Abstract
GAS6 is a ligand for the tyrosine kinase receptors Rse, Axl, and Mer, but its function is poorly understood. Previous studies reported that both GAS6 and Axl are expressed by vascular endothelial cells (EC), which play a key role in leukocyte extravasation into tissues during inflammation through adhesive interactions with these cells. The aim of this work was to evaluate the GAS6 effect on the adhesive function of EC. Treatment of EC with GAS6 significantly inhibited adhesion of polymorphonuclear cells (PMN) induced by phorbol 12-myristate 13-acetate (PMA), platelet-activating factor (PAF), thrombin, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), but not that induced by FMLP and IL-8. GAS6 did not affect adhesion to resting EC. Titration experiments showed that high concentrations of GAS6 were needed to inhibit PMN adhesion and that inhibition was dose-dependent at the concentration range of 0.1 to 1 μg/mL. One possibility was that high concentrations were needed to overwhelm the effect of endogenous GAS6 produced by EC. In line with this possibility, treatment of resting EC with soluble Axl significantly potentiated PMN adhesion. Analysis of localization of GAS6 by confocal microscopy and cytofluorimetric analysis showed that it is concentrated along the plasma membrane in resting EC and treatment with PAF induces depletion and/or redistribution of the molecule. These data suggest that GAS6 functions as a physiologic antiinflammatory agent produced by resting EC and depleted when proinflammatory stimuli turn on the proadhesive machinery of EC.
THE EXTENSIVE sequence similarity shared by tyrosine kinase domains of tyrosine kinase receptors (RTK) has allowed homology-based cloning of a large number of RTK-like proteins.1 Three of these proteins, designated Rse (also known as Sky, brt, tif, and tyro 3),2-6 Axl (also known as UFO, Ark, or Tyro 7),7-10 and Mer11-13 display similar extracytoplasmic domains, consisting of two Ig-like domains followed by two fibronectin type III repeats7 and share the ability to bind GAS6,14-18 a protein upregulated in response to growth arrest in murine and human fibroblast cell lines.19,20 Sequence analysis has shown that human GAS6 has 44% identity with protein S,20 a natural anticoagulant serving as cofactor for protein C in inactivation of factors V and VIIIa.21 It was initially suggested that GAS6 functions as a negative regulator of cell proliferation. Subsequent observation that hyperexpression of its receptors Axl and Rse7,22-24 have transforming capacity, however, indicated that it acts as a growth factor. This function has been documented in rat vascular smooth muscle cells,25 NIH 3T3 fibroblasts,15,26 and Schwann cells,27 although GAS6's mitogenic effect was always weak or limited to potentiation of other activating stimuli. Moreover, it has been reported that both GAS6 and Axl are expressed by bone marrow cells,28,29 but GAS6 does not function as either growth or survival factor for bone marrow hematopoietic progenitors and stromal cells.28 Despite the many efforts to characterize it,10,15,25-27,30 31 GAS6's biological activity is still poorly understood.
Both GAS6 and Axl are highly expressed by vascular endothelial cells (EC)20 (and unpublished data), which play a key role in leukocyte release from the bone marrow and extravasation into tissues during inflammation. These processes depend on activity of specific adhesion receptors by leukocytes and EC. Activity of adhesion receptors can be regulated by modulating their expression or their intrinsic adhesive function.32-34 Leukocytes maintain several of these receptors in an inactive state during transit in the blood stream and extracellular fluids and activate them only when proper specific stimuli are delivered. An important role in this signaling is played by molecules belonging to the chemoattractant family, which comprises classic chemoattractants, such as leukotriene B4, platelet-activating factor (PAF), N-formyl peptides and C5a, and chemokines, which are cytokines with chemoattractant capacity and a high level of sequence homology.35 36 Chemoattractants bind to the surface of EC that present them to circulating leukocytes. Leukocyte interaction with chemoattractants promotes integrin adhesiveness via inside-out signaling, activates cell motility, and stimulates degranulation and respiratory burst of phagocytes. The aim of this work was to evaluate involvement of the GAS6 system in the adhesive function of vascular endothelium.
MATERIALS AND METHODS
Cells.
Polymorphonuclear cells (PMN) were isolated from citrated blood of healthy volunteers by standard dextran sedimentation followed by Ficoll-Hypaque gradient centrifugation; residual erythrocytes were removed by hypotonic lysis. The PMN were resuspended in buffered salt solution (BSS) (138 mmol/L NaCl, 2.7 mmol/L KCl, 8.1 mmol/L Na2HPO4, 1.5 mmol/L KH2PO4, 1 mmol/L MgCl2, 1 mmol/L CaCl2, pH 7.4) supplemented with 1 mg/mL glucose and 1 mg/mL human serum albumin (HSA). Purity of the final cell suspension averaged 98%. PMN viability (as assessed by the trypan blue exclusion test) was always greater than 95%.
EC were isolated from human umbilical vein within 4 hours of delivery by trypsin (Difco Laboratories Inc, Detroit, MI) treatment (1%) and cultured in M199 (endotoxin tested, Sigma Chemical Co, St Louis, MO) with the addition of 20% bovine calf serum (BCS, endotoxin tested, Hyclone Laboratories Inc, Logan, UT) and 10 ng/mL human fibroblast growth factor (FGF, Santa Cruz Biotechnology Inc, Santa Cruz, CA). Purity of the EC preparation was evaluated by morphologic criteria and positive immunofluorescence for factor VIII. Contamination with blood leukocytes was assessed by immunofluorescence with an anti-CD45 antibody. EC were grown to confluence in flasks, trypsinized, and grown to confluence in 24-well plates. EC proliferation assay was performed by incubating 104 cells/well in 96-well plates in M199 containing 0.5% bovine serum albumin (BSA) in the presence or absence of recombinant human GAS6 (rhGAS6) (400 ng/mL), FGF (50 and 100 ng/mL), 10% fetal calf serum (FCS), and rhGAS6 (400 ng/mL) plus FGF (50 ng/mL) for 24 hours. Proliferation was evaluated upon growth factor stimulation by incubating cells for 4 hours in the presence of 1 μCi/well of 3HTdR (Dupont NEN, Cologno Monzese, Italy). Incorporated radioactivity was measured by scintillation counting of harvested cells and expressed as counts per minute (CPM). RhGAS6 was obtained from Amgen (Amgen, Thousand Oaks, CA) and produced as described.15
M07e, a growth factor-dependent cell line previously described,37,38 was grown in Iscove modified Dulbecco's medium (IMDM) supplemented with 5% FCS plus rhIL-3 (10 ng/mL) (Sandoz, Basel, Switzerland). Transfection of the Axl gene was performed by electroporation (Bio-Rad, Hercules, CA): 1 × 106 M07e cells were electroporated (250 mF and 400 V) with 20 μg of XhoI-linearized pLXSN-Axl DNA (generous gift of C. Schneider, Dipartimento di Scienze e Tecnologie Biomediche, University of Udine, Italy) and grown in IMDM plus 5% FCS, plus rhIL-3 (10 ng/mL) and G418 (400 μg/mL) (Sigma). Selection of the G418-resistant M07e cells expressing Axl (M07e.Axl) was performed by indirect immunofluorescence and fluorescence-activated cell sorting (FACS) analysis (FACScan, Becton Dickinson, Milano, Italy) using affinity-purified anti-Axl serum15 and fluorescein isothiocyanate (FITC)-conjugated goat antirabbit Ig serum (Dako, Milano, Italy).
Monoclonal antibody (MoAb) production.
Hydrophilicity of the amino acid sequence of human GAS6 was analyzed by the Pharmacia PC-Gene software (Pharmacia, Uppsala, Sweden). The oligopeptide INPRLDGCMRSW, which showed the best antigenicity index, was synthesized and coupled with keyhole limpet hemacyanin (KLH) (Sigma) according to Reichlin et al.39 Mice were immunized with the peptide-KLH complex in complete Freund's adjuvant and boosted three times with the same amount of antigen in incomplete Freund's adjuvant. Spleen cells were fused with the Ag8.28 myeloma cell line by polyethylene glycol (PEG), and hybridomas were cultured in hypoxanthine-aminopterine-thymide (HAT; Sigma)–supplemented RPMI (Sigma) medium and cloned by limiting dilution. Hybridomas 3C12 (IgM) and 5H7 (IgG1) producing MoAb to GAS6 were selected by enzyme-linked immunosorbent assay (ELISA) using rhGAS6 as the antigen. The MoAb specificity was confirmed by immunoprecipitation using 125I-labeled rhGAS6 (data not shown).
Adhesion assay.
EC were grown to confluence in 24-well plates, washed, and rested for 2 days in M199 plus 10% BCS without FGF. PMN (106 cells/mL) were labeled with fluoresceine diacetate (5 μg/mL) (Sigma) for 30 minutes at 37°C, washed with BSS, and plated at 106cells/well in a final volume of 0.25 mL BSS on EC, which had been treated or not with thrombin (2 U/mL) (Sigma), phorbol 12-myristate 13-acetate (PMA) (10−7 mol/L) (Sigma), 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (PAF) (10−7 mol/L) (Bachem, Bubendorf, Switzerland), IL-8 (10−8 mol/L) (Bachem), or FMLP (10-6mol/L) (Sigma) for 10 minutes and with IL-1β (10 ng/mL), tumor necrosis factor-α (TNF-α) (10 ng/mL) for 1 hour. After incubation, unbound PMN were removed by washing three times with 1 mL BSS, and the center of each well was analyzed at 100× magnification by fluorescence image analysis using a Leitz Diavert (Leitz, Germany) inverted microscope with DM 510G-2A filter, a Ikegami ICD-42E,CCD monochromatic video camera (Ikegami, Tokyo, Japan), a Matrox IP8/AT videographics digitizer board (Matrox Electronics System, Dorval, Canada), and two Packard Bell VL MF, 1024′800 0.28 mm dot pitch (Packard Bell, Downers Grove, IL). Cells were counted by the Image Pro Plus Software for microimaging (Media Cybernetics, version 1.3 for Windows 95; Silver Springs, MD). Single experimental points were assayed in quadruplicate and standard deviation (SD) of the four replicates was always lower than 10%. Data are presented as relative adhesion %, calculated as follows:
where control adhesion was measured in the absence of any treatment of EC. In preliminary experiments, this assay was found to produce results in line with those obtained by a standard adhesion assay performed with51Cr-labeled PMN (data not shown).
Homotypic aggregation assay.
M07e and M07e.Axl cells were labelled with ethidium bromide (100 μg/mL) (Sigma) and acridine orange (10 μg/mL) (Sigma) in IMDM containing 0.5% BSA. Cells were washed after staining and seeded at 1 × 106 cells/mL and incubated in the presence or absence of rhGAS6 (400 ng/mL) for 1 hour on a rocking shelf. They were then dropped on microscope slides, covered with coverslips, and aggregates of 20 fields were counted with a fluorescence microscope. Yellow-green (acridine orange) color aggregates of at least five living cells were counted. Aggregates of dead cells were red-stained by ethidium bromide and excluded.
Immunofluorescence analysis.
A total of 5 × 105 resting EC were incubated with anti-GAS6 MoAb at 4°C for 30 minutes, washed with phosphate-buffered saline (PBS) and incubated for 30 minutes at 4°C with a fluorescein conjugated goat antimouse antibody (Southern Biotechnology, Birmingham, AL). After washing with PBS, cells were analyzed in a FACScan (Becton Dickinson, Milano, Italy).
EC were seeded on microscope coverslips and incubated overnight in 3 cm Petri dish, washed in PBS, and incubated in M199 (Sigma) with 0.5% BSA for 2 hours. Cells were then fixed and permeabilized with paraformaldehyde 4% in PBS for 20 minutes and Triton X100 0.1% in PBS for an additional 10 minutes. They were then incubated at 37°C with the 3C12 MoAb for 30 minutes, or the control UCHLI (CD45R0) MoAb, washed with PBS, and stained at 37°C with FITC-conjugated goat antimouse serum (Southern Biotechnology) for 30 minutes. In some experiments before fixation and permeabilization, cells were stimulated for 10 minutes with 10-7 mol/L PAF. Slides were then washed with PBS, mounted on glass coverslips, and observed with a laser scanner confocal microscope (Bio-Rad MRC 600, equipped with a Nikon Diaphot inverted microscope and a Nikon Plan Apo 60/1.40 oil objective). Seven to nine focal frames were taken along the Z axis at 1-μm intervals and merged to obtain a reconstructed image.
Statistical analysis.
Results are expressed as mean ± SD. Statistical analysis was performed using the Mann-Whitney test for paired samples and statistical significance was set at P < .05.
RESULTS
In a previous report20 (and unpublished data), we found that GAS6 is highly expressed by EC. To evaluate the possibility that it functions as a growth factor for these cells, we assessed EC proliferation in vitro in the presence and absence of rhGAS6. These experiments showed that rhGAS6 does not modulate spontaneous EC proliferation and does not potentiate proliferation induced by FGF (data not shown).
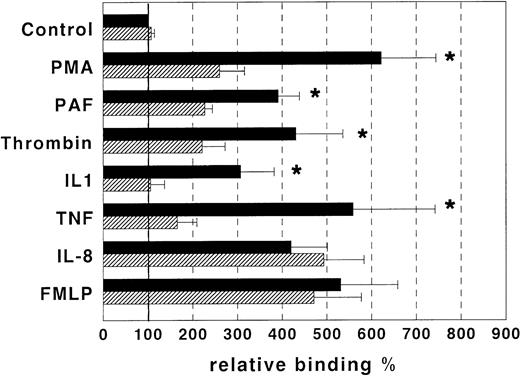
EC play a crucial role in leukocyte homing into tissues through adhesive interactions with these cells. These interactions are finely regulated by several factors, some of which are produced by EC themselves. Therefore, we investigated the possibility that GAS6 is involved in the adhesion function of EC. To confirm in a human model the observation by Bellosta et al10 that Ark may mediate homotypic/homophylic cell aggregation, we transfected Axl in M07e cells and performed aggregation assays in the presence and absence of rhGAS6. M07e.Axl cells displayed homotypic aggregation, whereas wild-type M07e cells did not. Moreover, addition of rhGAS6 to the aggregation assay completely inhibited cell aggregation (Fig1). To assess the rhGAS6 effect on EC adhesivity, we performed adhesion assays in vitro between cultured EC and peripheral blood PMN cells. EC were treated or not with rhGAS6 for 10 minutes and FITC-labeled PMN were then seeded onto EC in the presence and absence of PMA, PAF, thrombin, IL-1β, TNF-α, FMLP, or IL-8 and cell adhesion was assessed. Figure 2 shows that PMA, PAF, thrombin, IL-1β, TNF-α, FMLP, and IL-8 strikingly increased PMN adhesion to EC. RhGAS6 significantly inhibited the effect mediated by PMA, PAF, IL-1β, TNF-α, and thrombin, but not that mediated by FMLP or IL-8. By contrast, rhGAS6 did not affect PMN adhesion to untreated EC.
Aggregation assay of M07e and M07e.Axl cells. M07e and M07e.Axl cells were labeled with ethidium bromide and acridine orange and incubated in the presence or absence of rhGAS6 (400 ng/mL). Yellow-green (acridine orange) color aggregates of at least five living cells were counted in 20 microscopic fields, using a fluorescence microscope. Aggregates of dead cells were red-stained by ethidium bromide and excluded. The mean ± SD of five experiments is reported.
Aggregation assay of M07e and M07e.Axl cells. M07e and M07e.Axl cells were labeled with ethidium bromide and acridine orange and incubated in the presence or absence of rhGAS6 (400 ng/mL). Yellow-green (acridine orange) color aggregates of at least five living cells were counted in 20 microscopic fields, using a fluorescence microscope. Aggregates of dead cells were red-stained by ethidium bromide and excluded. The mean ± SD of five experiments is reported.
In vitro adhesion assays between cultured EC and peripheral blood PMN cells. EC were treated (▨) or not (▪) with GAS6 (1 μg/mL) and fluorescein-labeled PMN were then seeded on EC in the presence and absence of PMA (10−7 mol/L), PAF (10−7 mol/L), thrombin (2 U/mL), IL-8 (10−8 mol/L), IL-1β (10 ng/mL), TNF-α (10 ng/mL), FMLP (10-6 mol/L). Relative adhesion shows that PMA, PAF, thrombin, IL-8, IL-1β, TNF-α, and FMLP strikingly increased PMN adhesion to EC. GAS6 significantly inhibited the effect mediated by PMA, PAF, thrombin, IL-1β, and TNF-α, but not that mediated by IL-8 and FMLP. By contrast, GAS6 did not affect PMN adhesion to untreated EC. Results are expressed as relative adhesion percent and represent the mean ± SD from five to eight experiments. Asterisks mark values that are significantly different from the respective control (P < .05) and 100% relative adhesion (bold horizontal line) corresponds to an absolute adhesion of 13 ± 3 cells/microscope field.
In vitro adhesion assays between cultured EC and peripheral blood PMN cells. EC were treated (▨) or not (▪) with GAS6 (1 μg/mL) and fluorescein-labeled PMN were then seeded on EC in the presence and absence of PMA (10−7 mol/L), PAF (10−7 mol/L), thrombin (2 U/mL), IL-8 (10−8 mol/L), IL-1β (10 ng/mL), TNF-α (10 ng/mL), FMLP (10-6 mol/L). Relative adhesion shows that PMA, PAF, thrombin, IL-8, IL-1β, TNF-α, and FMLP strikingly increased PMN adhesion to EC. GAS6 significantly inhibited the effect mediated by PMA, PAF, thrombin, IL-1β, and TNF-α, but not that mediated by IL-8 and FMLP. By contrast, GAS6 did not affect PMN adhesion to untreated EC. Results are expressed as relative adhesion percent and represent the mean ± SD from five to eight experiments. Asterisks mark values that are significantly different from the respective control (P < .05) and 100% relative adhesion (bold horizontal line) corresponds to an absolute adhesion of 13 ± 3 cells/microscope field.
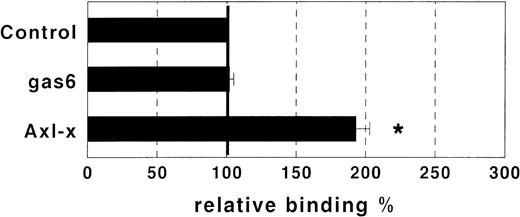
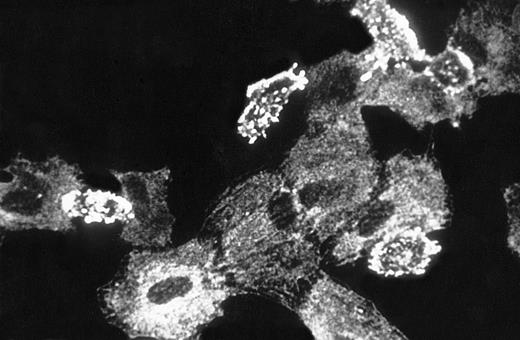
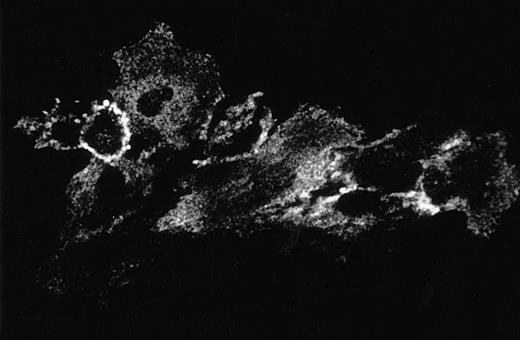
The observation that GAS6 did not inhibit adhesion driven by IL-8 and FMLP, which exert their effect by activating the PMN adhesive machinery, suggested that GAS6 acts on EC and not on PMN. To assess this possibility, we evaluated the GAS6 effect on PMN adhesion to FCS-coated culture wells. In this experimental system, PAF potentiated PMN adhesion, but GAS6 did not inhibit the PAF-driven adhesion (Fig 3). Moreover, to confirm the action of GAS6 on EC, we pretreated EC with GAS6 for 10 minutes and with PAF for an additional 10 minutes. EC were then washed three times and PMN were seeded. These experiments showed that the inhibitory effect of GAS6 on PAF-driven PMN adhesion to EC was unmodified by washing (data not shown). Titration experiments showed that high concentrations of rhGAS6 were needed to inhibit PMN adhesion to PAF-treated EC, and that inhibition was dose-dependent in the concentration range between 0.1 and 1 μg/mL (Fig 4). One possibility was that high concentrations of rhGAS6 were needed to overwhelm the effect of endogenous GAS6 produced by EC themselves. To assess this possibility, we evaluated the effect of a soluble ligand of GAS6 on PMN adhesion to EC. EC were treated or not for 10 minutes with a soluble form of Axl (Axl-x),15 and PMN adhesion was then assessed. Treatment of resting EC with Axl-x significantly potentiated PMN adhesion (Fig 5). These data suggested that GAS6 endogenously produced by resting EC functions as an antiadhesive molecule. We also investigated if EC activation by proadhesive factors modulates GAS6 expression by these cells. To assess this hypothesis, EC were treated or not with PAF, fixed, permeabilized, stained with the 3C12 MoAb, and examined by confocal microscopy. The fluorescence distribution pattern was rather granular, mainly organized in filament-like structures throughout the cytoplasm (Fig 6). In a proportion of EC, fluorescence localization was markedly different clustered in small patches close to the cell surface (Figs 6 and7). In PAF-treated cells, the fluorescence intensity was markedly lower and displayed a diffuse, granular pattern only with no evidence of surface patches (Fig 6). Moreover, analysis of unpermeabilized cells by immunofluorescence and FACS analysis showed that GAS6 is expressed on the surface of a variable proportion of resting EC (20% to 50%) and that treatment with PAF decreased surface expression of the molecule (data not shown).
In vitro adhesion assays between peripheral blood PMN cells and EC or FCS-coated plastic wells. EC and FCS-coated plastic wells were treated (▨) or not (▪) with GAS6 (1 μg/mL) for 10 minutes and fluorescein-labeled PMN were then seeded on the wells in the presence and absence of PAF (10−7mol/L). Cell adhesion was assessed after an additional 10 minutes. Relative adhesion shows that PAF strikingly increased PMN adhesion to EC and FCS-coated wells. However, GAS6 significantly inhibited the PAF-driven PMN adhesion to EC, but not that to FCS-coated wells. Moreover, GAS6 did not affect PMN adhesion in the absence of PAF. Results are expressed as relative adhesion (%) and represent the mean ± SD from five experiments. Asterisks mark values that are significantly different from the respective control (P < .05) and 100% relative adhesion (bold horizontal line) corresponds to an absolute adhesion of 16 ± 4 (EC) and 11 ± 2 (FCS-coated wells) cells/microscope field.
In vitro adhesion assays between peripheral blood PMN cells and EC or FCS-coated plastic wells. EC and FCS-coated plastic wells were treated (▨) or not (▪) with GAS6 (1 μg/mL) for 10 minutes and fluorescein-labeled PMN were then seeded on the wells in the presence and absence of PAF (10−7mol/L). Cell adhesion was assessed after an additional 10 minutes. Relative adhesion shows that PAF strikingly increased PMN adhesion to EC and FCS-coated wells. However, GAS6 significantly inhibited the PAF-driven PMN adhesion to EC, but not that to FCS-coated wells. Moreover, GAS6 did not affect PMN adhesion in the absence of PAF. Results are expressed as relative adhesion (%) and represent the mean ± SD from five experiments. Asterisks mark values that are significantly different from the respective control (P < .05) and 100% relative adhesion (bold horizontal line) corresponds to an absolute adhesion of 16 ± 4 (EC) and 11 ± 2 (FCS-coated wells) cells/microscope field.
Effect of GAS6 soluble ligands on PMN adhesion to resting EC. Resting EC were treated or not for 10 minutes with either GAS6 (1 μg/mL) or Axl-x (1 μg/mL), and adhesion of PMN was then assessed. Treatment with Axl-x significantly potentiated PMN adhesion, whereas the control MoAb did not display any effect. Results are expressed as relative adhesion percent and represent the mean ± SD from five experiments. Asterisks mark values that are significantly different from the control (P < .05) and 100% relative adhesion (bold horizontal line) corresponds to an absolute adhesion of 11 ± 5 cells/microscope field.
Effect of GAS6 soluble ligands on PMN adhesion to resting EC. Resting EC were treated or not for 10 minutes with either GAS6 (1 μg/mL) or Axl-x (1 μg/mL), and adhesion of PMN was then assessed. Treatment with Axl-x significantly potentiated PMN adhesion, whereas the control MoAb did not display any effect. Results are expressed as relative adhesion percent and represent the mean ± SD from five experiments. Asterisks mark values that are significantly different from the control (P < .05) and 100% relative adhesion (bold horizontal line) corresponds to an absolute adhesion of 11 ± 5 cells/microscope field.
Immunofluorescent localization of GAS6 in resting and PAF stimulated EC. EC on glass coverslips were treated for 10 minutes without (upper panel) or with (lower panel) PAF (10−7mol/L), fixed, permeabilized, immunostained as described in Materials and Methods, and observed using a laser scanning confocal microscope. Frames taken at 1-μm intervals along the Z axis were merged to obtain reconstructed images (600× final magnification).
Immunofluorescent localization of GAS6 in resting and PAF stimulated EC. EC on glass coverslips were treated for 10 minutes without (upper panel) or with (lower panel) PAF (10−7mol/L), fixed, permeabilized, immunostained as described in Materials and Methods, and observed using a laser scanning confocal microscope. Frames taken at 1-μm intervals along the Z axis were merged to obtain reconstructed images (600× final magnification).
Perimembrane localization of GAS6 in a resting EC. EC on glass coverslips were fixed, permeabilized, and immunostained as described in Materials and Methods. The individual frames obtained following confocal microscopy analysis of 1-μm intervals along the Z axis are progressively depicted from left to right and top to bottom. The first frame refers to the section taken at the glass attachment. One typical example is shown.
Perimembrane localization of GAS6 in a resting EC. EC on glass coverslips were fixed, permeabilized, and immunostained as described in Materials and Methods. The individual frames obtained following confocal microscopy analysis of 1-μm intervals along the Z axis are progressively depicted from left to right and top to bottom. The first frame refers to the section taken at the glass attachment. One typical example is shown.
DISCUSSION
This work shows that GAS6 inhibits PMN adhesion to EC promoted by PAF, thrombin, IL-1β, TNF-α, and PMA and that a soluble ligand of GAS6 potentiates PMN adhesion to resting EC. By contrast, GAS6 does not affect PMN adhesion to resting EC, or that promoted by FMLP and IL-8. The differential effect of GAS6 on EC activated with different stimuli was not surprising, as the mechanisms used by PAF, thrombin, IL-1β, TNF-α, and PMA to potentiate PMN adhesion to EC are in part different from those used by FMLP and IL-8. Resting EC contain low amounts of PAF, which is not released. PAF synthesis and release by EC is induced by thrombin, PMA, and PAF itself within 2 to 5 minutes.40Therefore, activity of thrombin and PMA may be mediated by endogenous PAF, which is both an autocrine and paracrine modulator of EC adhesiveness. By acting on PMN, PAF upregulates integrin adhesivity and cell polarization, whereas by acting on EC, it induces expression of P-selectin, rapid loss of sulphated proteoglycans, and change of cell shape by rearrangement of cytoskeletal structures, with rapid decrease in F-actin content and redistribution of vinculin. The role of PAF in PMN interactions with IL-1β– and TNF-α–treated EC is less clear, but both stimuli display an action on EC.41-44 By contrast, IL-8 is also secreted by EC, which present it to PMN, but has no autocrine effect on EC and exerts its effect by activating the PMN adhesive machinery. A selective effect on PMN is displayed also by the chemotactic peptide FMLP.45-47 Therefore, GAS6's ability to inhibit the proadhesive activity of PAF, PMA, IL-1β, TNF-α and not that of IL-8 and FMLP suggests that it acts on EC and not on PMN. In line with this possibility, we found that GAS6 does not inhibit PMN adhesion to FCS-coated plastic wells, and the inhibitory effect of GAS6 on PMN adhesion is not altered by washing the GAS6-pretreated EC before addition of PMN. These results are open to several, not mutually exclusive interpretations. One possibility is that GAS6 interacts with its receptor on EC and triggers a signal inhibiting EC response to the proadhesive stimuli. Another is that GAS6 sterically blocks adhesion molecules activated by the proadhesive stimuli. This is supported by the observation that Axl mediates homotypic/homophilic cell aggregation, which is inhibited by GAS6. PMN do not express Axl, but this molecule belongs to a family sharing high levels of structure homology and ligand promiscuity. Therefore, the hypothesis that GAS6 sterically blocks heterophilic interactions between Axl and unknown ligands expressed by PMN cannot be ruled out.
Leukocyte extravasation is due to the cooperative activity of several molecules acting on at least four steps33,34: (1) leukocyte rolling on endothelium, which is mediated by constitutively functional adhesion receptors, such as selectins and mucin-like molecules; (2) activation of inactive adhesion receptors by chemoattractants presented on EC; (3) leukocyte arrest mediated by molecules, such as the integrins, activated in the previous step; (4) extravasation mediated by molecules, such as CD31 and integrins, which direct leukocyte motility towards the tissue. It has been suggested that these steps identify codes that must be “dialed” to trigger extravasation in a specific tissue. EC control leukocyte migration by expressing distinct patterns of adhesion molecules and producing and/or presenting chemoattractants to leukocytes. This model suggests that “resting” EC are characterized by low production of proadhesive molecules. Our work expands this model and suggests that resting EC actively produce antiadhesive factors, such as GAS6, which are novel players in leukocyte migration control. The antiadhesive activity displayed by GAS6 suggests that it may function as a physiologic antiinflammatory agent. We suggest that resting EC may produce and secrete GAS6, which inhibits leukocyte/EC interaction. When EC are activated by proinflammatory stimuli, GAS6 is depleted and the EC proadhesive machinery is activated. In line with this model, cytofluorimeter analysis showed that GAS6 is expressed on the surface of a proportion of resting EC and expression is decreased on EC activation by PAF (data not shown). Moreover, exogenous GAS6 does not inhibit PMN adhesion to resting EC, which is, on the contrary, potentiated by a GAS6 ligand. We have been unable to detect GAS6 in supernatants of resting and activated EC by ELISA (data not shown), which suggests that GAS6 secreted by EC is not released in the supernatants, but immediately bound by the nearby GAS6-receptors. This behavior is displayed by other molecules that modulate EC adhesiveness, such as PAF.48
The possibility that GAS6 also functions as an antiaggregant has been extensively evaluated. However, we did not detect any GAS6-mediated inhibition of platelet aggregation induced by PAF, thrombin, ristocetin, and ADP in vitro (G.C. Avanzi, personal observation). GAS6 and Axl are expressed by several cell types beside EC.7 20Because interaction with EC is a key process not only in leukocyte extravasation, but also in cancer metastasis, expression of these molecules may perhaps modulate the metastatic capacity of tumors.
ACKNOWLEDGMENT
We gratefully acknowledge Dr Guido Tarone for oligopeptide supply and Dr Federico Bussolino for helpful discussion and revision of the manuscript.
Supported by AIRC (Milano) and AIDS Project (Istituto Superiore di Sanità, Roma). G.C. was supported by Comitato Gigi Ghirotti (Torino), L.G. was supported by AIRC, M.B was supported by ANLAIDS (Roma), and F.B. was supported by Cassa di Risparmio di Vercelli.
Address reprint requests to Gian Carlo Avanzi, MD, Dipartimento di Scienze Mediche, Via Solaroli 17, 28100, Novara, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.