long latent period. These results clearly demonstrate that the expression of p210bcr/abl by an appropriate promoter in transgenic mice can induce not only ALL but also MPD resembling human CML. Considering that both diseases developed in a single line, the difference in disease phenotypes is obviously not due to an integration site effect. Further studies will be required to clarify the mechanism of how the expression of p210bcr/abl in early hematopoietic progenitors causes two clinically distinct Ph1-positive human leukemias. In addition, our findings strongly suggest that the transcriptional mechanism regulating the expression of the bcrgene does not play an essential role in causing Ph1-positive human hematopoietic disorders. This is not in line with the hypothesis that regulatory sequences within thebcr promoter would contribute to the occurrence of myeloid leukemia in humans and presumably in mice,20 but rather supports the conclusions drawn from the BMT experiments, in which recipient mice expressing the p210bcr/abl driven by retrovirus-derived sequences developed human ALL- and CML-like diseases.13-15
The finding of marked thrombocytosis in the peripheral blood of mice in the present study, as frequently shown by CML patients,1implies that the tec promoter functions in megakaryocytic as well as myeloid and lymphoid lineages. Alternatively, the thrombocytosis might have been due to the structure of thebcr/abl cDNA used (b3a2 type), since some reports suggested a positive correlation between thrombocytosis and b3a2 typebcr/abl transcripts in human CML patients.36 37 The observed progressive anemia, frequently associated with deformity and fragmentation of red blood cells, could be explained by the impaired erythropoiesis due to the proliferation of myeloid and megakaryocytic cells in the bone marrow and spleen. No obvious fibrosis was observed in the bone marrow and/or spleen on staining with Azan (data not shown). In addition, prominent infiltration of mature and immature myeloid cells in the spleen and all other tissues examined might be indicative of the terminal stage of chronic phase. Thus, a long duration of chronic phase might be fatal without application of antiproliferative agents. In particular, one mouse, 4-5-2, showed massive proliferation of blast cells along the vessels in the lung. Although the precise origin of the blast cells remains to be identified, it is very likely that an extramedullar blast crisis occurred after a duration of chronic phase in that mouse.
The transgenic model described in the present report has several advantages over BMT experiments. (1) Since the latter are markedly influenced by the experimental procedures such as infection and culture conditions of the bone marrow cells, the results vary with the mouse and the experimental group. With transgenic mice, each line contains the transgene in the same integration site and expresses the transgene product in the same manner so that the results are reproducible and not affected by extrinsic factors. (2) Since the transgenic mice transmit the transgene stably to the progeny, they can be cross-mated with other transgenic or knockout mice for investigating the possible synergistic or suppressive effects of different genes in vivo. (3) The recipient mice in BMT experiments develop erythroleukemias, macrophage tumors, or reticulum cell sarcomas that are virtually unknown as human Ph1-positive leukemias. In contrast, our transgenic mice develop ALL and CML-like diseases within the same disease spectrum as human Ph1-positive leukemias. In this respect, our transgenic mice may more accurately reflect the leukemogenic property of p210bcr/abl in vivo.
In summary, we present a novel transgenic model for Ph1-positive human leukemias. This model will be valuable not only for investigating the biological properties of p210bcr/ablin vivo but also for analyzing the mechanism involved in the progression from chronic phase to blast crisis. In addition, they should find application for examination of the effectiveness of therapeutic regimens for human Ph1-positive leukemias.
ACKNOWLEDGMENT
We thank Dr T.W. Mak for providing the mouse TCRγ probe and thank Motoya Katsuki and the other staff of the Central Institute of Experimental Animal for technical instructions.
Supported by Grants-in-Aids from the Ministry of Education, Science and Culture of Japan.
Address reprint requests to Hisamaru Hirai, MD, Third Department of Internal Medicine, Faculty of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.