Abstract
Platelet factor 4 (PF4) serves as a lineage-specific marker of megakaryocyte development. We previously identified two positively acting sequences in the human platelet factor 4 (hPF4) gene promoter that synergized to drive high-level luciferase reporter gene expression in vitro. Using portions of the hPF4 5′-flanking region linked to the lacZ reporter gene, we observed in this investigation that constructs with −245 bp of 5′-flanking region were more active than constructs with −2 kb of 5′-flanking region in vitro. We created two independent transgenic mouse lines with a −245-bp hPF4/lacZ construct. Cells from these mice were tested for β-galactosidase (β-gal) expression at the mRNA level by Northern blot and semiquantitative reverse transcription polymerase chain reaction (RT-PCR) and at the protein level by immunohistochemistry assay. Mice from one line showed β-gal expression specifically in all megakaryocytes of all ploidy classes from bone marrow and in platelets. Expression level was comparable to that driven by the 1.1-kb rat PF4 promoter in other transgenic mouse lines. Those in the second line showed no β-gal expression in megakaryocytes, platelets, or any of the eight organs tested. The −245-bp hPF4 promoter is capable of driving reporter gene expression in a megakaryocyte-specific manner in transgenic mice. The small size of this megakaryocyte-specific promoter is compatible with that required in some viral vectors and may provide a model for targeting gene expression to megakaryocytes.
HEMATOPOIESIS IS the process of differentiation of pluripotential bone marrow stem cells to mature progeny. Normal differentiation is accompanied by distinct changes in gene expression. The platelet factor 4 (PF4) gene encodes an abundant α-granule protein and serves as a lineage-specific marker of megakaryocyte development.1 PF4 is a protein that is synthesized by megakaryocytes,2 packaged into α-granules of platelets, and released during platelet activation.3-6PF4 inhibits megakaryocyte differentiation, affects angiogenesis and tumor growth, neutralizes the anticoagulant activity of heparan sulfate on endothelial cells, and functions as a chemokine, an immunomodulatory substance that induces the chemotaxis of blood cells.1 7-9
The transcriptional regulation of the rat (r) PF4 gene in vitro and in vivo has been studied.10-15 These results identified a 1104-bp fragment containing three positively acting sequences. A prominent poly-T stretch, homologous to one in the human PF4 gene, was defined as a negative element. This 1.1-kb fragment was used to create transgenic mice in which the promoter drove expression of lacZ or other reporter genes. Recently, we reported our study of the transcriptional regulation of the hPF4 gene in vitro using transient transfection of reporter genes driven by its promoter.16 Twocis-acting sequences located from −107 to −239 were critical and synergized high-level expression in TPA-stimulated human erythroleukemia cell line (HEL) cells. One sequence, at −134 and including GATTA, may bind a GATA factor, whereas the other binds an as-yet unknown factor to a poly-T stretch (unpublished observations, December 1995). In contrast to the rPF4 findings, the poly-T sequence acted positively, and only one of the three other positively acting sequences identified for rPF4 was necessary for expression mediated by the hPF4 promoter in vitro.
The transcriptional regulation of hPF4 in vivo has not been reported. In studies in vitro, leukemic cell lines like HEL cells are not completely representative of the normal megakaryocyte, because they express gene products of other hematopoietic lineages.17 18How accurately the data from studies in vitro reflect the regulation of the hPF4 gene during normal megakaryocyte development remains to be determined. Given the differences with the rPF4 promoter, we were interested in investigation of the hPF4 promoter in vivo. In this study, a 245-bp fragment of the hPF4 promoter, the portion most active in vitro in comparison with fragments up to 2 kb in length, was linked to the lacZ reporter gene and used to create transgenic mice. β-gal expression in megakaryocytes and other tissues of the transgenic mice was examined to determine hPF4 promoter function in vivo.
MATERIALS AND METHODS
Creation of Transgenic Mice
A 2-kb fragment encompassing the hPF4 5′-flanking region15,19 was introduced into the pNASSβ vector at theXho I site (Clontech, Pal Alto, CA). This −2-kb hPF4/pNASSβ plasmid DNA was digested with Xba I andSty I to create the −245-bp hPF4/lacZ DNA. The latter plasmid included the sequence of the hPF4 gene from −245 bp to +49 bp and 3882 bp of the lacZ gene (Fig1). Before using the lacZ reporter gene in vivo in transgenic mice, we first tested these two lacZ constructs in vitro, in the way we had done previously with the luciferase reporter gene in TPA-induced HEL cells.16 Our rationale was to use the highest expression construct for the transgenic studies and to verify the utility of the expression assays we planned to use for the mice. The transfected HEL cells were stained with X-gal11 and also examined by measurement of the enzymatic activity of β-gal (Galacto-Light Kit; Tropix, Bedford, MA) following the manufacturer's instructions.
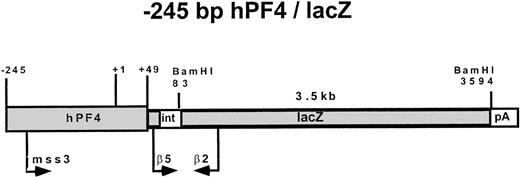
The −245 hPF4/lacZ DNA construct used in the creation of transgenic mice. Primers used in PCR screening (mss3 and β2), semiquantitative RT-PCR (β5 and β2), and the probe for Southern blots (3.5 kb BamHI fragment) are indicated with approximate locations of a spliceable intron (int) and polyadenylation signal (pA) sequences.
The −245 hPF4/lacZ DNA construct used in the creation of transgenic mice. Primers used in PCR screening (mss3 and β2), semiquantitative RT-PCR (β5 and β2), and the probe for Southern blots (3.5 kb BamHI fragment) are indicated with approximate locations of a spliceable intron (int) and polyadenylation signal (pA) sequences.
The purified −245-bp hPF4/lacZ DNA lacking exogenous vector sequences was microinjected into the pronuclei of fertilized eggs from superovulated female mice (C57Bl/6). The injected eggs were transferred to the oviducts of pseudopregnant female mice (CD-1) by the Transgenic Mouse Core Facility of the Children's Hospital of Philadelphia. All studies were approved by the Institutional Animal Care and Use Committee. Pups were analyzed for incorporation of the hPF4/lacZ transgene by PCR and Southern blot analyses as described below.
Genomic DNA was isolated from a tail biopsy of 3-week-old mice and amplified using one primer located in the 5′-flanking region of the hPF4 gene (mss-3, sense, 5′-GGTAATCTTGGCTGGCCAGAACCC-3′) and a second primer (β-2, antisense, 5′-TTAACAGGCTCTTTCGATCCC-3′) located in the lacZ gene (Fig 1). PCR was done for 25 cycles, with each cycle consisting of denaturing at 94°C for 45 seconds, annealing at 65°C for 45 seconds, and extension at 72°C for 1 minute using GeneAmp PCR System 9600 (Perkin Elmer, Norwalk, CT). The products were analyzed by agarose gel electrophoresis for the presence of the appropriately sized band (417 bp). As an internal control, a fragment of the mouse endogenous whey acidic protein (WAP) gene was also amplified using a second set of primers, WAP-S (sense, 5′-ATCCATGTCTCCATGCCTTCTTCT-3′) and WAP-A (antisense, 5′-TGTTGACAGGAG TTTTGCGGGTCC-3′).20
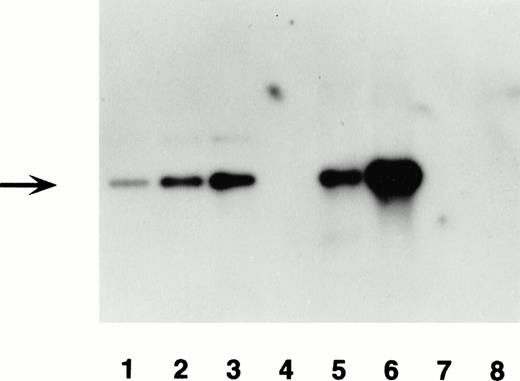
Southern blot analysis was used to confirm PCR-positive mice and to estimate transgene copy number. Genomic DNA (10 μg) was digested withEcoRV or BamHI, separated in a 1% (w/v) agarose gel, and transferred to a Zetabind filter (Cuno Inc, Meridien, CT).BamHI-digested plasmid DNA was used as a standard for estimating copy number by application of 1, 2, and 4 genome-equivalent copies. The filter was prehybridized in Rapid-hyb buffer (Amersham, Arlington Heights, IL) and then hybridized at 65°C overnight to a radiolabeled probe made with the Random Primers Labeling Kit (Boehringer Mannheim, Indianapolis, IN) using a 3.5-kb BamHI fragment of the β-gal gene as a template. The filter was washed in 2× SSC/0.1%(w/v) sodium dodecyl sulfate (SDS), 0.2× SSC/0.1% (w/v) SDS, and finally 0.1× SSC/0.1% (w/v) SDS at 65°C, and then exposed to film at −70°C with an intensifying screen. Three bands, the 5′-junction band, the 3′-junction band, and a 4.2-kb internal band, were present if the mouse genomic DNA had incorporated the transgene at more than one copy. The intensity of bands on film was analyzed by Imagequant PhosphorImager software (Molecular Dynamics, Sunnyvale, CA). The 5′- or 3′- junction band had an intensity that was considered equivalent to one copy per genome. Transgene copy number was calculated by comparing the intensity of the internal band with each junction band. This calculation was also verified by comparing transgene intensity with known amounts of plasmid DNA on the same Southern blot (Fig 2). Equivalent loading of DNA from genomic samples was verified by hybridization to the endogenous mouse β-actin cDNA.21
Transgene detection by Southern blot analysis of two transgenic mouse lines. Lanes 1, 2, and 3 contain 30, 60, and 120 pg of transgene construct DNA, BamHI-digested. Lanes 4 and 7 are blank. Lane 5 contains 10 μg of BamHI-digested genomic DNA from F0 no. 3, and lane 6 F0 no. 16. Lane 8 contains 10 μg of BamHI-digested genomic DNA from a negative littermate control. The blot was hybridized with the labeled lacZ probe shown in Fig 1. The expected 3.5-kb band for the transgene is denoted by the arrow. Hybridization with an endogenous mouse gene showed the expected band for lanes 5, 6, and 8 (data not shown).
Transgene detection by Southern blot analysis of two transgenic mouse lines. Lanes 1, 2, and 3 contain 30, 60, and 120 pg of transgene construct DNA, BamHI-digested. Lanes 4 and 7 are blank. Lane 5 contains 10 μg of BamHI-digested genomic DNA from F0 no. 3, and lane 6 F0 no. 16. Lane 8 contains 10 μg of BamHI-digested genomic DNA from a negative littermate control. The blot was hybridized with the labeled lacZ probe shown in Fig 1. The expected 3.5-kb band for the transgene is denoted by the arrow. Hybridization with an endogenous mouse gene showed the expected band for lanes 5, 6, and 8 (data not shown).
Collecting Bone Marrow and Tissues
The transgenic mice and normal littermates were killed. Tissues, including brain, lung, heart, liver, spleen, kidney, and adrenal, were immediately fixed in 4% (v/v) paraformaldehyde and 0.2% (v/v) glutaraldehyde in phosphate-buffered saline (PBS) at 4°C for 4 hours. Tissues were dehydrated, embedded in paraffin, and 5-μm sections were made. Femoral and humeral bones were fixed in 4% (v/v) paraformaldehyde and decalcified in Decal F (Stephens Scientific, Riverdale, NJ) for 30 minutes. Serial 5-μm sections of fixed, decalcified bone marrow were prepared for subsequent labeling with β-gal and antiplatelet antibodies as described below.
Immunohistochemistry
Transgene detection.
Deparaffinized 5-μm sections were hydrated with deionized water and briefly air dried. After antigen retrieval the tissues were blocked with CAS block (Zymed, San Francisco, CA) and incubated with the primary antibody, a rabbit anti–Escherichia coliβ-gal (1:10; 5′-3′, Inc, Boulder, CO), in a humidity chamber overnight at room temperature. Normal goat serum (10% v/v) was used in place of the primary antibody as a negative control. The slides were washed three times in PBS and treated with gold conjugated IgG (H + L) for the rabbit primary antibody (Zymed) for 1 hour at room temperature. The slides were rinsed in deionized water and treated with silver enhancement according to the manufacturer's protocol (Zymed). Slides were rinsed with deionized water and lightly counterstained with hematoxylin and mounted in Advantage aqueous medium (Inovex, Richmond, CA).
In some experiments paraffin sections of tissues and bone marrow on slides were stained with immunogold-silver stain (IGSS). Slides were blocked with 1% (w/v) bovine serum albumin (BSA)/0.3%(v/v) Triton X-100 for 30 minutes and incubated with primary antibody, a rabbit anti–E coli β-gal (1:100; 5′-3′, Inc) in a humidity chamber for 1 hour at room temperature. Normal rabbit serum was used in place of the primary antibody as a negative control. The slides were washed three times with PBS before incubation with secondary antibody, gold-labeled goat anti-rabbit IgG (1:30; Amersham), in the humidity chamber for 1 hour at room temperature. Slides were subsequently treated with silver enhancement (as per manufacturer's protocol; Amersham) after washing three times with PBS. Finally they were lightly counterstained with hematoxylin and visualized by light microscopy.
Megakaryocyte detection.
Deparaffinized sections were rehydrated with deionized water and briefly air dried. After antigen retrieval the tissues were blocked with Peroxo block (Zymed) and CAS block. The slides were incubated with 4A5, a rat anti-mouse platelet antibody22 in a humidity chamber overnight at room temperature. The 4A5 antibody was purified from the supernatant of a culture of 4A5 hybridoma cells (generously provided by Dr S. Burstein22) using a MAb Trap G II kit (Pharmicia, Piscataway, NJ) according to the manufacturer's instructions. Normal goat serum (10%) was used in place of the primary antibody as a negative control. Slides were rinsed three times in PBS and incubated with the second antibody, a goat anti-rat IgG (1:100; Jackson Immuno Labs, West Grove, PA) for 1 hour at room temperature. Slides were rinsed in PBS and developed in horseradish peroxidase (HRP) streptavidin and aminoethyl carbazole (AEC) chromogen (Zymed). Slides were rinsed with deionized water and lightly counterstained with hematoxylin and mounted in Advantage aqueous medium.
As a positive control for the function of the reporter construct and the immunohistochemistry methods, pCMV/lacZ and −245hPF4/lacZ reporter constructs were transiently transfected into TPA-stimulated HEL cells. Mice expressing β-gal in the liver under the control of a viral promoter (kindly provided by Dr J. Wilson, Institute for Human Gene Therapy, University of Pennsylvania) served as a positive control for tissue analysis.
RNA Isolation and Northern Blot Analysis
Total RNA was isolated from bone marrow and spleen using RNA STAT-60 (Tel-Test, Inc, Friendswood, TX) according to the manufacturer's protocol. Thirty micrograms of total RNA was fractionated on a 1% (v/v) formaldehyde/agarose gel and then transferred by capillary action to a Zetabind filter (Cuno, Inc). The filter was prehybridized at 68°C for 1 hour, hybridized at 68°C overnight with32P-labeled probe made from the 3.5-kb BamHI fragment of the β-gal gene, and washed in 0.5× SSC/0.1% (w/v) SDS and in 0.2× SSC/0.1%(w/v) SDS at 68°C. The filter was exposed to film at −70°C with an intensifying screen. To ensure the loading of equivalent amounts of RNA per lane and the quality of the all RNA samples, the same filter was stripped and rehybridized with a 32P-labeled probe of mouse endogenous β-actin cDNA.21
RT-PCR
RT of known input amounts of RNA from bone marrow was performed with random hexamers and SuperScript II reverse transcriptase (Life Technologies, Gaithersburg, MD). Then PCR of β-gal cDNA was performed with primers (β5, sense, GAGGAACTGAAAAACCAGAAAG-3′; β2, antisense, 5′-TTAACAGGCTCT TTCGATCCC-3′) designed to span a potentially spliceable intron (Fig 1). We observed with bone marrow RNA that this intron was not spliced out. Additional studies, including use of primers for mouse endogenous genes, which spanned small introns, verified that RT-PCR was working appropriately on RNA and not on contaminating DNA. The control primers for murine glyceraldehyde phosphate dehydrogenase (GAPDH) were the same as those reported by Guy et al.13 RT-PCR was performed for 25 cycles (94°C × 45 seconds, 60°C × 30 seconds, 72°C × 60 seconds) using 50, 100, 200, 300, or 1,000 ng of input RNA. The PCR products were analyzed by 2% agarose gel electrophoresis and visualized by ethidium bromide staining.
RESULTS
Generation of Transgenic Mice Carrying −245 bp hPF4/lacZ Gene
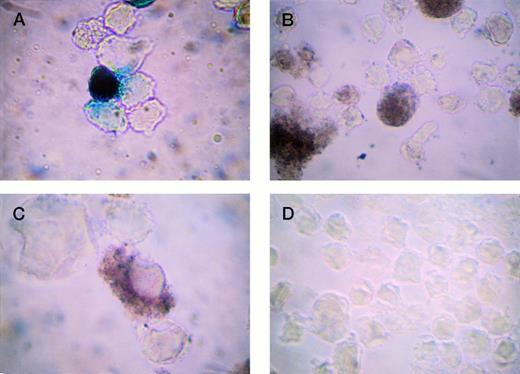
Based on our prior work in which we used a luciferase reporter gene,16 we created two constructs (−2 kb and −245 bp hPF4) linked to the lacZ gene. These were then transiently transfected into TPA-stimulated HEL cells. β-gal expression was analyzed in one of three ways: X-gal staining, enzymatic assay, and immunohistochemistry. The results verified that (1) both hPF4 promoters drove expression of the lacZ reporter gene; (2) the −245-bp hPF4 promoter was more active than the 2 kb promoter; and (3) that immunohistochemistry provided a valid alternative to X-gal staining and enzymatic assays (Fig 3).
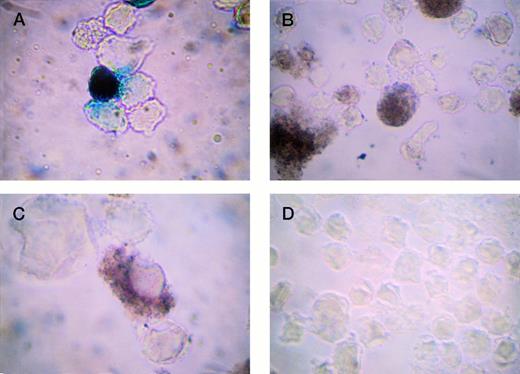
Detection of β-gal expression in TPA-stimulated HEL cells. LacZ was driven by cytomegalovirus (CMV) (A and B) or −245 bp hPF4 promoters (C and D). (A) X-gal staining (positive cells blue); (B) IGSS staining (positive cells brown); (C) IGSS staining; and (D) negative control, IGSS with no primary antibody (no brown signal). Original magnification × 500.
Detection of β-gal expression in TPA-stimulated HEL cells. LacZ was driven by cytomegalovirus (CMV) (A and B) or −245 bp hPF4 promoters (C and D). (A) X-gal staining (positive cells blue); (B) IGSS staining (positive cells brown); (C) IGSS staining; and (D) negative control, IGSS with no primary antibody (no brown signal). Original magnification × 500.
The −245-bp hPF4 promoter linked to the lacZ gene was chosen for use as the transgene construct. Two of 20 founder mice (F0no. 3 and F0 no. 16) contained the transgene as assessed by PCR and Southern blot analysis. These two founders were bred with normal mice (B6SJLF1) to produce offspring, which were screened by PCR with transgene specific primers (Fig 1). A second set of primers for the endogenous WAP gene was used as a PCR control for the DNA quality. Positive transgenic mice exhibited two bands, which were 417 bp for the transgene and 216 bp for the endogenous gene on agarose gel (data not shown). Negative transgenic mice exhibited only the 216-bp band. Both founders showed germline transmission of the hPF4 transgene. Selected PCR-positive offspring were confirmed by Southern blot, which was also used to assess transgene copy number (Fig 2). F0 no. 3 and F0 no. 16 contained 5 and 25 copies of the transgene, respectively.
The −245-bp hPF4 Promoter Is Sufficient to Direct Tissue-Specific Expression of a β-gal Reporter Gene in Transgenic Mice
Nine tissues (blood, bone marrow, brain, heart, lung, liver, spleen, kidney, and adrenal) were obtained and analyzed from positive and negative offspring of the two founders. Expression was analyzed at the protein and mRNA levels. Immunohistochemistry was performed to detect β-gal protein expression in these tissues. An anti–E coliβ-gal polyclonal antibody was used to compare stained positive and negative controls by immunohistochemistry (Figs 4 and5).
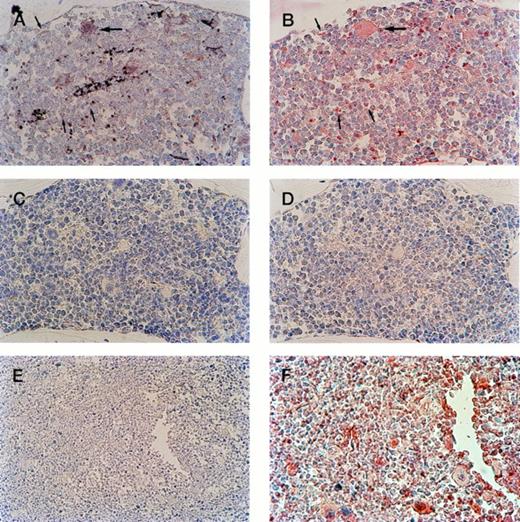
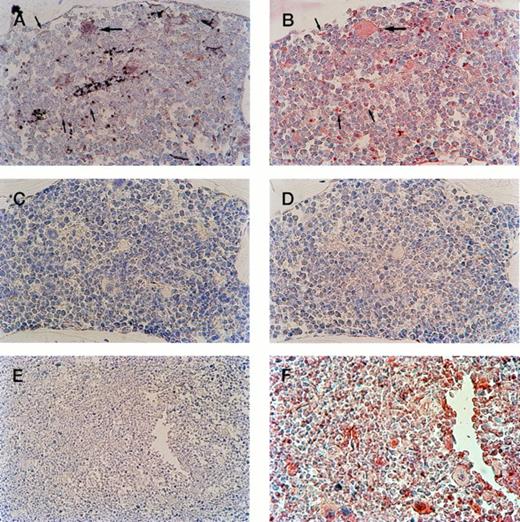
Serial sections of bone marrow from transgenic progeny of F0 no. 16 show megakaryocyte-specific β-gal expression. (A) In the bone marrow of transgenic mice, positive megakaryocyte staining by IGSS (brown color) is shown. Hematoxylin counterstaining provides the nuclear blue staining. (B) 4A5-labeled (red stained) bone marrow shows the location of immature and mature megakaryocytes. Comparison of (A) and (B) documents transgene expression in all identifiable immature (small arrows) and mature (large arrows) megakaryocytes. (C) IGSS negative control; IGSS staining of transgenic mouse without primary antibody shows absence of brown color. (D) 4A5 negative control; 4A5 staining of transgenic mouse without primary antibody shows absence of red color. (Original magnification × 400). (E, F) Nontransgenic negative control; IGSS (E, wider field under lower magnification) and 4A5 (F) staining of the bone marrow of a wild-type mouse under identical conditions as (A) and (B), respectively. IGSS is negative, whereas 4A5 is positive as expected.
Serial sections of bone marrow from transgenic progeny of F0 no. 16 show megakaryocyte-specific β-gal expression. (A) In the bone marrow of transgenic mice, positive megakaryocyte staining by IGSS (brown color) is shown. Hematoxylin counterstaining provides the nuclear blue staining. (B) 4A5-labeled (red stained) bone marrow shows the location of immature and mature megakaryocytes. Comparison of (A) and (B) documents transgene expression in all identifiable immature (small arrows) and mature (large arrows) megakaryocytes. (C) IGSS negative control; IGSS staining of transgenic mouse without primary antibody shows absence of brown color. (D) 4A5 negative control; 4A5 staining of transgenic mouse without primary antibody shows absence of red color. (Original magnification × 400). (E, F) Nontransgenic negative control; IGSS (E, wider field under lower magnification) and 4A5 (F) staining of the bone marrow of a wild-type mouse under identical conditions as (A) and (B), respectively. IGSS is negative, whereas 4A5 is positive as expected.
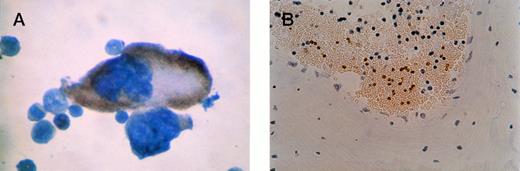
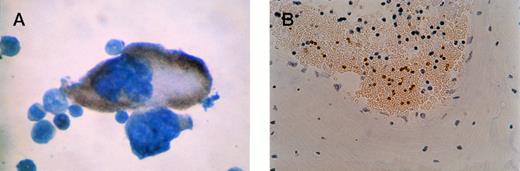
β-gal staining of megakaryocyte and platelets. (A) Close-up of positive megakaryocyte. (B) Platelets seen in a cross-section of a blood vessel from bone marrow of an F0no. 16 mouse. The unstained cells are red blood cells (anucleuate) and white blood cells (blue nuclei). Original magnification × 400.
β-gal staining of megakaryocyte and platelets. (A) Close-up of positive megakaryocyte. (B) Platelets seen in a cross-section of a blood vessel from bone marrow of an F0no. 16 mouse. The unstained cells are red blood cells (anucleuate) and white blood cells (blue nuclei). Original magnification × 400.
Positive yellow-brown cells were visualized in the bone marrow from transgene-positive offspring of F0 no. 16. Counterstaining was performed to identify cell morphology after IGSS and 4A5 staining. All morphologically identifiable megakaryocytes in the bone marrow from positive offspring of F0 no. 16 stained positively with IGSS (Figs 4A and 5A). No other tissues were positive. In particular, no staining was noted in the adrenal gland in the medulla or cortex. No cells in any tissue of transgene-positive offspring of F0no. 3 or in any transgene-negative littermates were positive for staining with IGSS. Figure 5B is a cross-section of a blood vessel from the bone marrow from an F0 no. 16 mouse showing β-gal–stained platelets surrounded by unstained red blood cells. There were no β-gal–stained platelets observed in any F0no. 3 or nontransgenic offspring (data not shown).
Cells labeled with the 4A5 antibody appear red in the bone marrow sections and represent cells of the megakaryocytic lineage (Fig 4B and F). All morphologically distinct megakaryocytes, as well as smaller megakaryocytes, in both the transgene-positive and wild-type specimens are labeled with 4A5. Serial sections of bone marrow show that 4A5-lableled cells that are morphologically distinct megakaryocytes (Fig 4A and B; large arrows) are also stained with IGSS. The smaller 4A5-labeled (red stained) cells that also stain with β-gal document transgene expression in less mature megakaryocytes (Fig 4A and B; small arrows).
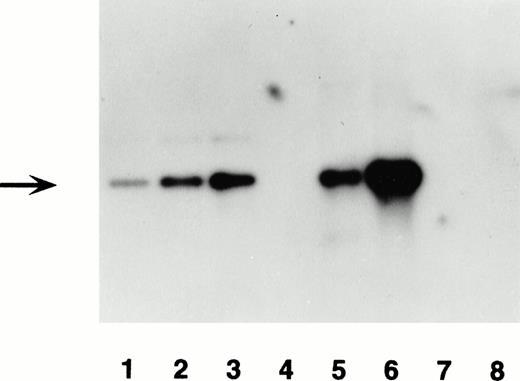
Expression of the transgene in transgenic mice was detected at the mRNA level as well as at the protein level. Total RNA was isolated from bone marrow and spleen from positive and negative offspring of the two founders. A 32P-labeled 3.5-kb fragment of the lacZ gene (Fig 1) was used as a probe to detect β-gal mRNA expression in bone marrow and spleen on Northern blots. As an internal control, a mouse endogenous β-actin cDNA probe was used to rehybridize the same filter after the 3.5-kb β-gal probe was stripped from the filter. All RNAs were detectable after rehybridization with the β-actin cDNA probe, indicating that high quality RNA was loaded in each lane in amounts that were approximately the same (Fig 6). Expression of β-gal mRNA was detected clearly in the bone marrow from offspring of F0 no. 16 (Fig 6). Expression was undetectable in the spleen of mice from either line and in the bone marrow of the F0 no. 3 line, in agreement with the protein analysis by immunohistochemistry.
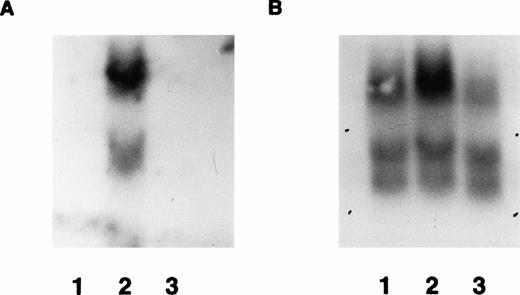
Demonstration of mRNA expression in transgenic mice by Northern blot. (A) RNA from wild-type mouse bone marrow (lane 1) and transgenic line F0 no. 16 bone marrow (lane 2) and spleen (lane 3) probed with radiolabeled β-gal probe. The major expected 5-kb upper band is shown, along with a lower cross-hybridizing band of uncertain origin. In (B), the same blot is shown as in (A), after it was stripped and reprobed with control mouse β-actin cDNA probe. Several bands hybridizing to the β-actin probe are present in each lane, allowing relative quantification of the amounts of hybridizable RNA in each lane.
Demonstration of mRNA expression in transgenic mice by Northern blot. (A) RNA from wild-type mouse bone marrow (lane 1) and transgenic line F0 no. 16 bone marrow (lane 2) and spleen (lane 3) probed with radiolabeled β-gal probe. The major expected 5-kb upper band is shown, along with a lower cross-hybridizing band of uncertain origin. In (B), the same blot is shown as in (A), after it was stripped and reprobed with control mouse β-actin cDNA probe. Several bands hybridizing to the β-actin probe are present in each lane, allowing relative quantification of the amounts of hybridizable RNA in each lane.
Level of Expression With RT-PCR
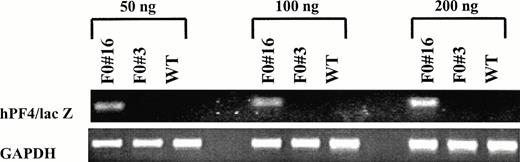
Semiquantitative RT-PCR was used to compare the level of expression of the human−245 bp PF4 promoter with that of the rat 1.1-kb rat PF4 promoter, described by Guy et al.13 F0 no. 16, F0 no. 3, and wild-type mice were examined for expression of hPF4/lacZ mRNA and GAPDH mRNA (Fig 7). The amplified products for the hPF4/lacZ and GAPDH primers are 164 bp and 352 bp, respectively. GAPDH primers were used to show that similar amounts of RNA were added and that the efficiency of amplification was similar among the samples.13 We detected expression of β-gal mRNA in the bone marrow from the F0 no. 16 line with 50 ng of input RNA at 25 cycles. No β-gal mRNA signal was detected in the F0 no. 3 line or the wild-type mice, even at 20-fold increases in the amount of input RNA (data not shown). These results confirm and extend the Northern blot analysis. The steady-state mRNA levels of β-gal driven by the −245 hPF4 promoter and that reported for E2F-1 driven by the −1.1-kb rat PF4 promoter are equivalent.
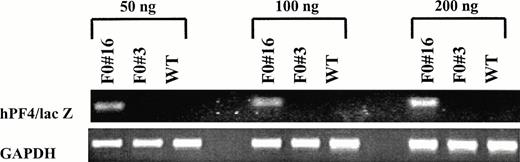
Semiquantitative RT-PCR analysis of −245 hPF4/lacZ expression in bone marrow RNA. Increasing amounts of total input RNA (50, 100, and 200 ng) from bone marrow of transgenic (F0no. 16 and F0 no. 3) and wild-type (WT) mice were reverse transcribed and amplified for 25 cycles. The PCR products for the −245 hPF4/lacZ (164 bp) and GAPDH primers (392 bp13) were visualized by ethidium bromide staining with 2% agarose gels.
Semiquantitative RT-PCR analysis of −245 hPF4/lacZ expression in bone marrow RNA. Increasing amounts of total input RNA (50, 100, and 200 ng) from bone marrow of transgenic (F0no. 16 and F0 no. 3) and wild-type (WT) mice were reverse transcribed and amplified for 25 cycles. The PCR products for the −245 hPF4/lacZ (164 bp) and GAPDH primers (392 bp13) were visualized by ethidium bromide staining with 2% agarose gels.
DISCUSSION
We showed expression of hPF4/lacZ reporter gene constructs by transient transfection in vitro of TPA-stimulated HEL cells in a way similar to our previous report for hPF4/luciferase reporter gene constructs.16 Using the −245-bp hPF4/lacZ DNA, we created and characterized new transgenic mouse lines. We showed that the −245-bp hPF4 promoter is sufficient for driving expression of a linked lacZ reporter gene in megakaryocytes in the bone marrow of transgenic mice.
Expression driven by the −245-bp hPF4 promoter was megakaryocyte specific, as no other tissue examined showed transgene expression. This −245-bp hPF4 5′-flanking region includes positivecis-acting megakaryocyte-specific elements, in agreement with our previous studies in vitro.16 A critical region from −107 to −239 includes a poly-T stretch and a putative GATA-binding site. GATA sites are seen as functional elements in gene regulation in erythroid, megakaryocyte, and mast cell lineages.23-27 The identity of transcription factors in the megakaryocyte binding to the critical region, including the poly-T sequence, is under investigation. In combination, these sites may play a central role in megakaryocyte-specific expression.
In addition to PF4, a number of genes expressed in the megakaryocyte/platelet lineage have had their promoters characterized in vitro, including GPIIb,26, 28-30 GPIbα,31GPIbβ,32 FcγRIIA,33 and PECAM,34 among others. Several classes of sites for interaction with DNA-binding proteins, notably GATA and ets, have been identified. Elucidation of the factors regulating differentiation is important, as abnormal differentiation of megakaryocytic cells may be related to both thrombocytopenic disorders and megakaryocytic leukemia.12-14,33 For platelet genes studied in transgenic mice (rPF4, hGPIIb, hGPIbα, and hFcγRIIA11-14, 31, 35-37 [and unpublished observations, December 1996]), to date no construct has been studied for its ability to provide copy-number–dependent expression in transgene-positive mice independent of the position of chromosomal integration. In the β-globin and other gene clusters, the sequences that confer position-independent expression in vivo are found in locus control regions outside of the immediate gene promoter.38 39 In this study, no reporter gene expression was detectable in one founder line that had five copies of the transgene. The sensitivity of our RNA analysis was such that, despite the lower copy number in that line versus the line with megakaryocyte-specific expression, we would have seen expression had it occurred. This suggests that −245-bp hPF4 promoter-driven expression of the reporter gene in vivo may depend on the position of chromosomal integration, but analysis of a large number of transgenic lines is necessary to show conclusively that expression is position dependent.
Both protein and RNA analyses show that −245-bp hPF4 directs β-gal reporter gene expression in the megakaryocytes in the bone marrow but not in spleen. A study of rat PF4 gene regulation in transgenic mice found that the transgene was expressed in all ploidy classes of megakaryocytes from bone marrow, but at extremely low frequency in the spleen.11 Further studies are needed to identify reasons for the difference in transgene expression in the megakaryocytes in bone marrow versus spleen. It is known that exogenous factors can induce functional, morphological, and biochemical changes in megakaryocytic cell lines.11, 17, 40-42 Soluble extracellular factors and/or cell-cell contacts in the microenvironment of the spleen may affect the properties of megakaryocytes there such that they differ from those developing in bone marrow.
X-gal staining is a common method to detect β-gal in tissues and was used in studies with the 1.1-kb rat PF4 promoter.11 In our study, X-gal staining of megakaryocytes from transgenic line no. 16 was seen, but high background was observed. When using IGSS immunohistochemistry to stain tissues we observed very clear, robust positive cells in the bone marrow of transgenic mice. The use of the anti–E coli β-gal antibody to detect transgene expression results in high specificity and distinguishes transgene-encoded β-gal from endogenous β-gal. With X-gal staining, we and others have found endogenous β-gal activity difficult to block completely, especially when measuring low levels of E coli β-gal activity.43 Our study suggests that immunohistochemistry is more specific and sensitive than X-gal staining, and others have had similar experience.44 We did not see expression of β-gal in the adrenal gland, in contrast to the observation with the rPF4 promoter.11 This may reflect differences in the human versus the rat promoter and/or differences in the sensitivity of the detection methods.
There are a number of potential reasons why β-gal expression in our mice could not be detected above background using X-gal staining. The −245-bp hPF4 promoter may drive lower level expression than the 1.1-kb rat PF4 promoter in the mouse. To investigate this possibility, we compared our transgene RNA level of expression with the report in which transgene E2F-1 was driven by the 1.1-kb rPF4 promoter.13 We found an equivalent level of expression in our F0 no. 16 and their highest expressing line in that the products were detected with 50 ng of input RNA and 25 cycles of RT-PCR. Formal comparison of RNA expression levels on a per-gene-copy basis was not possible because in the rPF4 work no correlation between expression and copy number was seen (M.O. Robinson, personal communication, June 1997). Within the limits of current technology, we cannot with certainty invoke chromosomal integration site effects, promoter strength effects, or other factors as the cause of any differences in the level of protein expression as manifested by different X-gal staining properties of megakaryocytes in the hPF4 and rPF4 transgenic mouse lines.
Our results show that the −245-bp hPF4 construct contains elements that drive megakaryocyte-specific expression in vivo. It may be a good model to use for targeting gene expression to megakaryocytes. Some viral vectors used for human gene therapy, such as adeno-associated virus, have limitations on the size of DNA that can be accommodated. Thus, there is a real utility in defining short promoter fragments that drive megakaryocyte-specific expression in vivo, because more room is left for the structural gene of interest (eg, those for coagulation factors, etc). Work is in progress in our laboratory on the identification of sequences, which may confer high-level and position-independent expression in the megakaryocyte/platelet in vivo.
ACKNOWLEDGMENT
We thank Dr Mortimer Poncz for sharing reagents and critical input. We thank Drs Lin Hu and Toshio Asakura of the Transgenic Mouse Core Facility of the Children's Hospital of Philadelphia. Carol Barone and the staff of the Research Histology Core at the duPont Hospital for Children provided invaluable assistance. We thank Drs Diana Cassel and Chris Stoeckert for their advice and encouragement, and Scott M. Taylor, Chris Chien, and Marybeth Helfrich for technical assistance.
Supported by a grant from the Public Health Service, NIH RO1 DK16691.
Address reprint requests to Steven E. McKenzie, MD, PhD, duPont Hospital for Children, 1600 Rockland Rd, Wilmington, DE 19899.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.