Abstract
Urokinase-type-plasminogen activator (uPA) and its receptor are localized in the vessel wall where they are involved in cellular activation and remodelling processes. Besides the cell surface glycolipid (GPI)-anchored urokinase receptor (uPAR), which binds uPA with high affinity, recent evidence points to the existence of soluble uPAR (suPAR), as well. In the present study, the origin, binding mechanism, and cellular effects of suPAR were examined. Under basal conditions human vascular smooth muscle cells (HVSMC), human umbilical vein endothelial cells (HUVEC), and monocytic cells released 0.1 to 2 ng/mL suPAR, which was increased twofold to fivefold after phorbol ester (PMA) stimulation, as measured by a function-dependent enzyme-linked immunosorbent assay (ELISA). suPAR alone did not bind to HVSMC or HUVEC, but reduced cellular uPA binding by 50% to 70%. However, after removal of GPI-uPAR with phosphatidylinositol-specific phospholipase C, suPAR dose-dependently increased uPA binding by fourfold to fivefold. This increase in binding was completely inhibited by vitronectin (VN) and by a monoclonal antibody against VN, but not by other matrix proteins or antibodies. Thus, VN-mediated uPA binding to cells was regulated by the ratio of soluble to surface-associated uPAR. In a uPAR-deficient cell line (LM-TK−), suPAR increased uPA binding up to 10-fold, whereas the truncated receptor lacking the amino-terminal uPA-binding domain was ineffective. The formation of a ternary uPA/suPAR/VN-complex on the cell surface and the free extracellular matrix could be inhibited by a monoclonal antibody against VN, as well as by plasminogen activator inhibitor-1 (PAI-1). Moreover, VN-mediated binding of the uPA/suPAR-complex led to a fivefold increase in plasminogen activator activity. Through this novel pathway, VN concentrates the uPA/suPAR-complex to cell surfaces and extracellular matrix sites, leading to the accumulation of plasminogen activator activity required for cell migration and tissue remodelling processes.
THE BALANCE BETWEEN the levels of plasminogen activators, urokinase type plasminogen activator (uPA), and tissue type plasminogen activator (tPA) and their inhibitor, plasminogen activator inhibitor-1 (PAI-1), controls the formation and action of plasmin. These components are localized or accumulated at the cell surface through specific receptors and hence are able to regulate pericellular proteolysis-related events.1-3 This system plays an important role in cell migration and tissue remodelling in angiogenesis, atherogenesis, tumor cell metastasis, and ovulation.4-6
uPA binds to the cell surface through the uPA-receptor, which contains three similar domains and is anchored within the plasma membrane by a glycolipid (GPI-) moiety. The amino-terminal domain (domain 1) is primarily involved in the molecular contact with uPA.7 The uPA-uPAR interaction is implicated in several processes that require intracellular signal transduction. Independent of its enzymatic activity, uPA stimulates monocytic cell chemotaxis,8,9activation of monocytes and neutrophils with respect to cell adhesion,10,11 tumor necrosis factor-α release,12 superoxide-anion production,13 and expression of matrix metalloproteinases.14 In addition, phosphorylation of specific proteins in monocytes has been observed, suggesting that the involved kinases are physically associated with uPAR.15 uPAR is also able to form a complex with β2-integrins15,16 or interferes with β1-integrin ligation.17 In the absence of a cytoplasmic domain, however, it is not clear how transduction of intracellular signaling events can occur.
A soluble form of uPAR (suPAR) exists in plasma (1 to 10 ng/mL),18 yet its origin is unknown. Similarly, no information exists regarding the levels of suPAR in the extracellular space of the vessel wall or on the distribution between the soluble and the GPI-anchored forms. Moreover suPAR, by itself, is thought to directly mediate intracellular signaling by interacting with target proteins (adaptors) in the plasma membrane.19
The multifunctional adhesion protein vitronectin (VN) accumulates prominently in extracellular matrices associated with acute injury and tissue repair20 or several malignant tumors.21Through its interaction with β1 and β3integrins, VN is involved in adhesion and migration-related processes. VN is a linking molecule between the extracellular matrix and some of the uPA-dependent mechanisms, as it was shown to directly interact with uPA and uPAR.22,23 Our previous investigations have shown that uPAR is a cellular receptor for VN on vascular endothelial cells and that the affinity of this interaction is increased by uPA.22 These observations prompted us to investigate whether vascular cells produce suPAR and how suPAR can interact with cells or influence uPA binding to cells. We show that ternary uPAR-containing complexes are assembled in a VN-dependent manner on the cell surface and extracellular matrix thereby allowing focalization of plasmin formation. suPAR can thereby induce cellular functions at sites distant from its synthesis and release.
MATERIALS AND METHODS
Reagents.
Recombinant Gly158scuPA (noncleavable inactivable mutant of high molecular weight uPA) was produced in Chinese hamster ovary cells.24 Recombinant uPAR and the truncated form lacking domain 1 were produced as described before25,26 and provided by Dr N. Behrendt (Finsen Laboratory, Copenhagen, Denmark). VN was purified from human plasma and converted to the multimeric form as previously described,27,28 monoclonal antibody (MoAb) 13H1 against VN was from Dr P. Declerck (Leuven, Belgium) and its characteristics have been described elsewhere.29Thrombospondin-1 purified from platelet concentrate30 was provided by Dr P. Vischer (Münster, Germany), antithrombospondin-1 antibody31 was from Dr D.F. Mosher (Madison, WI), fibronectin and antifibronectin antibody were from Sigma (Munich, Germany). Anti-uPAR MoAb (R4) was provided by Dr G. Hoyer-Hansen (Finsen Laboratory, Copenhagen, Denmark). Active PAI-1 was from Dr J. Deinum (Astra Hässle AB, Mölndal, Sweden) and anti-avβ3 MoAb (LM609) was provided by Dr S. Goodman (Merck KGaA, Darmstadt, Germany).
Cell cultures.
Cultures of human vascular aorta smooth muscle cells (HVSMC) were established, characterized, and grown exactly as described previously.32 Human umbilical vein endothelial cells (HUVEC) were provided by Dr B. Pötzsch (Bad Nauheim, Germany).22 Monocytic cells (U937 and HL-60) and LM-TK− cells were from American Type Culture Collection (Rockville, MD) and cultured as described by the supplier. Serum-free cultures of LM-TK− cells were grown in Iscove's Dulbecco's modified Eagle's medium (DMEM) on gelatin- or fibronectin-coated dishes. The cells were harvested by trypsinization, and soya-bean trypsin inhibitor was used to stop the trypsinization process. All cell culture media were from GIBCO (Eggenstein, Germany).
Radiolabeling of proteins.
Gly158scuPA was used in the ligand binding studies because it has no proteolytic activity that might lead to secondary events in the binding assay. Gly158scuPA (20 μg) and suPAR (1.7 μg) were labeled with 1 mCi Na125I (Amersham-Buchler, Braunschweig, Germany) using Iodogen (Pierce, Oud Beijerland, The Netherlands) according to a previously outlined procedure.33 34 Free 125I was removed by gel filtration on Sephadex G25 (Pharmacia, Freiburg, Germany) followed by dialysis. The specific radioactivity of Gly158scuPA and suPAR was 50 or 500 μCi/μg, respectively.
Cell binding experiments.
HUVEC, HVSMC, and LM-TK− cells were grown to confluency in 48-well plates. The cells were washed extensively with serum-free medium and the binding buffer, DMEM/F-12, 25 mmol/L HEPES, 0.3% (vol/vol) bovine serum albumin (BSA), pH 7.4 was added to the wells. The plates were then maintained at 4°C, while the competitors, excess cold ligand (to measure nonspecific binding) and radiolabeled ligand, were added to a final volume of 0.2 mL. Typically, 105 cpm 125I-Gly158scuPA or125I-suPAR were added per well. For estimation of nonspecific binding, a final concentration of 250 nmol/L Gly158scuPA or 10 μg/mL suPAR was used. After 2 to 3 hours of incubation at 4°C, the wells were washed with phosphate-buffered saline (PBS), cells were solubilized with 1 mol/L NaOH and counted in a γ-counter. Pretreatment of cells with phosphatidylinositol-specific phospholipase C (piPLC) (Oxford Glyco Systems, Oxford, UK) was performed in serum-free medium containing 0.5 U/mL of piPLC for 2 hours at 37°C before proceeding with the standard binding assay.
suPAR-enzyme–linked immunosorbent assay (ELISA).
Analysis of suPAR was performed in 18-hour cell-conditioned media from different cell types containing 0.2% fetal calf serum as indicated. After collection, the conditioned media were supplemented with NaN3 (0.05% wt/vol), Tween-20 (0.05% wt/vol), and EDTA (5 mmol/L) before the assay. For some experiments, the samples were concentrated using concentrator tubes with a 10-kD cut-off membrane (Amicon, Beverly, MA). Maxisorp plates (Nunc, Roskilde, Denmark) were coated with 50 μL of the anti-uPAR MoAb R4 (2 μg/mL). Plates were blocked with 1% (wt/vol) BSA and recombinant suPAR as a standard (0.1 to 5 ng/mL) or cell conditioned media were incubated in these wells overnight at 4°C. After extensive washing, uPA (10 nmol/L) (Medac, Hamburg, Germany) was added to the wells for 30 minutes at room temperature, unbound uPA was washed away, and a mixture of plasminogen (20 μg/mL) and its substrate S2251 (800 μmol/L) (Chromogenix, Mölndal, Sweden) was added. The rate of plasmin formation was followed at 405 nm on a Thermomax reader (Molecular Devices, Menlo Park, CA) at 37°C. This assay allowed the quantitation of suPAR in samples with a detection limit of 0.2 ng/mL.
Cell adhesion assays.
Cell adhesion to VN-coated 48-well plates was tested according to our previously described protocol.22 Briefly, plates were coated with 2 μg/mL VN and blocked with 3% BSA. Cells were trypsinized, washed in serum-free medium and plated on to the VN-coated wells for 1 hour with or without competitors. After this incubation period, the wells were washed and the adherent cells were quantified by crystal violet staining.
Extracellular matrix preparation.
HUVEC, HVSMC, and LM-TK− cells were grown to confluency in 48-well plates and washed three times with PBS containing 2% (wt/vol) BSA and 1 mmol/L CaCl2. Cells were removed with PBS containing 0.5% (wt/vol) Triton X-100 for 15 minutes followed by incubation with 0.5% (wt/vol) Triton-X100 and 0.1 mol/L NH3 for another 15 minutes at 22°C.35Finally, wells were washed with PBS and blocked with PBS containing 3% (wt/vol) BSA before proceeding with a radioligand binding assay or a plasminogen activation assay.
Plasminogen activation assay.
LM-TK− cells or their free extracellular matrix were incubated in the absence or presence of uPA (0.1 to 50 nmol/L) at 4°C for 2 to 3 hours with other test substances as indicated in the figure legends. Unbound uPA was washed away and the rate of plasminogen activation was measured as described above.
RESULTS
suPAR in vascular cells.
To quantify suPAR in the conditioned media of vascular cells, a sensitive assay was developed. suPAR in the conditioned media was captured by MoAb-R4, that does not interfere with receptor binding of uPA, and was detected via the binding of uPA followed by a plasminogen activation test. Only biologically active suPAR was measured in this assay, whereas truncated two-domain suPAR was not (data not shown), and the detection limit of the assay was 0.2 ng/mL. The assay was specific (Fig 1A) in that it was not influenced by uPA, tissue plasminogen activator, PAI-1 (each 10 nmol/L), or up to 10% (vol/vol) fetal calf serum, respectively, in the conditioned medium in the initial step of suPAR capture. The levels of suPAR in the conditioned media of HVSMC, HUVEC, and monocytic cells (U937, HL-60) were in the range 0.1 to 2 ng/mL or 0.5 to 4 ng/18 h/106cells (Fig 1B). Stimulation of the cells with phorbol myristate acetate (PMA) increased the suPAR levels twofold to fivefold. The levels of suPAR were similar before and after ultracentrifugation of the conditioned medium indicating that suPAR was not associated with membrane fragments. Phase separation with Triton X-114 indicated that the majority of suPAR did not have an intact glycolipid anchor. In untreated and PMA-stimulated vascular cells, the ratio of secreted suPAR versus cell bound uPAR was in the range 1:0.5 to 1:10 (details to be published elsewhere). These results show that suPAR can be released from vascular cells and accumulates in the extracellular space.
Presence of suPAR in conditioned media of vascular cells. (A) suPAR was captured by immobilized anti-uPAR MoAb-R4 and subsequently detected by measuring the extent of uPA binding using a plasminogen activation assay. Recombinant suPAR was used to generate the standard curve (rate of plasmin formation; Vmax, mOD/min at 405 nm) against which the unknown samples were quantified. (B) The production of suPAR by HVSMC, HUVEC, HL-60, and U937 cells was measured under basal conditions (hatched bars) or after stimulation by PMA (100 ng/mL) (filled bars). Data are shown as ng/18 h/106 cells (mean ± standard error of mean [SEM] of triplicate wells) and similar results were observed in three separate experiments.
Presence of suPAR in conditioned media of vascular cells. (A) suPAR was captured by immobilized anti-uPAR MoAb-R4 and subsequently detected by measuring the extent of uPA binding using a plasminogen activation assay. Recombinant suPAR was used to generate the standard curve (rate of plasmin formation; Vmax, mOD/min at 405 nm) against which the unknown samples were quantified. (B) The production of suPAR by HVSMC, HUVEC, HL-60, and U937 cells was measured under basal conditions (hatched bars) or after stimulation by PMA (100 ng/mL) (filled bars). Data are shown as ng/18 h/106 cells (mean ± standard error of mean [SEM] of triplicate wells) and similar results were observed in three separate experiments.
Binding of the uPA/suPAR-complex to HVSMC and HUVEC.
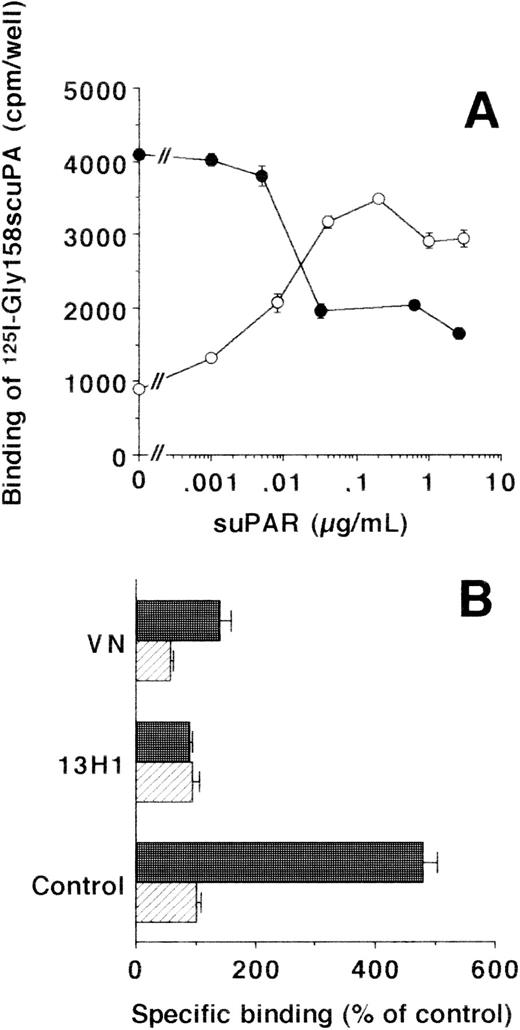
The interaction of suPAR with different cell types was studied in direct binding assays. While there was no binding of125I-suPAR to HVSMC and HUVEC, these cells bound125I-Gly158scuPA through the GPI-anchored uPAR. As expected, this binding was inhibited by uPA and Gly158scuPA (IC50:0.5 nmol/L for both isoforms) or by the addition of unlabeled suPAR (IC50:10 ng/mL) in a dose-dependent way. Although excess uPA could completely inhibit125I-Gly158scuPA binding, only 50% to 70% inhibition was observed with excess suPAR. Pretreatment of HVSMC and HUVEC with piPLC to remove the GPI-anchored uPAR resulted in a decrease of uPA binding by 75% in HVSMC and 90% in HUVEC, respectively. In contrast, this low residual binding of 125I-Gly158scuPA to piPLC-treated cells was increased fourfold to fivefold after addition of suPAR (Fig 2A).
Effect of suPAR on the binding of125I-Gly158scuPA to vascular cells. (A) The specific binding of 125I-Gly158scuPA to HVSMC was determined in the absence and presence of increasing suPAR concentrations as indicated. Binding experiments were performed with untreated cells (•) or with piPLC-treated cells (○). Data represent mean ± SEM (cpm/well) of triplicate wells from a typical experiment. Similar results were obtained in three separate experiments on HVSMC or HUVEC, respectively. (B) The effects of MoAb-13H1 against VN (25 μg/mL) and multimeric VN (20 μg/mL) on the binding of 125I-Gly158scuPA to piPLC-treated HVSMC were tested in the absence (hatched bars) or presence (filled bars) of suPAR (1 μg/mL). Data are expressed as percentage of control (mean ± SEM) of three different experiments, where 100% (control) is represented by the specific binding of125I-Gly158scuPA in the absence of suPAR. Similar results were obtained in experiments with HUVEC.
Effect of suPAR on the binding of125I-Gly158scuPA to vascular cells. (A) The specific binding of 125I-Gly158scuPA to HVSMC was determined in the absence and presence of increasing suPAR concentrations as indicated. Binding experiments were performed with untreated cells (•) or with piPLC-treated cells (○). Data represent mean ± SEM (cpm/well) of triplicate wells from a typical experiment. Similar results were obtained in three separate experiments on HVSMC or HUVEC, respectively. (B) The effects of MoAb-13H1 against VN (25 μg/mL) and multimeric VN (20 μg/mL) on the binding of 125I-Gly158scuPA to piPLC-treated HVSMC were tested in the absence (hatched bars) or presence (filled bars) of suPAR (1 μg/mL). Data are expressed as percentage of control (mean ± SEM) of three different experiments, where 100% (control) is represented by the specific binding of125I-Gly158scuPA in the absence of suPAR. Similar results were obtained in experiments with HUVEC.
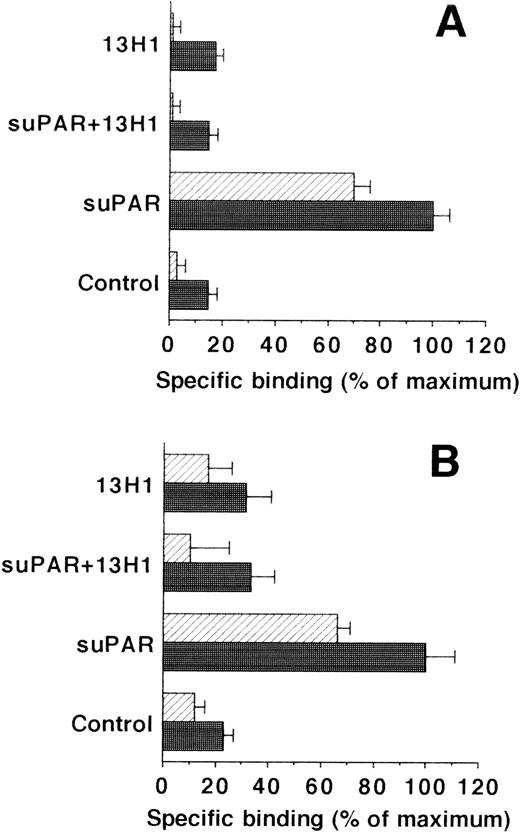
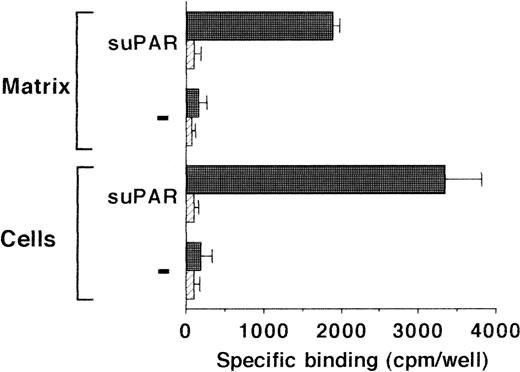
Because uPA and uPAR directly interact with VN, the possibility was tested that VN was the cellular binding site for the uPA/suPAR-complex mediating the formation of a ternary product. Binding of the uPA/suPAR-complex to piPLC-treated cells was dose-dependently inhibited by soluble VN or MoAb-13H1 against VN (Fig 2B). In contrast, both competitors had no influence on the binding of125I-Gly158scuPA alone to normal or piPLC-treated cells (data not shown). Thrombospondin-1 or antibodies to it were by far less effective, and other matrix proteins such as fibronectin, tenascin, fibrinogen, osteonectin, type I collagen, as well as antibodies against fibronectin as competitors were ineffective (data not shown). Excess suPAR could not completely inhibit the binding of uPA to normal cells alone, but this residual binding could be blocked by MoAb-13H1 or excess VN, implicating that suPAR added to untreated cells partially inhibits the uPA binding to its receptor, but also binds, in a complex form with uPA, to cell-associated VN. The cell-associated VN could be in two compartments, either freely associated with some cell surface molecules or incorporated into the extracellular matrix surrounding the cells. Hence, an analysis of the distribution of complex binding to cells versus matrix was performed. The binding to extracellular matrix of HUVEC and HVSMC was 60% and 70%, respectively, of the total uPA/suPAR-complex binding (Fig 3). Thus, not only cell-associated but also matrix-associated VN is able to bind the uPA/suPAR-complex.
Binding of uPA/suPAR-complex to piPLC pretreated vascular cells and their isolated extracellular matrix. The binding of 125I-Gly158scuPA to piPLC pretreated HVSMC (A) and HUVEC (B) (filled bars) and their respective extracellular matrix preparations (hatched bars) is compared in the absence or presence of suPAR (1 μg/mL) or MoAb-13H1 (25 μg/mL). For both cell types, data represent mean ± SEM of a typical experiment in triplicate where the maximal binding to piPLC pretreated cells in the presence of suPAR is set at 100%. Similar results were obtained in three separate experiments.
Binding of uPA/suPAR-complex to piPLC pretreated vascular cells and their isolated extracellular matrix. The binding of 125I-Gly158scuPA to piPLC pretreated HVSMC (A) and HUVEC (B) (filled bars) and their respective extracellular matrix preparations (hatched bars) is compared in the absence or presence of suPAR (1 μg/mL) or MoAb-13H1 (25 μg/mL). For both cell types, data represent mean ± SEM of a typical experiment in triplicate where the maximal binding to piPLC pretreated cells in the presence of suPAR is set at 100%. Similar results were obtained in three separate experiments.
uPA/suPAR-complex binding to LM-TK− cells.
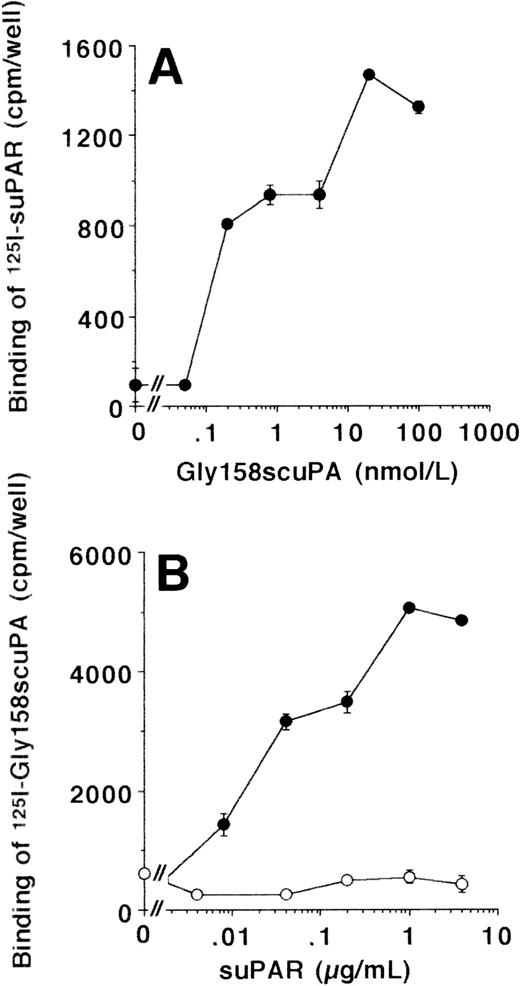
The interaction between uPA/suPAR and VN was further investigated in LM-TK− cells, a mouse fibroblastic cell line that has been shown in earlier studies to be negative for uPAR surface expression.36125I-suPAR did not bind to LM-TK− cells, and also 125I-Gly158scuPA alone showed very little specific binding. In contrast, the uPA/suPAR-complex bound specifically to these cells, as the binding of125I-suPAR was substantially induced after coaddition of Gly158scuPA (Fig 4A). Likewise, in the converse experiment, the binding of 125I-Gly158scuPA was stimulated dose-dependently up to 10-fold in the presence of suPAR (Fig4B). In contrast, the truncated form of uPAR that lacks domain 1, ie, the binding site for uPA, did not enhance the binding of uPA at all (Fig 4B).
Binding of uPA/suPAR-complex to LM-TK−cells. (A) The binding of 125I-suPAR to LM-TK− cells was tested in the absence or presence of increasing concentrations of unlabeled Gly158scuPA as indicated. No specific binding of 125I-suPAR alone was observed. (B) The binding of 125I-Gly158scuPA to LM-TK− cells was tested in the absence or presence of increasing concentrations of unlabeled suPAR (•) or the truncated, domain 1-lacking suPAR (○) as indicated. Data represent mean ± SEM (cpm/well) of triplicate wells. Similar results were observed in five separate experiments.
Binding of uPA/suPAR-complex to LM-TK−cells. (A) The binding of 125I-suPAR to LM-TK− cells was tested in the absence or presence of increasing concentrations of unlabeled Gly158scuPA as indicated. No specific binding of 125I-suPAR alone was observed. (B) The binding of 125I-Gly158scuPA to LM-TK− cells was tested in the absence or presence of increasing concentrations of unlabeled suPAR (•) or the truncated, domain 1-lacking suPAR (○) as indicated. Data represent mean ± SEM (cpm/well) of triplicate wells. Similar results were observed in five separate experiments.
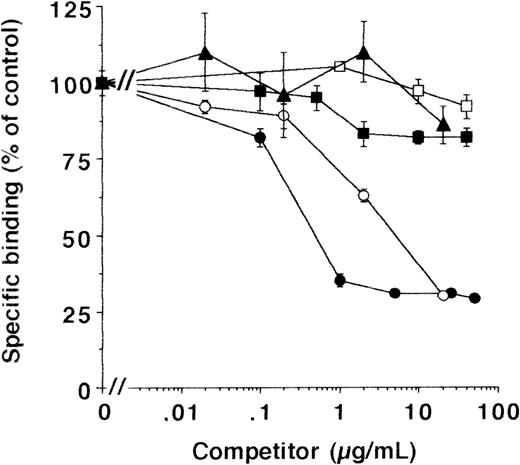
As with HVSMC and HUVEC, the binding of the uPA/suPAR-complex was inhibited by MoAb-13H1 and exogenous VN (Fig 5), while other matrix proteins were not effective. To quantitate the binding to cell- and extracellular matrix-associated VN, experiments were performed on matrix preparations of LM-TK− cells in parallel. Binding of uPA/suPAR-complex to matrix was approximately half the binding to intact cells and showed the same characteristic inhibition by excess soluble VN or MoAb-13H1, respectively (see below). In addition, active PAI-1, which has been shown to disrupt the interaction between uPAR and VN22,37 and which forms a complex with active uPA, completely inhibited the binding of uPA to cells in the presence of suPAR (data not shown) and thereby abrogated ternary complex formation. These data indicate that VN concentrates uPA on the cell surface and the extracellular matrix in the presence of suPAR. This cell- and matrix-associated VN could arise from the serum in the culture medium. To test this hypothesis, LM-TK− cells were grown in serum-free medium on gelatin- or fibronectin-coated surfaces. No binding of the uPA/suPAR-complex to these cells was observed. However, preincubation of cells for 48 hours with 10 μg/mL multimeric VN (equivalent to the serum-concentration28) resulted in binding of uPA/suPAR complex to cells and matrix (Fig 6), which was inhibited by MoAb-13H1 (not shown), indicating that VN was the binding partner.
Effect of different competitors on the binding of uPA/suPAR-complex to LM-TK− cells. MoAb-13H1 against VN (•), multimeric VN (○), monomeric VN (▴), an MoAb against thrombospondin-1 (□), or soluble thrombospondin-1 (▪) were tested for their effect on the binding of the uPA/suPAR-complex to LM-TK− cells. Data are expressed as percentage of control (mean ± SEM) from three different experiments. The binding of125I-Gly158scuPA in the presence of suPAR and in the absence of any competitor served as the 100% control, and the binding of 125I-Gly158scuPA alone was about 20% of the binding of the complex.
Effect of different competitors on the binding of uPA/suPAR-complex to LM-TK− cells. MoAb-13H1 against VN (•), multimeric VN (○), monomeric VN (▴), an MoAb against thrombospondin-1 (□), or soluble thrombospondin-1 (▪) were tested for their effect on the binding of the uPA/suPAR-complex to LM-TK− cells. Data are expressed as percentage of control (mean ± SEM) from three different experiments. The binding of125I-Gly158scuPA in the presence of suPAR and in the absence of any competitor served as the 100% control, and the binding of 125I-Gly158scuPA alone was about 20% of the binding of the complex.
Binding of 125I-Gly158scuPA to serum-free cultures of LM-TK− cells and their extracellular matrix. Cells were grown for 14 days in completely serum-free medium on gelatin- or fibronectin-coated dishes. Before the binding experiment, the cells were preincubated with buffer only (hatched bars) or with 10 μg/mL multimeric VN for 48 hours (filled bars). Binding of 125I-Gly158scuPA to cells and extracellular matrix in the absence or presence of suPAR (1 μg/mL) was performed as indicated. Results are mean ± SEM (cpm/well) of triplicate wells. Similar results were obtained in three separate experiments.
Binding of 125I-Gly158scuPA to serum-free cultures of LM-TK− cells and their extracellular matrix. Cells were grown for 14 days in completely serum-free medium on gelatin- or fibronectin-coated dishes. Before the binding experiment, the cells were preincubated with buffer only (hatched bars) or with 10 μg/mL multimeric VN for 48 hours (filled bars). Binding of 125I-Gly158scuPA to cells and extracellular matrix in the absence or presence of suPAR (1 μg/mL) was performed as indicated. Results are mean ± SEM (cpm/well) of triplicate wells. Similar results were obtained in three separate experiments.
Plasminogen activation on the surface of LM-TK−cells.
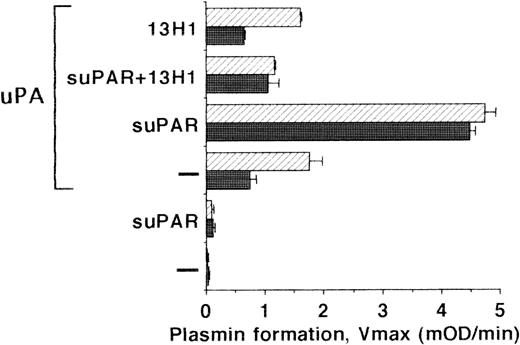
To define whether the VN-mediated binding of the uPA/suPAR-complex has physiologic consequences, we determined the plasminogen activation potential on the surface of LM-TK− cells and on their extracellular matrix. Under basal conditions, no plasminogen activation was detectable. Addition of uPA alone to cells or to isolated matrix resulted in low plasminogen activation representative of the small portion of uPA binding seen before. After coaddition of suPAR, plasminogen activation was increased 5- to 10-fold showing that suPAR stabilized the ternary complex with VN on the LM-TK−cell surface and the matrix (Fig 7). In contrast, suPAR added to uPA (without cells or matrix) followed by a washing step did not initiate any plasminogen activation. Similar to the above binding data, MoAb-13H1 could inhibit plasmin formation due to interference with uPA/suPAR-complex binding to VN.
Plasminogen activation on LM-TK− cells. LM-TK− cells (filled bars) and their extracellular matrix (hatched bars) were incubated in the absence or presence of uPA (10 nmol/L), as well as in the absence or presence of suPAR (1 μg/mL) and MoAb-13H1 (25 μg/mL) against VN for 2 to 3 hours at 4°C as indicated. The unbound uPA was then washed away and the rate of plasmin formation was measured (Vmax, mOD/min at 405 nm). Results are mean ± SEM of triplicate wells and similar results were obtained in three separate experiments.
Plasminogen activation on LM-TK− cells. LM-TK− cells (filled bars) and their extracellular matrix (hatched bars) were incubated in the absence or presence of uPA (10 nmol/L), as well as in the absence or presence of suPAR (1 μg/mL) and MoAb-13H1 (25 μg/mL) against VN for 2 to 3 hours at 4°C as indicated. The unbound uPA was then washed away and the rate of plasmin formation was measured (Vmax, mOD/min at 405 nm). Results are mean ± SEM of triplicate wells and similar results were obtained in three separate experiments.
LM-TK− cell adhesion.
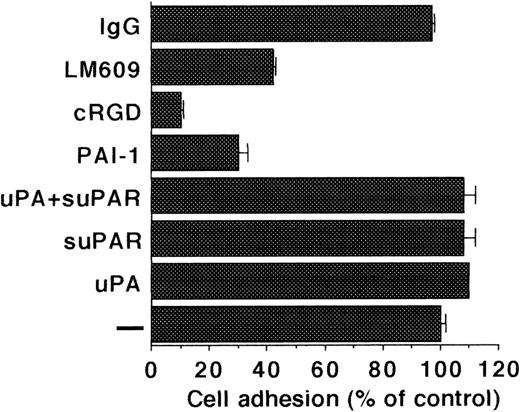
Because uPAR in complex with uPA and VN has been implicated in mediating cell adhesion, the effect of the uPA/suPAR-complex on adhesion was tested. The adhesion of LM-TK− cells to VN was completely dependent on integrins as evidenced from inhibition by cyclic RGD peptides, PAI-1 and avβ3blocking MoAb LM609. Addition of uPA or suPAR on their own and also of the uPA/suPAR-complex did not influence the adhesion of LM-TK− cells onto a VN substrate (Fig 8). Conversely, LM-TK− cells did not adhere onto uPA or uPA/suPAR-complex coated plates (data not shown).
Adhesion of LM-TK− cells to immobilized VN. Adhesion of cells to VN-coated wells was performed in the absence of any competitor (−) or in the presence of uPA (100 nmol/L), suPAR (1 μg/mL), cRGD (10 μg/mL), PAI-1 (200 nmol/L), anti-avβ3 MoAb-LM609 (25 μg/mL), or control MoAb-IgG (25 μg/mL) and measured by crystal violet staining. Results are expressed as percentage of adhesion to VN without competitor (mean ± SEM of triplicate wells). Similar results were obtained in six separate experiments.
Adhesion of LM-TK− cells to immobilized VN. Adhesion of cells to VN-coated wells was performed in the absence of any competitor (−) or in the presence of uPA (100 nmol/L), suPAR (1 μg/mL), cRGD (10 μg/mL), PAI-1 (200 nmol/L), anti-avβ3 MoAb-LM609 (25 μg/mL), or control MoAb-IgG (25 μg/mL) and measured by crystal violet staining. Results are expressed as percentage of adhesion to VN without competitor (mean ± SEM of triplicate wells). Similar results were obtained in six separate experiments.
DISCUSSION
Adhesion receptors such as integrins together with the extracellular matrix provide the basic molecular framework for virtually all adhesion and migration related cell functions. In addition, cell movement requires partial breakdown of the surrounding matrix macroscopically and dissociation of attachment points microscopically in a specific spatiotemporal manner. The plasminogen activation system seems to be crucially involved in the latter processes.38,39 We and others15-17,22,40 41 have recently shown that there is considerable cross-talk between the adhesion and the proteolytic systems: (1) VN is a ligand for uPAR and the affinity of this interaction is regulated by uPA and PAI-1; (2) PAI-1 inhibits VN binding to integrins and uPA reverses this effect; (3) uPAR can interact with β1 and β2 integrins. In the present study, we show that vascular cells produce appreciable quantities of suPAR and that VN serves as a receptor for uPA/suPAR-complexes on cells and extracellular matrix thereby concentrating and redistributing uPA-related plasminogen activation. These cross-regulatory interactions provide additional points of control for cellular processes pertinent to cell adhesion and migration.
suPAR is present in the media of tumor cell lines and in the plasma of patients suffering from paroxysmal nocturnal hemoglobinuria,42 yet its origin is not clear. Due to its glycolipid-anchored linkage the existence of suPAR could arise in different ways: (1) cleavage of the glycolipid-anchor by specific lipases; (2) vesicle shedding; or (3) incomplete synthesis of the glycolipid anchor. We provide evidence that suPAR is secreted from vascular endothelial, smooth muscle, and monocytic cells and does not have an intact glycolipid anchor. The basal release (0.1 to 2 ng/mL) could be enhanced after activation of the protein kinase C pathway by PMA. In this assay, only the levels of the functionally intact suPAR are measured and they are in the concentration range found in plasma (1 to 10 ng/mL).18
The existence of soluble receptors such as suPAR can have a number of consequences.43 In principle, soluble receptors can: (1) stabilize the ligand without having an intrinsic role in signal transduction, thereby preventing degradation of the ligand until it is delivered to the membrane-associated receptor; (2) improve the presentation of the ligand to the cells; (3) compete with their membrane-bound counterparts for binding to the ligand; (4) be an integral participant in ligand-induced signaling allowing cells without the receptor to interact with the ligand-receptor complex and thereby imparting responsiveness in cells that do not express the membrane receptor.43 Most of these properties could be ascribed to suPAR in the present study.
In uPAR lacking LM-TK− cells we observed no direct binding of suPAR, whereas specific binding was seen in the presence of uPA. The uPA/suPAR-complex bound exclusively to VN, but not to other matrix or cell surface-associated proteins. The VN, which is responsible for mediating the binding of the complex, originates from the serum and is associated with cells or becomes incorporated into the extracellular matrix.20 Hence, we propose that the binding of the complex to LM-TK− cells is mediated predominantly by VN, whereas in a previous report, partial interaction was also attributed to thrombospondin-1.36 The plasminogen activator potential on the cell surface or the matrix of cells was drastically enhanced by the uPA/suPAR-complex and could be inhibited by MoAb-13H1 or active PAI-1. These observations underline the central role of VN also as adaptor component for the control of pericellular proteolysis, which is relevant for tissue remodelling. Yet, uPA alone or uPA in complex with suPAR did not promote the direct adhesion of LM-TK− cells to VN-coated surface. This is in accordance with our previous observations that the uPA/suPAR-complex together with VN is not involved in static adhesion phenomena.22
Intact HUVEC and HVSMC bound uPA with high affinity, and uPAR blocking antibodies or piPLC pretreatment, respectively, reduced uPA binding. suPAR significantly inhibited binding of uPA, acting in this case as a competitive soluble receptor, thereby impairing the biologic effects of uPA as a cell surface-associated plasminogen activator or inducer of signal transduction events. However, piPLC pretreatment of these cells removed the glycolipid-anchored uPAR and unmasked a binding pattern of the uPA/suPAR-complex that was very similar to that on LM-TK− cells. The binding was abolished by soluble VN or MoAb-13H1 against VN emphasizing the formation of a ternary uPA/suPAR/VN-complex in which VN served as the recognition component on the cell surface or the extracellular matrix. These data show that suPAR not only competes with membrane-bound uPAR for binding to its ligand, but can also redistribute the presentation of uPA on the cell surface and the extracellular matrix.
With the formation of a ternary complex, suPAR or the uPA/suPAR-complex could initiate proteolysis-independent signal transduction and biologic events over and above those mediated through the classical glycolipid-anchored uPAR. In fact, receptor cross-talk with, eg, integrins was found not to be necessarily dependent on glycolipid-anchorage of uPAR.16,17 Additional results from our laboratory indicate that piPLC-treated differentiated monocytic cells lose their ability to adhere to endothelium via β2-integrins, whereas maximal cell-to-cell contact could be reconstituted by the addition of suPAR.41 Finally, through a putative membrane adaptor, suPAR has been shown to directly activate chemokinesis.9
The description of VN as a novel receptor for uPA in complex with suPAR adds another example to the list of VN-containing ternary complexes in association with serine proteases and a protease binding protein.44 In contrast, the predominant receptor that serves to bind and endocytose uPA-PAI–1 complexes, is the α2-macroglobulin receptor or low-density lipoprotein receptor related protein.45-47 This complex contributes to the inhibition of plasminogen activation, whereas VN mediates redistribution of plasminogen activation. Thus, the respective binding partner for uPA is able to direct the subsequent fate of the protease.
The binding mechanism for the uPA/suPAR-complex uncovered in this study might play an important role in processes where VN is accumulated extravascularly as in angiogenesis,48,49 atherosclerotic plaques,50 during wound healing,20 or in association with tumors.21,51 Similar situations could arise where suPAR is overexpressed and found in increased concentrations in the circulation or extracellularly, in clinical sepsis syndrome, or in inflammatory and malignant diseases.18 As a novel adaptor, VN thereby harbors uPA/suPAR-complex at cell surfaces or extracellular matrix at sites very distant from their release and contributes to tissue remodelling events.
ACKNOWLEDGMENT
We gratefully acknowledge the technical assistance of T. Schmidt and the generous gift of reagents from Drs G. Hoyer-Hansen and N. Behrendt (Finsen Laboratory, Copenhagen, Denmark). We also thank Drs A.E. May, K.D. Wohn, and M. Germer for critical comments.
Supported in part by Grant No. Pr 327/1-3 from the Deutsche Forschungsgemeinschaft, Bonn, Germany (to K.T.P.).
T.C. and S.M.K. contributed equally to this study.
This work is part of the M.D. thesis of T.C. at the Department of Medicine, Justus-Liebig Universität Giessen, Germany.
Address reprint requests to Klaus T. Preissner, PhD, Max-Planck-Institut, Kerckhoff-Klinik, Sprudelhof 11, D-61231 Bad Nauheim, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Presence of suPAR in conditioned media of vascular cells. (A) suPAR was captured by immobilized anti-uPAR MoAb-R4 and subsequently detected by measuring the extent of uPA binding using a plasminogen activation assay. Recombinant suPAR was used to generate the standard curve (rate of plasmin formation; Vmax, mOD/min at 405 nm) against which the unknown samples were quantified. (B) The production of suPAR by HVSMC, HUVEC, HL-60, and U937 cells was measured under basal conditions (hatched bars) or after stimulation by PMA (100 ng/mL) (filled bars). Data are shown as ng/18 h/106 cells (mean ± standard error of mean [SEM] of triplicate wells) and similar results were observed in three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/7/10.1182_blood.v91.7.2305/3/m_blod4073801.jpeg?Expires=1768724290&Signature=S6FxVEmnBFBCo5wHSH75Kj6aCpFrqISoc4hkZ-fqdXvtZdmOAZmM237~amu-sf3wdcKjgZLDy4xOFKc2sm2J2MOtaGY5Gzb0Eg0B-6CFR7MLY6ZlpEETYX4IOL4DWqvNmgu8LvAwMdyDTQypMJGyIkP3Zku3Y~caAxoBrxizs3efqGiuKUtVE-HBTEjy2AgZ4cgTA~T218bM9AmikO7IdtzH6OhocBxOBwY34BW1vp-rwbqvu6skKtcViUK9LLWtgxBvtGxkJ9d1yWibOWYTHGjDR4ZTZduF3L6ZyFAADlZfz8pAdcal6optEaJV1wF5hlmcA8iXYEH39tnuN7lHqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)