Abstract
The role of human T-cell leukemia virus type II (HTLV-II) in human lymphoproliferative and hematopoietic abnormalities in which the retrovirus can be isolated is still elusive. Here we show that the C344 T-cell–derived lymphotropic HTLV-II type IIa Mo strain acts directly on CD34+ hematopoietic precursors by rescuing them from apoptosis induced by interleukin-3 (IL-3) deprivation. This effect is viral strain-specific, as it is not observed with the B-lymphotropic HTLV-II type IIb Gu strain, it does not require infection of the hematopoietic precursors, and, interestingly, it is strongly dependent on the infected cellular host from which the virus was derived. Indeed, growth adaptation of the Mo strain to the permissive B-cell line, BJAB, renders the virus no longer capable of mediating the antiapoptotic effect. However, pretreatment of the BJAB-adapted Mo strain with antibodies specific for HLA class II, but not class I, histocompatibility antigens restores the antiapoptotic potential of the virus. These results constitute the first evidence that HTLV-II retrovirus can directly influence the homeostasis of human progenitors, without infecting them, and that this crucial activity is strongly inhibited by the presence of host-derived envelope-associated HLA class II antigens.
THE HUMAN T-CELL leukemia-lymphoma viruses types I and II (HTLV-I and -II) are closely related oncogenic retroviruses. Infection is lifelong and transmissible vertically by breast feeding and horizontally by sexual intercourse, transfusion of infected blood, and intravenous drug usage. There is strong evidence associating HTLV-I infection with subsequent development of adult T-cell leukemia/lymphoma (ATLL), tropical spastic paraparesis (TSP), and other conditions of inflammatory nature.1 No definitive relationship between HTLV-II infection and specific human diseases has been established, although neurodegenerative and lymphoproliferative disorders can be observed.2 HTLV-II infection has been shown to be endemic in a large number of native American Indian populations and high rates of infection have also been shown in intravenous drug abusers in North America and Europe.2
HTLV infections lead to several immune dysfunctions, including spontaneous proliferation of T cells, which have been attributed to the effect of the trans-regulatory protein tax, encoded by the pxviral gene.3,4 In fact, beside the action on the viral long terminal repeat (LTR) to regulate viral RNA transcription, tax is also capable of trans-activating heterologous eukaryotic promoters of genes involved in T-cell activation and proliferation, including a large array of cytokines.3 Further studies have shown that antibodies to tax are often found in the serum of infected individuals,5,6 and a cell-mediated immune response directed toward the tax protein is detected in a large proportion of HTLV-associated myelopathy (HAM) and TSP patients7; in addition, tax can be found in the cell-free supernatant of cells expressing the viral product.8 Taken together these observations suggest that HTLV-I tax can have important biologic functions outside the infected cell.
Very little is known of the function of HTLV virus-encoded envelope structures, as well as the actual composition of the viral envelope, particularly with regard to host cell membrane-derived components. In other retroviruses, such as human immunodeficiency virus (HIV)-1, cell-derived molecules have been shown to be present in large amounts, in a rather selective fashion, and to influence the biologic characteristics of the virus life cycle.9 HTLV-I and -II are mostly T lymphotropic showing an in vivo preferential targeting to CD4 and CD8 T cells, respectively. However, in some patients, it has been recently shown that both HTLVs can infect non–T-cell populations, including monocytes and B cells.10-12 In light of these findings, it would be pertinent to assess the presence and the relative proportion of envelope-associated cell-derived structures.
It has not been firmly established whether HTLVs can infect hematopoietic precursors, and the hematologic abnormalities present in ATLL patients can simply reflect alterations in environmental factors such as cytokine,13 some of which, however, are essential for maintaining survival and/or for promoting differentiation of hematopoietic precursors. For example, withdrawal of interleukin-3 (IL-3) from immature hematopoietic cells has been shown to lead to internucleosomal DNA cleavage and other events characteristic of apoptosis.14,15 In various viral infections, either an accelerated apoptosis or a protection from it can be observed.16-19
For all these reasons, we set out to investigate the biologic effects of HTLV-II virus on a specific cell lineage, which could be involved in the hematologic abnormalities associated with HTLV infection. We focused our study on CD34+ cells either directly isolated from healthy bone marrow (BM) donors or as a cell line (TF-1). The effect of two distinct HTLV-II viral strains, Mo and Gu, belonging to subtype IIa and IIb, respectively, were investigated. Mo and Gu differ in their proviral genomic sequence by 4.8%; at the level of theenv region, a 95.4% nucleotide sequence identity is observed, resulting in 2.9% divergence in the predicted amino acid sequence (14 amino acid substitutions, of which five are nonconservative).20 The two viral subtypes encode different tax proteins, as shown by an additional 25 amino acids in the carboxy terminus of the Gu-derived protein, and this may lead to differences in tax function in vivo.20 Several parameters were analyzed: (1) CD34+ cell viability in response to HTLV-II treatment, as assessed by apoptotic cell death analysis after IL-3 deprivation; (2) CD34+ cell permissivity to infection, as assessed by polymerase chain reaction (PCR) of viral genes in the treated cells; (3) correlation between virus-derived, as well as virus-associated cell host-derived, structures and biologic effects on CD34+hematopoietic precursors. The results show a critical role for HTLV-II virions in mediating survival and growth effects on CD34+hematopoietic precursors. Importantly, membrane components of the cellular type generating the virus assumed significance in the virus-mediated effect on hematopoietic progenitors, as host-derived envelope-associated HLA class II, but not class I, molecules appeared to interfere directly with the capacity of the virus to inhibit apoptosis of CD34+ cells.
MATERIALS AND METHODS
Cells.
The IL-3–dependent human erythromyeloid cell line, TF-1, was obtained through the courtesy of Dr B.R. Davis (Medical Research Institute, San Francisco, CA). TF-1 expresses the CD34 marker in greater than 95% of the cells.21 It was routinely cultured in RPMI-1640 (GIBCO, Grand Island, NY) plus 10% fetal calf serum (FCS) (GIBCO) and 1 ng/mL of human rIL-3 (Genzyme, Boston, MA) in 24-multiwell plates (Falcon, Oxnard, CA) at the optimal density of 3 to 6 × 105cells/mL. The C344 cell line, kindly provided by Dr R.C. Gallo (National Cancer Institute, Bethesda, MD) is an HTLV-II–infected T-cell line harboring the HTLV-II Mo provirus.22 The BJAB cell line is an Epstein-Barr virus (EBV) negative B-cell line able to support the HTLV-II replication.23 C344 and BJAB were used as producers for the viral isolates Mo and Gu, respectively. All cultures were maintained in RPMI 1640 plus FCS and were kept at 37°C in a 5% CO2 humidified atmosphere.
BM samples.
Samples were obtained from five HIV-1 and HTLV seronegative subjects after informed consent, according to the Helsinki Declaration of 1975. BM CD34-positive (BM CD34+) cells were purified as described previously.24 Briefly, after phycoll separation and washings in Iscove's modified Dulbecco's medium (IMDM) (GIBCO) plus 10% FCS, light density mononuclear cells were deprived of adherent cells by two successive 1-hour adherence steps in plastic petri dishes (Costar, Cambridge, MA). A total of 6 × 106 cells were then reacted with mouse monoclonal antibody (MoAb) anti-CD2, -CD3, -CD8, -CD11, -CD19, and -CD20 (Becton Dickinson, Mountain View, CA) for 1 hour in ice under agitation. After washing, cells were incubated for 30 minutes on ice with immunomagnetic beads coated with IgG antimouse (MCP 450 Dynabeads; Dynal, Oslo, Norway) at a immunomagnetic bead/target cell ratio of 10:1 in a final volume of 400 μL. Lineage-positive cells were then removed by a magnet (MPC1 Dynabeads). The remaining cells were positively selected by My-10 anti-CD34 MoAb (Tecnogenetics, Milan, Italy) and the immunobead procedure described above, using an immunomagnetic bead-to-cell ratio of 3:1. CD34+ cells were collected by magnet and resuspended in 1 mL IMDM plus 10% FCS. After overnight incubation at 37°C, CD34+ cells were gently pipetted 30 to 40 times to mechanically separate them from immunomagnetic beads, which were eliminated by a rapid passage on MPC1. The CD34+-enriched populations were determined to be more than 95% pure at FACScan analysis by a subsequent staining with a mouse MoAb that recognizes a separate epitope of CD34 (HPCA-2; Becton Dickinson).
Viral stocks.
Supernatants obtained from HTLV-II–infected C344 and BJAB cell lines were clarified at low speed centrifugation (1,000g for 10 minutes) and passage through 0.45-μm filters. To obtain concentrated virus preparations, the clarified supernatants were centrifuged at 50,000g and the sedimented particles then purified by ultracentrifugation on sucrose gradient (25% to 60%). The 1.16 to 1.18 g/mL density fractions were pooled, dialyzed, and pelleted by centrifugation. Virus particles were resuspended in medium at one tenth of the original volume. The viral titers are defined by cpm reverse transcription (RT) activity and levels of p24Ag in culture fluids. In the supernatants, p24Ag assay showed values ranging between 200 to 600 pg/mL corresponding to 3 to 8 × 103 cpm/mL of RT activity; the HTLV-II Mo virus preparation expressed 310 × 103 cpm/mL of RT and contained 48 ng/mL of p24 HTLV-II core antigen. Purified virus from the BJAB cell line infected with Gu viral strain,20 23 contained 370 ng/mL of p24, whereas those from the BJAB cell line infected with Mo strain, expressed 40 ng/mL of p24. Heat inactivation of HTLV-II was performed by incubating the viral preparations at 56°C for 30 minutes.
Inoculation of the human progenitor TF-1 and BM CD34+cells with HTLV-II.
Cells to be tested for their susceptibility to HTLV-II were washed twice in serum-free RPMI-1640 medium and plated at a concentration of 5 ×105 cells/mL in virus-containing medium (0.5 to 1 ng/mL HTLV-IIp24 Ag equivalent, see below). The following virus preparations were used: (1) purified Mo viral particles generated from C344 cell line; (2) clarified supernatant from C344 culture containing Mo virus; (3) purified virions obtained from BJAB cells infected with HTLV-II Gu strain; (4) clarified supernatant from BJAB cells infected with HTLV-II Gu strain; (5) clarified supernatant from uninfected BJAB cell line; (6) purified virions generated from BJAB cells infected with HTLV-II Mo strain; (7) clarified supernatant from BJAB cells infected with HTLV-II Mo strain. After a 24-hour incubation at 37°C with the different inocula, the cells were washed and resuspended in RPMI-1640 medium plus 10% FCS and cultured in absence of IL-3. Due to the low number of purified BM CD34+ cells obtainable after negative and positive immunomagnetic bead selection, the HTLV-II action on these cells was only performed by treatment with HTLV-II Mo strain.
Detection of HTLV-II in Cell Cultures
Antigen detection.
Supernatants from TF-1 cultures were checked for the presence of HTLV-I/II p24 core antigen by an enzyme immunoassay (Retrovirology Coulter Corp, Hialeah, FL). The concentration of antigen in the samples was determined by a linear regression analysis using different p24 standard amounts (from 250 to 15.6 pg) performed and interpreted according to the manufacturer's instructions.
PCR analysis.
To verify the presence of a viral infection after HTLV-II inocula, 3 × 105 TF-1 or BM CD34+ cells were analyzed twice a week for proviral DNA presence by PCR. Briefly, DNA was extracted from cells using a commercial kit (IsoQuick, Microprobe Corp, Bothell, WA). PCR amplification of a 159-bp HTLV-II taxsequence was performed using 50 pmol of each primer SK43-SK44 (Perkin Elmer Cetus, Norwalk, CT) in 50 μL volume of reaction solution containing Tris-HCl 10 mmol/L, pH 8.3, KCl 50 mmol/L, MgCl22.5 mmol/L, gelatine 1 mg/mL, dNTPs 0.2 mmol/L each, Taq polymerase 1.25 U. Each amplification was performed by using 1.5 μg of cellular DNA. The reactions were performed in a System 9600 thermal cycler (Perkin Elmer Cetus) programmed for 35 cycles of denaturation at 94°C for 10 seconds, annealing at 58°C for 13 seconds, and extension at 72°C for 10 seconds (first denaturation step: 1 minute; last extension step: 2.30 minutes). For Southern blot analysis, one third of each amplified sample was subjected to electrophoresis on 2.8% agarose gel (NuSieve:SeaKem 3:1, FMC; Rockland, ME) and then transferred to a nylon membrane (HyBond N, Amersham, Little Chalfont, Buckinghamshire, UK). The specific SK45 probe (Perkin Elmer Cetus) was labeled with fluorescein (3′-oligolabeling system, Amersham) and after overnight hybridization and filter washes, a detection chemiluminescent technique (ECL, Amersham) was used. The light signal was detected by exposure to Hyperfilm-MP (Amersham) at room temperature for about 1 hour. PCR runs included several samples containing all the reagents except DNA or DNA from uninfected cells as negative controls. Positive control samples consisted of DNA extracted from C344 cells.
Apoptosis assays.
Apoptosis was induced by culturing the CD34+ cells in absence of their growth factor. Apoptosis was measured by three different methods. Flow cytometry analysis: cells were fixed in 2 mL cold 70% ethanol at 4°C for 1 hour, washed in PBS, and treated with 0.5 mg RNAse (Type I-A, Sigma, St Louis, MO) in 0.4 mL of PBS for 1 hour at 37°C. Propidium iodide (PI; Sigma) was added to a final concentration of 40 μg/mL, and the samples incubated in the dark at 4°C for 10 minutes. The PI fluorescence of individual nuclei was measured using a FACStar plus Flow cytometer (Becton Dickinson) equipped with an Argon ion laser (Spectra Physics 2020) tuned to 514 nm wavelength, 300 mW output. PI fluorescence was measured on a log instead of a linear scale to allow for better identification of apoptotic cells as a subdiploid cell peak. The threshold was triggered on the same F12 (PI fluorescence) signal in which a clear-cut distinction between cell debris and apoptotic cells could always be identified. Quantitative evaluation of apoptotic cells was performed by the lysis II analysis software (Becton Dickinson) and data were expressed as the percentage of apoptotic versus nonapoptotic cells, regardless of the specific cell cycle phase. DNA laddering: the absence or the presence of fragmentation of chromosomal DNA to the size of oligonucleosomes was measured according to the method of Facchinetti et al.25 Briefly, 5 × 106 cells were resuspended in 500 μL of ice-cold lysing buffer (NaCl 150 mmol/L, Tris 50 mmol/L [ph 7.6], Triton X-100 1%, EDTA 10 mmol/L) containing 1 μg DNase-free Rnase; after 10 minutes on ice, the sample was low speed centrifuged and the supernatant recovered. After phenol extraction and ETOH precipitation, the samples were resuspended in 25 μL TE. After 5 minutes at 60°C, the fragments were loaded on a 1.5% agarose gel and run at 7.5 V/cm for 3 hours. Giemsa-stained cytospin preparations: cytospin of CD34+cells were analyzed by light microscopy and the percentage of cells with the morphologic features of apoptosis were calculated based on a sample of 200 cells.
Proliferation assay.
The capacity of HTLV-II–treated CD34+ cells to overcome apoptotic death after IL-3 withdrawal was further assayed by3H-thymidine incorporation during time to assess potential viral-dependent mitogenic effect. Briefly, cells were incubated with 1 μCi/mL of 3H-thymidine (6 Ci/mmol) for 6 hours starting at different times after virus treatment. Cells were then harvested, washed, and 3H-thymidine DNA content measured by liquid scintillation counter.
Sequence analysis of the env gene of proviral DNA from T- and B-HTLV-II-infected cells.
The genomic DNA of isolates Mo and Gu was prepared from infected cells as described previously.23 Sequencing of the envgp46 region of these isolates was performed by cloning the PCR product of the env gp46 region using the pCRTM11 plasmid (Invitrogen Corp, San Diego, CA) under conditions recommended by the manufacturer. The two primers used were nucleotides (nt) 5100-5130 upstream and nt 6170-6200 downstream. The reaction mixture contained, in a 100-μL volume, 200 μmol/L dNTPs, 20 pmol of each primer, 2.5 U of Taq polymerase, 1 mmol/L MgCl2, 50 mmol/L KCl, 10 mmol/L Tris-HCl (pH 8.3), and 20 to 40 ng of genomic DNA as template. The PCR reaction was performed for 60 cycles (denaturation at 92°C for 60 seconds, annealing at 62°C for 60 seconds, extension at 72°C for 60 seconds). DNA sequencing with M13 reverse and forward primers was performed using the dideoxy chain termination method (T7 sequencing kit; Pharmacia, Uppsala, Sweden). At least three clones of each isolate were sequenced to ensure the reliability of the method.
Immunoblot analyses of HTLV-II virions.
Cell-free supernatants containing HTLV-II virions generated from C344 established cell line or in infected BJAB cells were ultracentrifuged at 150,000g for 1 hour and the pellets were resuspended in TNE buffer (Tris 10 mmol/L [pH 8.0], NaCl 100 mmol/L, EDTA 1 mmol/L) at 1/100 of initial volume. To conserve envelope glycoprotein integrity, the virion concentrates were purified on Sepharose CL-4B (Pharmacia) chromatography column and the fractions containing virus were pooled and centrifuged in microfuge for 90 minutes. The pellets were disrupted in lysis buffer containing 0.5% Triton X-100 and analyzed by immunoblot in a 10% polyacrylamide gel in nonreducing conditions. Blots of HTLV-II extracts from T and B cells were probed with: (1) MoAb to anti-HTLV-II gp46 (Cellular Products, Buffalo, NY) to determine structural changes of viral envelope protein; (2) MoAbs specific for common determinants of HLA class I, MoAb B9.12.1,26 for common determinants of HLA class II molecules, MoAb Tu35,27 or for the class II DR subset, MoAb D1.1228 to identify specific cellular histocompatibility antigens associated with virions. Immunoreactivity was visualized by ECL detection system.
RESULTS
HTLV-II prolongs CD34+ hematopoietic precursor cell survival and suppresses apoptosis in the absence of IL-3.
In a first set of experiments, TF-1 cells were deprived of IL-3 and after 24 hours of culture inoculated with either one of the two HTLV-II viral strains, Mo and Gu. Untreated TF-1 cells, maintained in the absence or presence of IL-3, were used as controls. To establish the relative extent of programmed cell death induced in the various experimental conditions, quantitative fluorescence staining of nuclear DNA was performed. Results of a typical experiment are reported in Table 1. As expected, IL-3–deprived TF-1 cells showed a dramatic increase in the percentage of apoptotic cells, already appreciable at day 3 of culture when compared with TF-1 cells grown in the presence of the cytokine. Interestingly, IL-3–deprived TF-1 cells inoculated with the Mo virus originated from the C344 T-cell line, either as clarified supernatant or as purified viral preparation, showed a drastically reduced percentage of apoptotic cells, closely approaching the values obtained in untreated TF-1 cells grown in the presence of IL-3. On the contrary, IL-3–deprived TF-1 cells inoculated with Gu virus originated from the B-cell line, BJAB, did not show significant protection from apoptosis. To investigate whether the findings obtained in the TF-1 cell line could be reproduced in normal hematopoietic precursors, experiments were performed on BM CD34+ cells obtained from five healthy subjects. The low number of CD34+ cells obtainable after the various purification steps prevented an extensive kinetics analysis of the normal BM-derived cells with both virus strains. Only three time points (0-, 3-, and 7-day point) after inoculation of C344-derived HTLV-II Mo strain were considered. As shown in Table2, the susceptibility of BM-derived CD34+ cells to apoptosis was reduced to near basal levels, similar to the conditions obtained in TF-1 cells grown in the presence of IL-3 and in IL-3–deprived TF-1 cells inoculated with the same virus. From these data, we conclude that the physiologic tendency to apoptosis of BM CD34+ cells cultured in the absence of IL-3 can be overcome by HTLV-II Mo virus strain.
These results show for the first time that HTLV-II virus can directly modify the cell growth of both a CD34+ cell line and, more importantly, of normal CD34+ hematopoietic precursors. They also point to a difference in the biologic properties of the two HTLV-II viral strains used.
HTLV-II virus adapted to a distinct susceptible cellular host no longer retains the capacity to protect hematopoietic precursors from IL-3 withdraw-dependent apoptosis.
The capacity of the Mo virus to protect CD34+ cells from apoptosis as opposed to the incapacity to do so of the Gu virus may be the consequence of several causes, either single or combined, including viral structural differences, cell type tropism, phenotype adaptation to distinct cellular environments, and capacity to infect the target. To examine these questions in more detail, the following experiments were performed. Uninfected BJAB cells, maintained in exponential phase of growth, were cocultured for 48 hours with the same share of C344 Mo-infected cells in transwell-col units to avoid direct cell-to-cell contact. After removal of C344 cells and washing, BJAB cells were expanded and controlled in purity by flow cytometric analysis. Transfer of infection from T- to B-cell line was obtained, as HTLV-II Mo produced by BJAB cells was evident after 20 days of culture in the absence of C344 T cells, as assessed by PCR analysis (data not shown) and detection of p24Ag in supernatants (80, 145, >220 pg/mL at 7, 14, and 20 day point). After various serial passages, purified virus or clarified supernatants from BJAB cells infected with HTLV-II Mo virus strain were used to inoculate IL-3–deprived TF-1 cells. The results of a typical experiment are shown in Table 1. It can be clearly seen that both forms of HTLV-II Mo virus derived from BJAB cells, in contrast to those derived from C344 T-cell line, were unable to protect from apoptosis. Indeed, apoptosis was accelerated because at day 6, only 17% and 28% of TF-1 viable cells, depending on the virus preparations used, remained in the culture. The above results were confirmed both by DNA analysis, which showed the characteristic features of DNA degradation observed in apoptotic dead and Giemsa-stained cytospins showing apoptotic nuclei (data not shown).
These data indicate that suppression of apoptosis in IL-3–deprived TF-1 cells is not only related to the HTLV-II viral strain used, but also to virus adaptation to the permissive cellular host.
Susceptibility of TF-1 and BM-derived CD34+ cells to HTLV-II infection.
The results reported above show that, in appropriate conditions, hematopoietic progenitors are protected from apoptosis when confronted with HTLV-II virus. To determine whether block of apoptosis requires active infection of the cells, culture of TF-1 cells treated with C344-derived HTLV-II Mo strain or BJAB-derived HTLV-II Gu strain were analyzed for the presence of p24 Ag in the supernatant twice a week beginning at day 5 and up to day 25 and by PCR for the presence of integrated proviral sequences in the cellular DNA. In experimental conditions allowing the infection of umbilical cord blood mononuclear cells with the above virus preparations, none of the samples tested showed a detectable amount of p24Ag (data not shown), indicating the lack of an active viral replication in these cells. Moreover, TF-1 cells analyzed at day 3 and 7 after the inoculum of the C344-derived Mo strain did not show the presence of HTLV-II tax-specific sequences in their genomes (Fig 1, lanes 5 and 3, respectively). Superimposable results were obtained with the normal BM CD34+ cells treated with the same viral preparation (data not shown). HTLV-II virus of the Mo strain adapted to BJAB cells, under the form of either purified particles or cleared supernatants, was also tested for the ability to infect TF-1. Interestingly, unlike its equivalent derived from the C344 T-cell line, the Mo strain adapted to BJAB cells, was infectious for hematopoietic progenitors. As shown in Fig 1, by day 3 (lane 4) and particularly by day 7 (lane 2) after virus inoculum, proviral DNA sequences were found in TF-1 cells. Furthermore, heat-inactivated HTLV-II Mo strain derived from the C344 T-cell line was still capable of protecting from apoptosis TF-1 cells (Table 1) and normal BM CD34+ cells (Table 2) deprived of IL-3.
Assessment of HTLV-II proviral DNA in TF-1 cell line by PCR amplification of a tax gene sequence. TF-1 cells treated with HTLV-II Mo strain derived from C344 T cells (lanes 3 and 5) or BJAB cells (lanes 2 and 4) were cultured for 3 days (lanes 4 and 5) or 7 days (lanes 2 and 3). DNA was extracted, amplified, and electrophoresed. Detection of the amplified product was performed by using a tax specific probe. Lane 1, negative control (untreated TF-1 cells); lane 6, positive control represented by PCR-amplified DNA from C344 T cells; lane 7, molecular weight markers.
Assessment of HTLV-II proviral DNA in TF-1 cell line by PCR amplification of a tax gene sequence. TF-1 cells treated with HTLV-II Mo strain derived from C344 T cells (lanes 3 and 5) or BJAB cells (lanes 2 and 4) were cultured for 3 days (lanes 4 and 5) or 7 days (lanes 2 and 3). DNA was extracted, amplified, and electrophoresed. Detection of the amplified product was performed by using a tax specific probe. Lane 1, negative control (untreated TF-1 cells); lane 6, positive control represented by PCR-amplified DNA from C344 T cells; lane 7, molecular weight markers.
These results show that infection is not required for generating HTLV-II–mediated protection from apoptosis of hematopoietic precursors and, by consequence, they rule out also the possibility that newly produced soluble tax protein is involved in this event. Taken together, these results show that integration of HTLV-II provirus is not required for blocking or suppressing apoptosis.
HTLV-II virus-dependent protection from IL-3 withdraw-induced apoptosis in TF-1 cells is related to host-derived HLA class II molecules associated with the virus.
As shown above, protection from apoptosis does not require infection of the cell. Thus, the results obtained raise the possibility that HTLV-II Mo virus adapted to BJAB cells may have undergone structural modifications, particularly in its envelope components that first contact the cellular membrane, which make it no more capable to exert its biologic function. To assess this possibility, HTLV-II Mo provirus isolates from C344 T cells and from BJAB cells were sequenced and compared in the env region. In addition, the two virus isolates were examined for structural changes or modification of gp46 envelope glycoprotein by immunoblot analysis. Neither differences between the proviral env gene sequences nor important posttranscriptional structural changes such as different glycosylation patterns in the corresponding gp46 were found (data not shown).
We then investigated whether host-derived cell surface structures, and particularly HLA-encoded glycoproteins, were differentially represented in the envelope of the two virus isolates. For these studies MoAbs directed against monomorphic determinants of either HLA class I or class II, were used in immunoblot analysis. Preliminary experiments had shown that both HLA class I and class II antigens were expressed on the cell surface of the C344 and BJAB cell lines, the expression of both markers being stronger in the B-cell line, particularly for HLA class II molecules (data not shown). Figure 2shows the results of these experiments. HTLV-II Mo strain isolates from both T- and B-cell lines presented in their envelope substantial amounts of HLA class I antigens represented by a major band of about 60 kD in nonreducing conditions. Of note and in agreement with the surface expression in the cellular hosts, HLA class I antigens were more abundant on the B-cell–derived Mo strain. Similarly, the MoAb D1.12 specific for monomorphic determinants of HLA class II DR molecules, recognized a viral envelope-associated protein of about 65 kD in the BJAB-derived isolate, consistent with the size of an HLA-DR antigen in nonreducing conditions. This antibody was not able to show sufficient amounts of a similar antigen in the C344-derived viral lysate. However, probing with the MoAb Tu35, recognizing all HLA class II antigens, including DR, DP, and DQ, resulted in a visible band of the same molecular weight as the one detected by the D1.12 antibody in both BJAB- and C344-derived viruses, suggesting that the lack of detection of DR antigens in the envelope of T cell-derived Mo strain could be due to qualitative more than quantitative differences.
Immunoblot analysis of HTLV-II virus lysates after reaction with MoAbs specific for HLA class-I (B9.12.1) or class-II (D1.12 and Tu35). Pellets of purified HTLV-II virions generated from C344 cell line (T) or from BJAB cells (B) were incubated in lysis buffer containing 0.2% sodium dodecyl sulfate (SDS) and equivalent concentrations of extracts, as shown by protein staining, analyzed by immunoblot in a 10% polyacrylamide gel containing 0.1% SDS. Immunoreactivity was visualized by chemiluminescence detection system. The size (in kilodaltons [k], indicated on the right) were determined by comparison with protein markers.
Immunoblot analysis of HTLV-II virus lysates after reaction with MoAbs specific for HLA class-I (B9.12.1) or class-II (D1.12 and Tu35). Pellets of purified HTLV-II virions generated from C344 cell line (T) or from BJAB cells (B) were incubated in lysis buffer containing 0.2% sodium dodecyl sulfate (SDS) and equivalent concentrations of extracts, as shown by protein staining, analyzed by immunoblot in a 10% polyacrylamide gel containing 0.1% SDS. Immunoreactivity was visualized by chemiluminescence detection system. The size (in kilodaltons [k], indicated on the right) were determined by comparison with protein markers.
It was then of interest to analyze whether the presence of HLA glycoproteins on the viral envelope of the two HTLV-II Mo isolates could be associated with the distinct apoptotic behavior observed in the TF-1 cell line inoculated with the two isolates. Table 3 summarizes the results of a typical experiment of susceptibility to apoptosis, as described in Table 1, in which the C344-derived and the BJAB-derived Mo strains were incubated with saturating concentrations of the various anti-HLA MoAbs, before using for inoculating TF-1 cells. The percentage of apoptotic cells was then calculated after 1, 4, and 8 days of culture. It can be seen that preincubation of the BJAB-derived Mo strain with anti-HLA class II, but not with anti-HLA class I, antibodies resulted in strong protection from apoptosis of TF-1 cells, similar to the protection observed when the C344-derived Mo strain was used. In this latter case, no significant further protection or opposite effects, such as block of protection from apoptosis, were observed.
From these data, we conclude that host-derived HLA-class II, but not class I, molecules present in the envelope of HTLV-II Mo strain originated from the B-cell line BJAB are associated with the block of protection from apoptosis observed in TF-1 hematopoietic progenitor cells.
Treatment with C344-derived Mo strain or with BJAB-adapted Mo strain treated with anti-class II antibody is mitogenic for CD34+TF-1 cells.
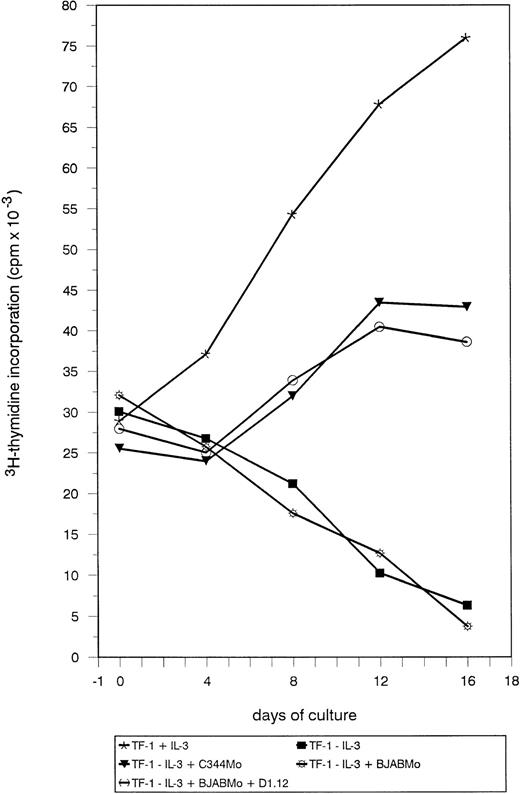
To assess whether CD34+ cell protection from apoptosis by HTLV-II virus was accompanied by a mitogenic effect induced by the virus, the DNA content of TF-1 cells undergoing different treatments was measured by 3H-thymidine incorporation. As shown in Fig 3, withdrawal of IL-3 resulted in a sharp decrease in cell proliferation analyzed over a 16-day period. Incubation with C344-derived Mo strain resulted in maintenance of cell proliferation, although the proliferative capacity evaluated in the whole cell population was slower than the one observed in the presence of IL-3. On the contrary, treatment of TF-1 cells with BJAB-derived Gu strain or Mo strain adapted to BJAB both resulted in a rapid decrease of 3H-thymidine incorporation. Incubation of TF-1 cells BJAB-derived Mo strain treated with anti-class II antibodies restored, instead, the capacity of the cells to proliferate in absence of IL-3, with a kinetics superimposable to the one observed for TF-1 cells treated with C344-derived Mo strain.
Measurement of TF-1 cell proliferation after incubation with HTLV-II virus. TF-1 cells were deprived of IL-3 for 24 hours and then seeded at a concentration of 2.5 × 105 cells/well in a volume of 300 μL of culture medium. At this time, cells were either supplemented with IL-3 (subsequently added every 4 days) or treated with the various stimuli listed in the bottom panel. Treatments with HTLV-II strains were for 24 hours. Cell proliferation was measured by3H-thymidine incorporation (cpm in the ordinate) over time (days in the abscissa).
Measurement of TF-1 cell proliferation after incubation with HTLV-II virus. TF-1 cells were deprived of IL-3 for 24 hours and then seeded at a concentration of 2.5 × 105 cells/well in a volume of 300 μL of culture medium. At this time, cells were either supplemented with IL-3 (subsequently added every 4 days) or treated with the various stimuli listed in the bottom panel. Treatments with HTLV-II strains were for 24 hours. Cell proliferation was measured by3H-thymidine incorporation (cpm in the ordinate) over time (days in the abscissa).
From these results, we conclude that the antiapoptotic effect on TF-1 cells by C344-derived Mo strain and by the same strain adapted to a B-cell environment and treated with anti-class II antibodies is accompanied by a mitogenic effect.
DISCUSSION
HTLV-II is known for its capacity to deregulate T-lymphocyte growth, most likely through constitutive activation of cellular genes mediated by the viral trans-regulatory protein tax. The present study was undertaken to investigate whether other cellular systems, and particularly hematopoietic precursors, could be sensitive to the HTLV-II action, as hematologic abnormalities other than T-cell proliferation are associated with the virus infection. Our results show unambiguously and for the first time that hematopoietic precursors, represented both by the TF-1 CD34+ positive cell line and, more importantly, by normal BM-derived CD34+ cells, can directly be affected by the HTLV-II virus. In fact, it was found that the HTLV-II Mo viral strain, belonging to the subtype IIa, grown in a T-cell host was able to block the apoptosis induced after IL-3 withdrawal in hematopoietic precursors represented by the TF-1 cell line and by normal BM-derived CD34+ cells. The contact with the virus was necessary and sufficient to mediate the biologic effect as suggested by the fact that heat-inactivated virus was still able to block apoptosis and that no integration and viral replication was demonstrated in TF-1 cells undergoing protection from apoptosis. Protection from apoptosis was accompanied by a direct mitogenic effect of the virus because TF-1 cells continued to proliferate after viral treatment in the absence of IL-3, although to a lesser extent with respect to cells treated with IL-3 alone. The relatively slower replication time of TF-1 cells treated with the virus should be framed, however, with the likely possibility of the more heterogeneous response of the cultured cells to the virus stimulus as compared with the IL-3 growth factor stimulus. Taken together, these results provide evidence that HTLV-II virions are critically involved in mediating survival and growth effects on CD34+ hematopoietic precursors and suggest that the hematologic abnormalities found during HTLV-II infection can depend, at least in part, on a direct virus-cell interaction.
The mitogenic activity triggered by HTLV-II contact with the cellular target is reminiscent of a similar effect of HTLV-I virus on resting T cells.29,30 Several studies have strongly suggested that HTLV-I tax protein is implicated in the virus-dependent mitogenic activity.31 32 In the present study, the HTLV-II mitogenic effect was exerted in the absence of infection, simply by contact with the virus; more importantly, the effect persisted even in the presence of heat-inactivated viral particles, indicating that tax did not play a significant role in the protection from apoptosis.
All of these observations suggested, instead, that structural components of the HTLV-II viral envelope could be involved in the triggering of the mitogenic effect on contact with CD34+cells. We first focused our attention on viral encoded structures such as gp46 envelope glycoprotein for several reasons: (1) the HTLV-I gp 46, closely related in sequence to HTLV-II counterparts, has been reported to be involved in the triggering of human peripheral T-lymphocyte proliferation when these cells are confronted with the virus32,33; (2) our results showed that the capacity to deregulate the programmed cell death of the hematopoietic cellular targets was not shared by a distinct HTLV-II isolate, the Gu strain, grown in the B-cell host BJAB; (3) more importantly, we found that the T-cell–derived HTLV-II Mo strain, adapted to infect and replicate the B-cell BJAB environment, was no more capable of protecting hematopoietic precursors from apoptosis. It is known that modification in HIV-1 gp120 acquired by passages through different cell types can produce few amino acid substitutions in specific protein domains that can affect binding, cell fusion, infectivity, and tropism of the virus.34 In contrast to HIV, whose frequency of mutations is very high, HTLV-II viral strains are very much conserved.2 Nevertheless, we decided to investigate whether the adaptation in two distinct cellular environments of the same HTLV-II virus, the Mo strain, could generate mutations in the HTLV-II gp46 encoding gene offering a hint to explain the distinct biologic behavior of BJAB-derived on hematopoietic precursors. Comparative sequence analysis of gp46 encoding env genes from HTLV-II Mo strain derived from T cells or from B cells showed, instead, no differences. We therefore concluded that a major component of the viral envelope such as gp46 was not responsible for the biologic effect observed.
We then turned our attention on cell host-derived membrane structures, which could be acquired by the HTLV-II virus during the budding from infected cells. In other human retroviruses such as HIV-1, a series of cell surface antigens have been detected as integral components on cell-free virions or have been found to be associated with virus preparations as contaminants that are difficult to remove.35 Among the various cell membrane components that could be present in the viral envelope, we set out to investigate HLA class I and class II antigens because they have been detected in substantial amounts in other retroviral envelopes such as HIV-1, in which they can be involved in several aspects of the virus life cycle and pathogenicity. MoAbs to HLA class I molecules inhibit virus propagation in in vitro cultures by neutralizing the virus through interaction with HLA class I molecules associated with the virion envelope.9,35 In addition, a second antiviral mechanism of anti-HLA class I antibodies, which affects a postbinding stage of the virus life cycle and which probably acts through a modulation of the target T-cell activation, has been recently reported.36HIV-1 envelope-associated HLA class II antigens can serve as superantigen-presenting structures to T cells, which are then activated and proliferate. Moreover, HLA-DR–bearing HIV could, in the continuous presence of certain superantigens, induce T-cell apoptosis.37 Little is known about the selective presence and corresponding functional implication of cell-derived components in the HTLV envelope, although preliminary studies indicate that HLA components can be found in the HTLV-I envelope.38 The results presented in this study clearly show the presence of both HLA class I and class II antigens on the HTLV-II virus envelope. A relationship existed between the amount of HLA molecules expressed in the infected host cell and corresponding detectability in the virus envelope. Indeed, the BJAB-derived HTLV-II Mo strain presented a more elevated concentration of both HLA class I and HLA class II antigens with respect to the T-cell–derived strain. Of particular interest was the fact that HLA class II, but not class I, molecules were functionally involved in the mechanism of HTLV-II–mediated protection from apoptosis. In fact, preincubation of the BJAB-derived virus with antibodies against HLA class II, but not class I, molecules reverted the functional phenotype of the virus to the one of the T-cell–derived virus, and protection from apoptosis of, and consequent mitogenic effect on, hematopoietic precursors was reestablished. The fact that C344 cells were chronically infected by the HTLV-II Mo strain prevented the analysis of the biologic effect of the BJAB-derived Gu strain after possible adaptation to this T-cell environment. It remains to be investigated whether Gu adaptation to a different T-cell environment could result in a virus with antiapoptotic potential on CD34+ cells.
The mechanism by which HLA class II, but not class I, molecules interfere with the mitogenic effect of HTLV-II is a matter of speculation. The concentration of class II molecules on the virus envelope may play a role, as class II molecules are barely appreciable in the envelope of the mitogenic HTLV-II Mo strain originated from the C344 T-cell line. However, the results presented in this study indicated also a differential presence of the class II subsets in the HTLV-II envelope of the T-cell–derived virus with the major subset, the HLA-DR, undetectable. This finding is at variance with the situation observed in the HIV viral envelope, in which, instead, a presence of DR antigen in the absence of DP and DQ antigens has been reported.9 Therefore, the possibility that DR, more than DP and DQ, is involved in the loss of mitogenic potential of HTLV-II Mo strain derived from BJAB cells cannot be ruled out. HLA class II antigens are crucial molecules for the correct functioning of the immune system. Expressed on appropriate antigen-presenting cells, they act as peptide antigen-presenting structures for the recognition by the clonotypically distributed T-cell receptor, delivering the specific first signal which triggers, in T-helper cells, the cascade of events leading to both cellular and humoral immune responses.39 In certain circumstances and particularly when stimulation via HLA class II molecules is not accompanied by accessory signals, T cells fail to respond, may become anergic, or even receive an apoptotic signal.40 Moreover, recent studies suggest that HLA class II itself can promote and transduce an apoptotic signal in the cells that express it.41 42 It is therefore tempting to speculate that the abrogation of the mitogenic effect of BJAB-derived HTLV-II Mo virus on hematopoietic precursors is not a purely passive phenomenon, but may be due to a direct interaction of viral envelope HLA class II molecules with CD34+ cell surface structures. If this interpretation is correct, the natural receptor of HLA class II molecules, the CD4 molecule, should not be involved, as TF-1 cells do not express detectable amounts of CD4 (data not shown). Thus, additional, still elusive, cell surface molecules of hematopoietic cells may serve as HLA class II functional ligands.
ACKNOWLEDGMENT
R.S.A. thanks Prof Lorenzo Moretta, IST, Genova for the invaluable scientific and economic support to his research.
Supported in part by Istituto Superiore Sanità (ISS) IX National Project AIDS No. 9403-16 and No. 9493-02 (to R.S.A.), by Associazione Italiana per la Ricerca sul Cancro (AIRC) programme 1996, and by ISS First National Project Tuberculosis (to R.S.A.). G.T. is the recipient of an I.S.S. AIDS fellowship.
Address reprint requests to Claudio Casoli, PhD, Istituto di Patologia Medica, Facoltà di Medicina Università di Parma, v. Gramsci 14, I-43100 Parma, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 2. Immunoblot analysis of HTLV-II virus lysates after reaction with MoAbs specific for HLA class-I (B9.12.1) or class-II (D1.12 and Tu35). Pellets of purified HTLV-II virions generated from C344 cell line (T) or from BJAB cells (B) were incubated in lysis buffer containing 0.2% sodium dodecyl sulfate (SDS) and equivalent concentrations of extracts, as shown by protein staining, analyzed by immunoblot in a 10% polyacrylamide gel containing 0.1% SDS. Immunoreactivity was visualized by chemiluminescence detection system. The size (in kilodaltons [k], indicated on the right) were determined by comparison with protein markers.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/7/10.1182_blood.v91.7.2296/3/m_blod4073202.jpeg?Expires=1766181722&Signature=kyehqFFZdKwu9ceA0gydDr-uqTYjAKNxJPNE82HUIflnHg2KbAdsHgbucoCdh9xqEKPAMrV0nb77s6kidIJT2bPqSA-7-I83TeO56wyAwzrxd8NH18zszcOG~UgQKYb25V3VL3R6qH8Q2-FlGUhJG6WejxbZTMnDbsirjAACEIh5PH68NXTV5SFJOwsOIaObPbHGpJhnETu4mo7m1ELpt0oHjTRu3F2Kbbo7L6PgX2JvThP94EzXUaUYmPJe4-jsJFXlJBXu8Sbon2kq1Kq1pHoNuXzTpp1itIqeTG1SmX7WD10mJNFSdizDNJvhVxgD69qDkVdJF-4albxiZeEkRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 2. Immunoblot analysis of HTLV-II virus lysates after reaction with MoAbs specific for HLA class-I (B9.12.1) or class-II (D1.12 and Tu35). Pellets of purified HTLV-II virions generated from C344 cell line (T) or from BJAB cells (B) were incubated in lysis buffer containing 0.2% sodium dodecyl sulfate (SDS) and equivalent concentrations of extracts, as shown by protein staining, analyzed by immunoblot in a 10% polyacrylamide gel containing 0.1% SDS. Immunoreactivity was visualized by chemiluminescence detection system. The size (in kilodaltons [k], indicated on the right) were determined by comparison with protein markers.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/7/10.1182_blood.v91.7.2296/3/m_blod4073202.jpeg?Expires=1766181723&Signature=fOZm0ecYUMphYZ6UPOpyb1p5eSB~etehLr0NfSYzEIA3qBYDCr3PT9zYu4WNVrEbS0rb5~CCAvISovlsn~Vw9luvmsgwb5LS54eiMefWv0GpeSXD~yTlus-VeGQU2PDqSk0PuryWkOgrCRsFE9jmvbbez8b9Fz~k9qbkh~tQX52YSmRsIwKLq159I0STw0SsbvDo~hGaserQRBvH55CF3uMfEHdppWoutT4aMacYB5fZtqLDvfAIzOJO2GuohqFUawmuFGwrhe68IEHBYdzy~tUAt~SlaVRD2bCOpCjUst5Z5qgOfDYknUflGjLikpXSgl0lZpqaSs6HK932ctq5UQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
