Abstract
Besides its well-established effects on granulocytopoiesis, granulocyte colony-stimulating factor (G-CSF) has been shown to have direct effects on the recruitment and bactericidal ability of neutrophils, resulting in improved survival of experimentally infected animals. We studied the effect of G-CSF on the course of experimental pneumonia induced by Klebsiella pneumoniae, an important gram-negative bacillary pulmonary pathogen. Using a highly reproducible murine model, we here show the paradoxical finding that mortality from infection was significantly increased when animals received G-CSF before induction of pneumonia. Administration of G-CSF promoted replication of bacteria in the liver and spleen, thus indicating an impairment rather than an enhancement of antibacterial mechanisms. By contrast, a monoclonal antibody against Klebsiella K2 capsule significantly reduced bacterial multiplication in the lung, liver, and spleen, and abrogated the increased mortality caused by G-CSF. In vitro studies showed a direct effect of G-CSF on K pneumoniaeresulting in increased capsular polysaccharide (CPS) production. When bacteria were coincubated with therapeutically achievable concentrations of G-CSF, phagocytic uptake and killing by neutrophils was impaired. Western blot analysis showed three binding sites of G-CSF to K pneumoniae. Binding of 125I-G-CSF to K pneumoniae was displaced by an excess of unlabeled G-CSF, whereas an unrelated cytokine, interleukin-1α, did not compete with G-CSF binding to the bacteria. Thus, in this model, the direct effect of G-CSF on a bacterial virulence factor, CPS production, outweighed any beneficial effect of G-CSF on recruitment and stimulation of leukocytes.
GRANULOCYTE COLONY-stimulating factor (G-CSF) is now widely used as a therapeutic agent for prevention or treatment of neutropenia induced by myelotoxic agents.1,2In addition, a number of experimental animal studies suggest its potential benefit in the treatment of infections by a variety of microorganisms. For example, G-CSF was able to augment the recruitment of polymorphonuclear leukocytes (PMN) into lungs of rats infected intratracheally with Klebsiella pneumoniae and to enhance intrapulmonary bactericidal activity against this pathogen, with an enhanced survival of treated animals.3 Fatal pneumococcal pneumonia in rats could also be prevented by pretreatment with G-CSF.4 Splenectomized mice challenged withStreptococcus pneumoniae aerosol had a significantly greater survival rate when treated with G-CSF.5 In Pseudomonas aeruginosa pneumonia after experimental hemorrhage, G-CSF improved survival when administered prophylactically.6 Finally, in neutropenic mice with systemic infections caused by P aeruginosa, Escherichia coli, Serratia marcescens, Staphylococcus aureus, or Caudida albicans, G-CSF significantly enhanced survival,7,8even when antibiotics failed to show therapeutic efficacy.8
K pneumoniae is one of the most frequently isolated gram-negative bacterial pathogens in severe nosocomial infections.9-11 The rapidly progressive clinical course ofKlebsiella pneumonia, which is often complicated by multilobular involvement, lung abscesses and high mortality,12,13 leaves little time to institute effective antibiotic treatment. In addition, an increasing proportion of nosocomial K pneumoniae isolates are resistant to multiple antibiotics commonly used in intensive care units.14-16Alternative approaches to the prophylaxis and supportive treatment of Klebsiella respiratory infections are therefore needed.
We have shown previously that a monoclonal antibody (MoAb III/5-1) directed against the K2 capsular polysaccharide (CPS) of K pneumoniae is protective in a mouse sepsis model as well as in experimental K pneumoniae pneumonia in rats.17 18However, antibodies may not be distributed equally within tissues and therefore may not be present in effective concentrations in certain organs. Moreover, to be effective, opsonophagocytic antibodies need the presence of phagocytic cells to enhance clearance of bacteria. We thus aimed to extend our studies using G-CSF in addition to MoAb III/5-1 to enhance the efficacy of nonantibiotic therapy against experimentalK pneumoniae pneumonia. Much to our surprise, we found a dramatic increase in mortality when mice were pretreated with G-CSF before induction of pneumonia. Subsequent studies showed that this adverse effect of G-CSF was most likely a result of a direct action of G-CSF on K pneumoniae. G-CSF bound specifically to the bacteria and enhanced the production of cell-bound CPS, enabling the bacteria to escape phagocytosis and to spread rapidly throughout the body.
MATERIALS AND METHODS
Animals.
Pathogen-free female BALB/c mice, 20 to 25 g, were obtained from the breeding facility of the Institute of Infectious Diseases, Klinikum Benjamin Franklin, Free University, Berlin, Germany, and used throughout the study. They were provided free access to standard laboratory animal diet and water and subjected to a 12-hour day/night rhythm.
Bacteria.
K pneumoniae B5055 (serotype O1:K2)19 and F201 (serotype O1:K-)20 were used throughout the study. For animal studies, we grew the bacteria to mid-log phase in trypticase soy broth (TSB; Difco, Detroit, MI) for 4 hours at 37°C with gentle shaking (100 rpm), washed them once with sterile physiological saline, and adjusted them spectrophotometrically to the desired concentrations, which were confirmed by plating serial 10-fold dilutions of the suspension on trypticase soy agar (TSA; Difco).
Reagents and injection protocol.
Sterile, pyrogen-free recombinant human G-CSF was obtained from AMGEN (München, Germany). The MoAb III/5-1 is a mouse IgM directed against K2-CPS and was described previously.17 18 Different dilutions from both MoAb III/5-1 and G-CSF were made with phosphate-buffered saline (PBS) under sterile conditions. All reagents as well as the PBS itself were endotoxin free as assessed using the limulus amebocyte lysate assay (Chromogenix, Mölndal, Sweden). The assay detected E coli O111:B4 lipopolysaccharide (LPS) at concentrations of 1 pg/mL and above. Animals were injected with either 50 μg/kg G-CSF subcutaneously (SC) and/or 10 mg/kg MoAb III/5-1 intravenously (IV) as detailed in Table1. Control animals received PBS only at all time points at the same volume as the treated mice (0.1 mL).
In a preliminary experiment, we confirmed that G-CSF was active in this mouse strain. Mice (three per group) were injected SC twice daily for 4 days with three different doses of G-CSF (15 μg/kg, 50 μg/kg, or 250 μg/kg, respectively), and blood was obtained from a tail vein once daily beginning 4 days before G-CSF treatment and throughout the treatment phase. Total leukocyte counts were determined with a Coulter counter (Model DN; Coulter Electronics LTD, Harpenten Herts, UK) and differential counts were obtained with smears stained with May-Grünwald solution. Absolute numbers of each cell type were calculated by multiplying the percentage obtained on differential counting by the total white blood cell count. Data showed a dose- and time-dependent increase in absolute neutrophil numbers as well as in total leukocyte counts comparable to previous reports,21 thus confirming that the G-CSF treatment used in this study had the expected effects on leukocytes (data not shown).
Experimental pneumonia.
Animals were anesthetized with 8 mg/kg of xylacine (Bayer, Leverkusen, Germany) and 80 mg/kg of ketamine hydrochloride (Parke, Davis & Co, Munich, Germany) administered intraperitoneally. The bacterial inoculum (total volume, 50 μL; containing 1 × 103colony-forming units [CFU]) was instilled intranasally into the left nasal opening while holding the mice upright. The animals were returned to their cages, and survival as well as body weight were assessed every 12 hours. Surviving animals were killed at 72 hours after challenge. Because both histology and culture of organ homogenates for quantitative detection of bacteria are subject to sampling error if only parts of the organs are examined, we decided not to perform both histology and organ cultures in the same animal. Instead, at the start of the study, three mice were randomly assigned to undergo histological examination. If animals died during the observation period, necropsy for histology was performed not later than 12 hours after death. All surviving mice were examined bacteriologically at the end of the study (72 hours after bacterial challenge). Quantitative culture of bacteria in lungs, liver, and spleen, as well as histological examination were performed as described.18 CFU detected in the organs and their log10 were standardized per 0.1 gram wet organ weight. As a parameter of bacterial dissemination from the lung, an index was computed for each investigated mouse according to the following formula:
Immunoblotting.
K pneumoniae B5055 was grown in TSB at 37°C overnight and collected by centrifugation (1,560g, 15 minutes, 4°C). The pellet was lysed in Tris/EDTA buffer (50 mmol/L Tris, 1 mmol/L EDTA; pH 8.0 [Sigma Chemical Co, St Louis, MO]) containing 1 mmol/L phenylmethylsulfonyl fluoride, 100 μg/mL Aprotinin, and 0.1 mmol/L Leupeptin (all Sigma) by three cycles of freeze-thawing. Samples were then reduced by heating the lysate for 10 minutes at 80°C in the presence of 0.5% sodium dodecyl sulfate and 1.25% mercaptoethanol (both from Sigma), subjected to polyacrylamide gel electrophoresis (PAGE; 12%) and blotted onto PVDF membranes (Millipore, Bedford, MA). An aliquot of the lysate was subjected to proteinase K treatment (Boehringer Mannheim, Indianapolis, IN) before reduction (60 minutes at 60°C). After blocking nonspecific binding sites with PBS containing 0.1% TWEEN 20 and 3% bovine serum albumin (BSA; Sigma), individual lanes were incubated in a Hoefer Decafold apparatus (Pharmacia Biotech, Piscataway, NJ) as indicated (4°C, overnight) with G-CSF, rmIL-1α (Genzyme, Cambridge, MA), or G-CSF which was preabsorbed with rabbit anti-human G-CSF–IgG (a kind gift from Dr P. Stevens and Ed Shatzen, AMGEN, Thousand Oaks, CA). After washing, bound G-CSF was detected by incubation (4°C, 4 hours) with a murine anti-human G-CSF MoAb (IgG1, also kindly provided by Dr P. Stevens and Ed Shatzen) which was preabsorbed with reduced bacterial lysate to reduce nonspecific binding (37°C, 1 hour, at a ratio of 1:1 [vol/vol]). After additional washing steps, bound MoAb was detected with a horseradish peroxidase-labeled goat anti-mouse IgG (Kierkegaard & Perry, Gaithersburg, MD) and subsequent enhanced chemiluminescence. Identical replicate samples were also stained after PAGE using conventional silver-staining procedures as described.22
Binding of 125I-G-CSF to K pneumoniae.
Single colony isolates of K pneumoniae B5055 or K pneumoniae F201 were grown to late log-phase and assessed for their ability to bind 125I-G-CSF at either 37°C or 4°C. Bacteria (1 × 108 CFU) were incubated in 0.5 mL of PBS containing 1% heat-inactivated fetal calf serum plus various concentrations of labeled and/or unlabeled G-CSF or interleukin-1α (IL-1α). Preliminary experiments showed that there was no difference in binding between samples with and those without sodium azide or between samples incubated at 37°C or 4°C. Thus, the final binding experiments were performed in 1.5-mL Eppendorf tubes at 4°C in the absence of sodium azide. The tubes were slowly rotated for 40 minutes, centrifuged for 5 minutes (14,250g), and the supernatants immediately removed. Bound radioactivity in the pellets as well as in the supernatants was counted. Measurements of bound and free ligand were converted to molar concentrations according to standard methods.23
In vitro production of CPS in the presence of G-CSF.
After incubation of bacteria overnight in TSB (37°C, 120 rpm), we transferred 2 × 105 CFU into 250 mL of fresh TSB with either G-CSF (10 ng/mL), recombinant murine IL-1α (2 ng/mL), or in TSB alone to obtain an initial concentration of approximately 850 CFU/mL (ie, a 1:230 dilution). Bacteria were incubated again (37°C, 120 rpm), and 50-mL samples were withdrawn at 2, 4, 6, 8, and 10 hours and immediately placed on ice for subsequent analysis. Colony counts, quantification of cell-bound CPS by means of a previously described enzyme-linked immunosorbent assay,18,24 as well as the cell disruption before the determination of total bacterial cell protein19 were performed exactly as described. Total bacterial cell protein was determined according to the method of Lowry et al25 by using different concentrations of BSA in distilled water as a standard. To account for possible increase in cell mass, CPS amounts were expressed as the ratio of cell-bound CPS/total protein rather than cell-bound CPS per colony counts.19 For use in the microphagocytosis and PMN killing assays, samples at 6 hours of incubation were used.
Preparation of PMN, serum, and microphagocytosis and PMN killing assays.
Heparinized (50 U/mL) venous blood was obtained from one healthy donor in all tests. PMN were isolated and residual erythrocytes were lysed as described earlier.26 Serum was obtained from one healthy volunteer under conditions that preserved complement activity, stored in small aliquots at −80°C, and used throughout all experiments at a final concentration of 60% vol/vol.
We performed the killing assay as described previously.17Briefly, PMN at a final concentration of 2.5 × 105cells/well, normal human serum as a complement source, bacteria (prepared as described above) at a final concentration of 2.5 × 104 CFU/well (thus, yielding a final PMN:bacteria ratio of 10:1), and Hanks' Balanced Salt Solution with Ca2+ and Mg2+ were incubated in 96-well round-bottom tissue culture plates (Costar, Cambridge, MA) in a total volume of 150 μL. Incubation was performed at 37°C with shaking (1,000 rpm). Immediately after mixing the ingredients, and after 60 and 90 minutes, samples (10 μL) were taken from each well and placed on ice into a glass tube containing sterile distilled H2O with 0.1% BSA (wt/vol) to lyse the PMN without killing the bacteria. Viable counts were determined by plating serial dilutions on TSA. We calculated the percentage of surviving bacteria according to the following formula:
Controls in each experiment included incubation of PMN and bacteria without serum; bacteria and serum without PMN; and PMN, bacteria and heat-inactivated serum (56°C, 30 minutes, to show that there were no antibodies or nonspecific serum activity against K2-CPS), each of which showed no killing of bacteria.
To assess phagocytosis, we incubated bacteria, PMN, and normal human serum as described above. After 60 minutes, samples (10 μL) were taken from each well and processed as described. Immediately after the samples were withdrawn, the 96-well plates were centrifuged (250g, 5 minutes) to separate the neutrophils out of the PMN-bacteria suspensions. Samples (10 μL) were withdrawn again and processed as above. The numbers of bacteria remaining in suspension after pelleting the PMN are given as percent of the total number of bacteria counted in the samples before centrifugation.
Statistical analysis.
Using a software package (Statistica for Windows, version 4.5; StatSoft, Tulsa, OK), we compared survival by the Cox-Mantel test, and all other parameters (body weights, bacterial counts, CPS/protein ratios, percentage of surviving bacteria) by the two-tailed Mann-Whitney U test.
RESULTS
Pretreatment with G-CSF worsened the outcome of experimental K pneumoniae pneumonia in mice.
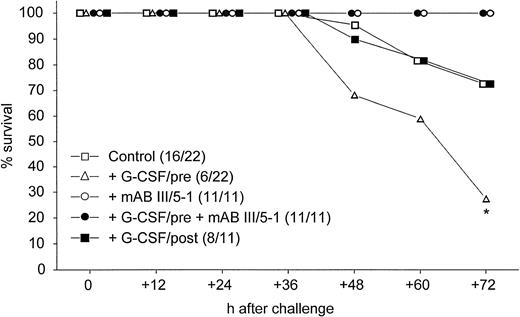
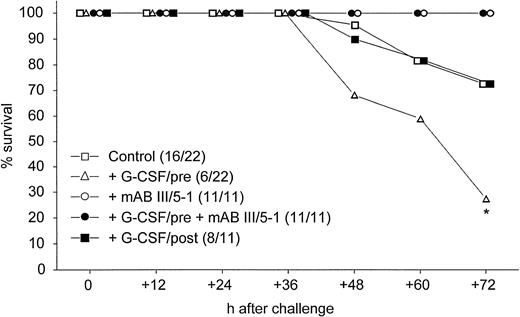
Non-neutropenic mice were infected intranasally with 0.5 × LD50 of K pneumoniae. When G-CSF was administered before induction of pneumonia, significantly fewer mice survived 72 hours than in the control group (Fig 1).This deleterious effect of G-CSF was not observed when treatment was started 24 hours after the bacteria were inoculated, but there was no improved survival compared with control animals (Fig 1). The enhanced lethality observed when mice were pretreated with G-CSF was completely abolished by cotreatment with MoAb III/5-1, which is specific for K2-CPS of K pneumoniae (Fig 1).
Survival after pulmonary infection via the intranasal route with 1 × 103 CFU of K pneumoniae B5055. Numbers in parentheses denote surviving animals/total number of animals. *, P = .0094 of treatment with G-CSF/pre compared with mice treated with MoAb III/5-1 (both groups) and P = .011 of treatment with G-CSF/pre compared with control animals and animals receiving G-CSF after induction of pneumonia (Cox-Mantel test).
Survival after pulmonary infection via the intranasal route with 1 × 103 CFU of K pneumoniae B5055. Numbers in parentheses denote surviving animals/total number of animals. *, P = .0094 of treatment with G-CSF/pre compared with mice treated with MoAb III/5-1 (both groups) and P = .011 of treatment with G-CSF/pre compared with control animals and animals receiving G-CSF after induction of pneumonia (Cox-Mantel test).
Because the body weight is a reliable parameter for the degree of illness in this type of pneumonia,18 we measured the change in body weight to obtain information about the course of infection in animals which did not die. Neither pretreatment with G-CSF nor the initiation of G-CSF treatment at 24 hours after bacterial challenge led to any improvement in weight compared with control animals (total weight loss [in grams] of control mice [median, −4.6; quartiles, −5.4 and −4.3]; total weight loss [in grams] of mice pretreated with G-CSF [median, −5.1; quartiles, −5.5 and −4.4]; total weight loss [in grams] of mice treated with G-CSF at 24 hours after bacterial challenge [median, −4.4; quartiles, −4.7 and −3.9]). However, weight loss could be reduced significantly by the IV administration of MoAb III/5-1 (total weight loss in grams [median, −2.9; quartiles, −4.4 and −1.1], P = .0045 as compared with control mice). The best outcome was observed when the treatment with MoAb III/5-1 was combined with pretreatment of the mice with G-CSF (total weight loss in grams [median, −1.9; quartiles, −2.4 and −0.9], P = .0003 as compared with control mice).
Pretreatment with G-CSF promoted bacterial spread to liver and spleen.
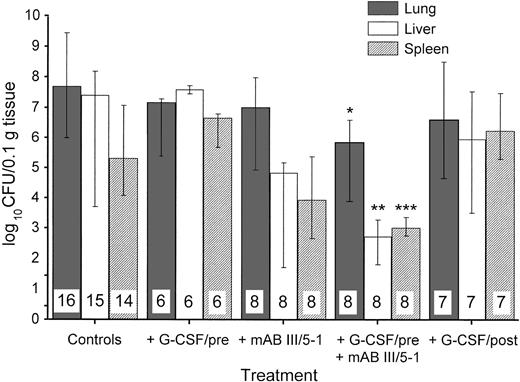
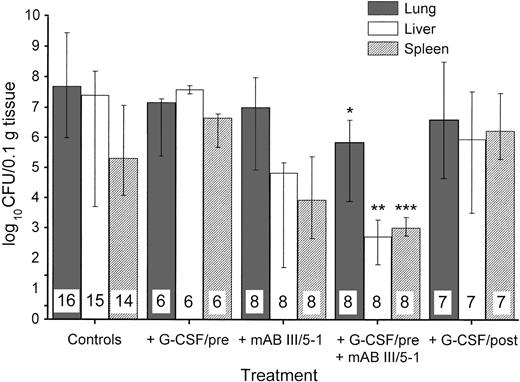
In addition to survival and degree of illness, we examined the bacterial spread in K pneumoniae pneumonia treated with or without G-CSF in our model. When organs were investigated in surviving animals 72 hours after bacterial challenge, the group pretreated with G-CSF had no significant decrease in colony counts in the lungs (Fig 2). However, pretreatment with G-CSF led to an increased colony count in liver and spleen compared with control animals. In contrast, MoAb III/5-1 administered with G-CSF–pretreatment was able to significantly decrease colony counts in all three organs investigated, thus reversing the effect of G-CSF–pretreatment. If treatment with G-CSF was started at 24 hours after bacterial challenge, there was no indication of enhanced bacterial spread (Fig 2). The presence of bacteria in other organs that originated from the lungs, indicative of spread of infection from a primary to a secondary site, can be expressed as an index. Table2 shows this effect of G-CSF more directly: only pretreatment with G-CSF showed a ratio of bacterial counts in distant organs to lung greater than 1. Thus, in G-CSF–pretreated animals, bacterial dissemination was promoted from the lung to other sites.
Effect of different treatments on the growth and spread of 1 × 103 CFU of K pneumoniae B5055 instilled into the lungs. Treatments are as outlined in Table 1. The number of animals investigated is given within the bars. Data are given as median ± quartiles. *, P = .017 compared with control animals; **,P = .0074 compared with control animals and .0019 compared with treatment with G-CSF/pre; ***, P = .014 compared with treatment with G-CSF/pre (Mann-Whitney U test).
Effect of different treatments on the growth and spread of 1 × 103 CFU of K pneumoniae B5055 instilled into the lungs. Treatments are as outlined in Table 1. The number of animals investigated is given within the bars. Data are given as median ± quartiles. *, P = .017 compared with control animals; **,P = .0074 compared with control animals and .0019 compared with treatment with G-CSF/pre; ***, P = .014 compared with treatment with G-CSF/pre (Mann-Whitney U test).
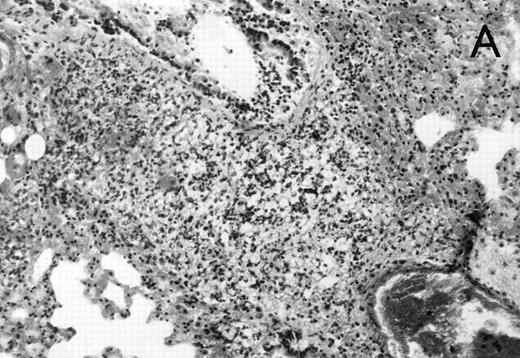
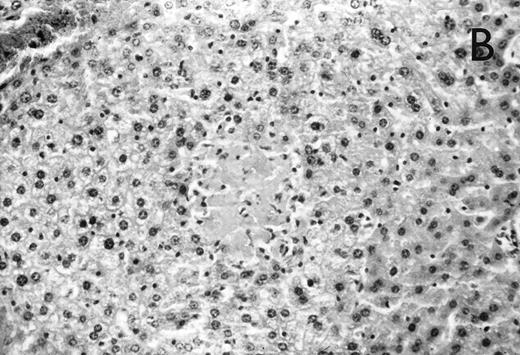
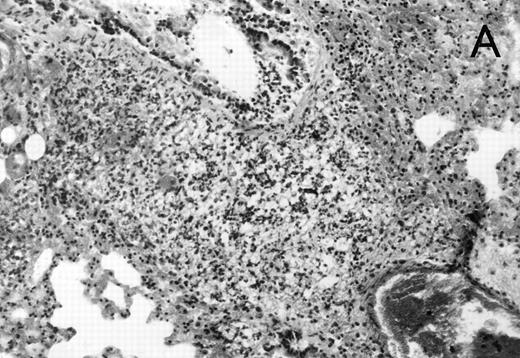
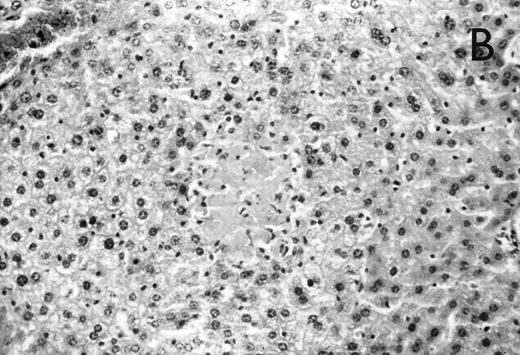
Histology further supported this observation. Control animals showed well-developed pneumonia with abscess formation (Fig3A) in their lungs, but in their livers, only small areas of inflammation with scattered microabscesses and foci of hepatic necrosis were observed (Fig 3B). In contrast, mice pretreated with G-CSF had only mild peribronchitic alterations in their lungs without signs of containment of inflammation such as abscess formation (Fig 4A). Livers and spleens of mice pretreated with G-CSF were severely altered, showing large abscesses which contained massive amounts of bacteria (Fig 4B). In addition, large necrotic areas surrounded by granulocytes were observed in the livers (Fig 4C). When MoAb III/5-1 was administered in addition to pretreatment with G-CSF, almost all changes observed in animals pretreated with G-CSF were reversed: there were only minor bronchial and peribronchial infiltrations by granulocytes (minimal change focal pneumonia), and the livers showed small microabscesses as observed in control mice. When treatment with G-CSF was started at 24 hours after bacterial challenge, a mixed pattern was observed. In the lungs, there was a beginning pneumonic reaction and a moderate perivascular and septal edema. However, in the liver there was almost no difference to the severe alterations observed in mice pretreated with G-CSF: huge abscesses loaded with bacteria joined extended necrotic areas.
(A) Micrograph of lung tissue 72 hours after intranasal infection with 1 × 103 CFU of K pneumoniae B5055. Lung abscess with severe tissue destruction in a PBS-treated control animal. Hematoxylin & Eosin (H&E); original magnification ×150. (B) Small foci of hepatic necrosis. H&E; original magnification ×600.
(A) Micrograph of lung tissue 72 hours after intranasal infection with 1 × 103 CFU of K pneumoniae B5055. Lung abscess with severe tissue destruction in a PBS-treated control animal. Hematoxylin & Eosin (H&E); original magnification ×150. (B) Small foci of hepatic necrosis. H&E; original magnification ×600.
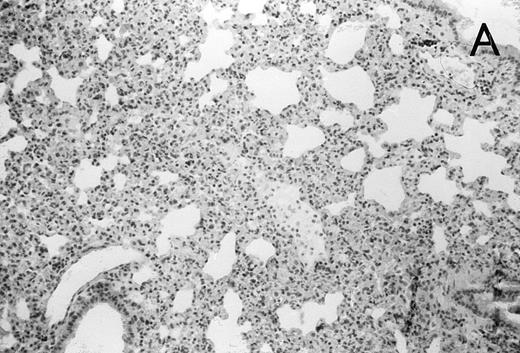
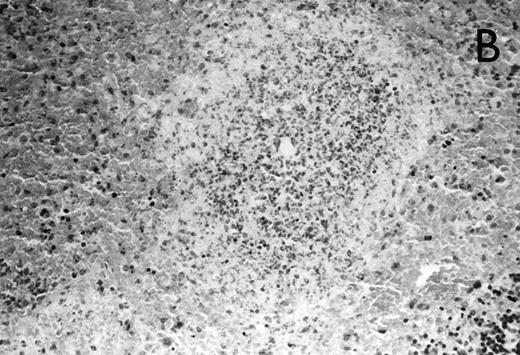
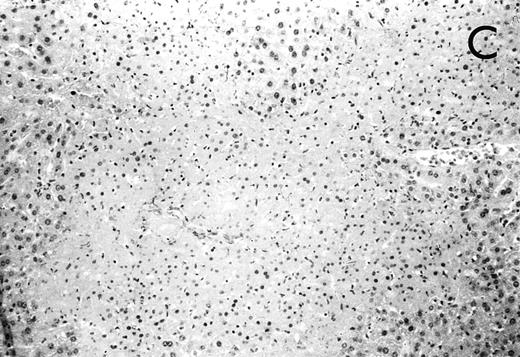
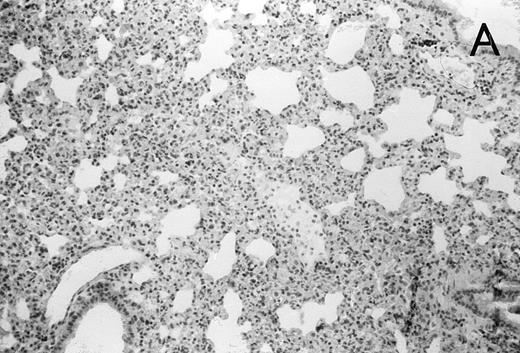
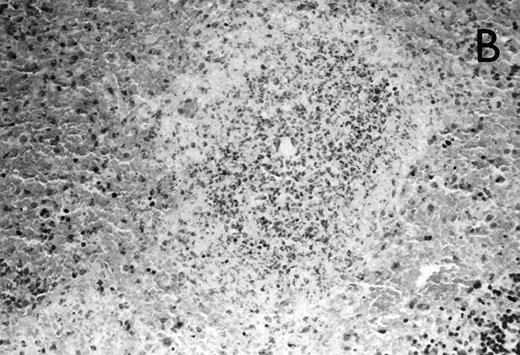
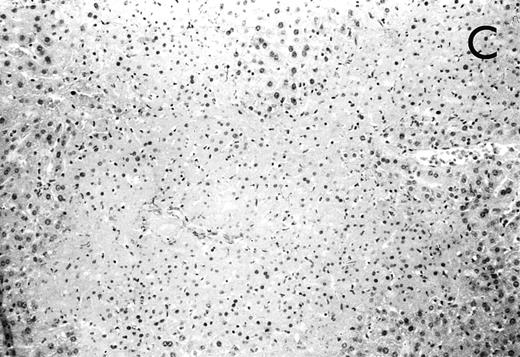
(A) Micrograph of lung tissue 48 hours after intranasal infection with 1 × 103 CFU of K pneumoniae B5055 and pretreatment with G-CSF (50 μg/kg sc) at −48 hours, −36 hours, −24 hours, and −12 hours before infection. Peribronchiolar neutrophils without destruction of lung parenchyma. H&E; original magnification ×150. (B) Splenic abscesses in the red pulp containing numerous gram-negative bacilli are found. H&E; original magnification ×370. (C) Confluent hepatic necroses. H&E; original magnification ×150.
(A) Micrograph of lung tissue 48 hours after intranasal infection with 1 × 103 CFU of K pneumoniae B5055 and pretreatment with G-CSF (50 μg/kg sc) at −48 hours, −36 hours, −24 hours, and −12 hours before infection. Peribronchiolar neutrophils without destruction of lung parenchyma. H&E; original magnification ×150. (B) Splenic abscesses in the red pulp containing numerous gram-negative bacilli are found. H&E; original magnification ×370. (C) Confluent hepatic necroses. H&E; original magnification ×150.
Coincubation of K pneumoniae B5055 with G-CSF resulted in enhanced capsular polysaccharide production and increased resistance against killing by neutrophils.
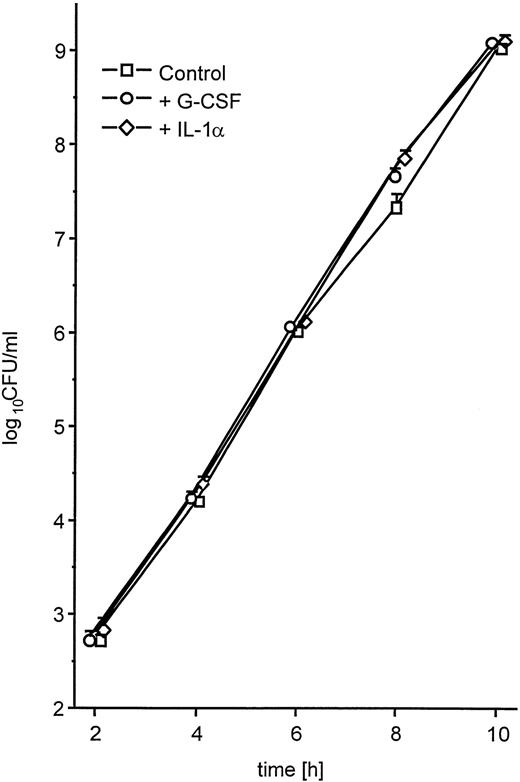
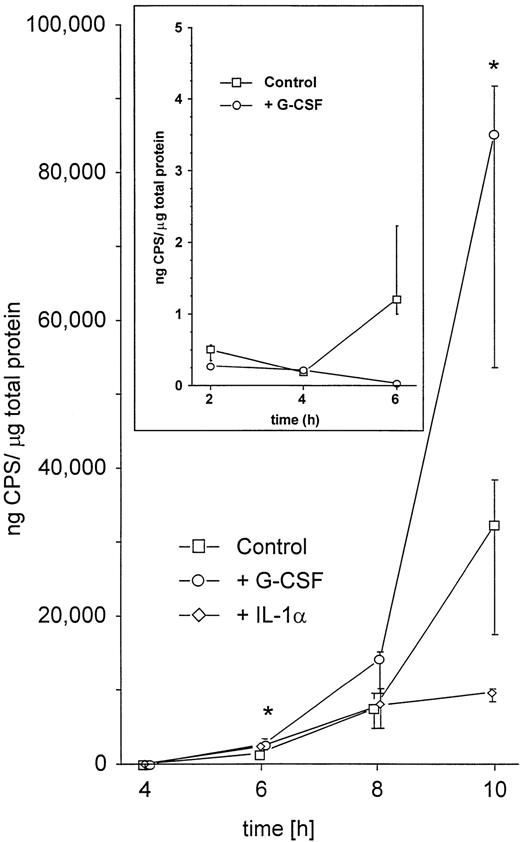
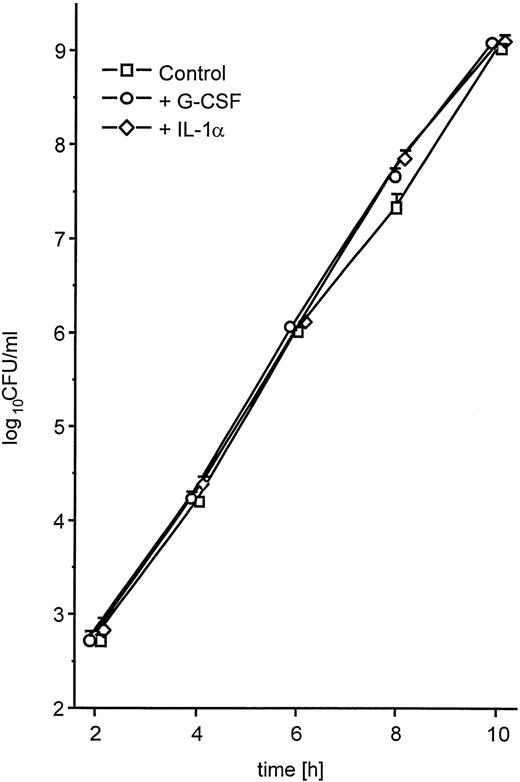
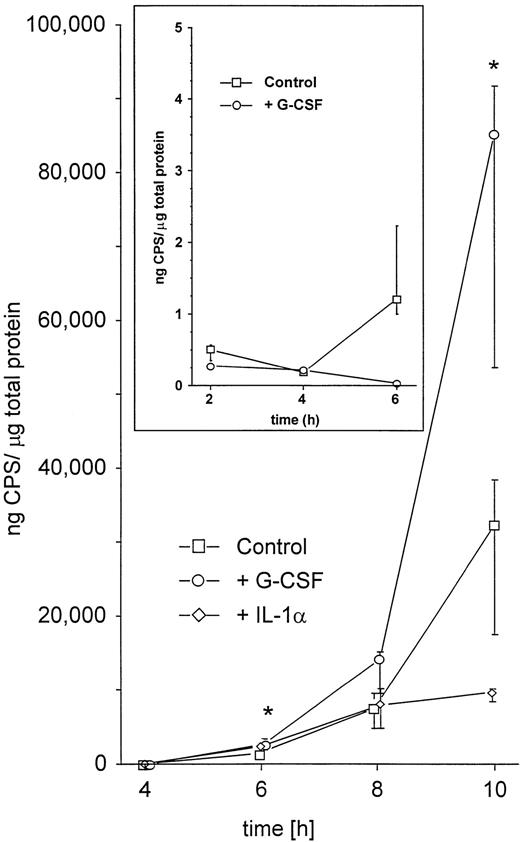
The enhanced spread to secondary sites in animals pretreated with G-CSF suggested that K pneumoniae in these mice were better able to evade phagocytic host defenses. This could be caused by either enhanced growth of the bacteria or enhanced production of antiphagocytic virulence factors. Therefore, we first investigated whether the presence of G-CSF had any effect on the growth kinetics of K pneumoniae. Bacteria grown in the presence of clinically relevant concentrations of G-CSF (10 ng/mL)27 showed no difference in the growth rate compared with controls grown in TSB only (Fig5). Second, we investigated the influence of G-CSF on the production of cell-bound CPS, the main mechanism by which K pneumoniae evades phagocytic host defenses.28 There was a marked increase in the production of cell-bound CPS when bacteria were incubated in the presence of G-CSF as compared with controls (Fig 6). In contrast, IL-1 used at a concentration previously shown to significantly bind to other gram-negative bacteria29 had no effect on the growth rate (Fig 5) or on the production of cell-bound CPS (Fig 6). A nonvirulent bacterial control strain (F201), producing only minimal amounts of K2-CPS, showed no increase in cell-bound CPS production when incubated under the same conditions (Fig 6, inset).
Growth of K pneumoniae B5055 in the absence or presence of either G-CSF (10 ng/mL) or IL-1α (2 ng/mL) as described in Materials and Methods. Data are from three independent experiments, each performed in duplicate, and are given as median ± quartiles.
Growth of K pneumoniae B5055 in the absence or presence of either G-CSF (10 ng/mL) or IL-1α (2 ng/mL) as described in Materials and Methods. Data are from three independent experiments, each performed in duplicate, and are given as median ± quartiles.
Enhancement by G-CSF of the production of cell-bound CPS.K pneumoniae B5055 was grown in the absence or presence of either G-CSF (10 ng/mL) or IL-1α (2 ng/mL) as described in Materials and Methods. Data are from four independent experiments, each performed in duplicate, and are given as median ± quartiles. *, P = .02 of bacteria grown in the presence of G-CSF as compared with controls (Mann-Whitney U test). Inset: Effect of G-CSF on the production of cell-bound CPS in the avirulent strain K pneumoniae F201. Bacteria were grown in the absence or presence of G-CSF (10 ng/mL) as described in Materials and Methods. Data are from four independent experiments, each done in duplicate, and are given as median ± quartiles. Note different scale on the y axis.
Enhancement by G-CSF of the production of cell-bound CPS.K pneumoniae B5055 was grown in the absence or presence of either G-CSF (10 ng/mL) or IL-1α (2 ng/mL) as described in Materials and Methods. Data are from four independent experiments, each performed in duplicate, and are given as median ± quartiles. *, P = .02 of bacteria grown in the presence of G-CSF as compared with controls (Mann-Whitney U test). Inset: Effect of G-CSF on the production of cell-bound CPS in the avirulent strain K pneumoniae F201. Bacteria were grown in the absence or presence of G-CSF (10 ng/mL) as described in Materials and Methods. Data are from four independent experiments, each done in duplicate, and are given as median ± quartiles. Note different scale on the y axis.
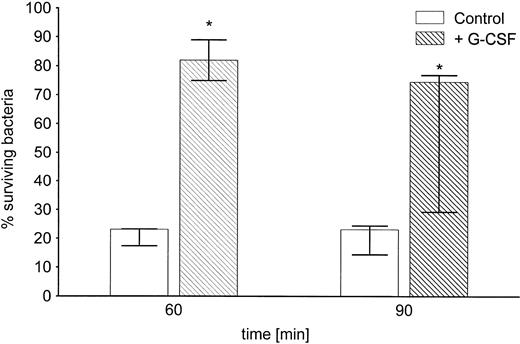
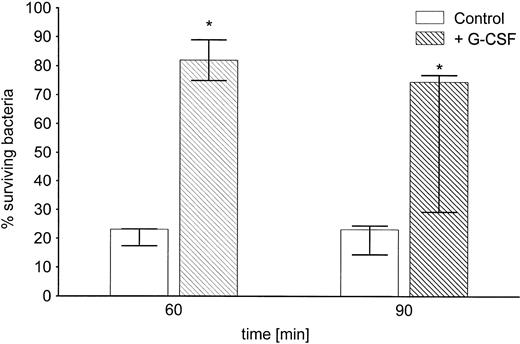
The increase in cell-bound CPS by the virulent strain B5055 grown in the presence of G-CSF resulted in a significant resistance to uptake and killing by neutrophils in vitro (Fig7). After coincubation for 6 hours with G-CSF, about 80% of the initial bacterial inoculum survived as compared with 23% of bacteria grown in the absence of G-CSF. This difference was still observed after 90 minutes of exposure to phagocytosis and killing by PMN.
Inhibition by G-CSF of in vitro phagocytosis and killing of Klebsiella by neutrophils. K pneumoniae B5055 was grown for 6 hours in the absence or presence of G-CSF (10 ng/mL) and subsequently subjected to phagocytosis of PMN in the presence of 60% normal human serum as described in Materials and Methods. Data are from four independent experiments, each performed in duplicate, and are given as median ± quartiles. *, P = .014 compared with the corresponding time point of controls grown in the absence of G-CSF (Mann-Whitney U test).
Inhibition by G-CSF of in vitro phagocytosis and killing of Klebsiella by neutrophils. K pneumoniae B5055 was grown for 6 hours in the absence or presence of G-CSF (10 ng/mL) and subsequently subjected to phagocytosis of PMN in the presence of 60% normal human serum as described in Materials and Methods. Data are from four independent experiments, each performed in duplicate, and are given as median ± quartiles. *, P = .014 compared with the corresponding time point of controls grown in the absence of G-CSF (Mann-Whitney U test).
To investigate whether this increased survival was caused by an increase in resistance to adherence and ingestion by the neutrophils, we measured the number of bacteria remaining in the cell-free supernatants of PMN-bacterial suspensions following centrifugation after 60 minutes of coincubation. Eighty-four percent (median; 82.7% and 85.5%, quartiles) of the total bacteria were recovered when K pneumoniae was grown in the presence of G-CSF. In contrast, only 53.9% (median; 52.9% and 59.2%, quartiles) of the total bacteria were recovered from the bacteria grown in TSB only (P = .014), suggesting that less bacteria were taken up by neutrophils when G-CSF was present during bacterial growth before the phagocytosis assay.
G-CSF bound to K pneumoniae B5055.
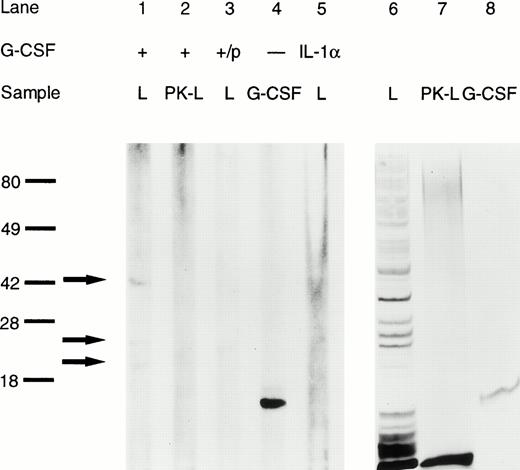
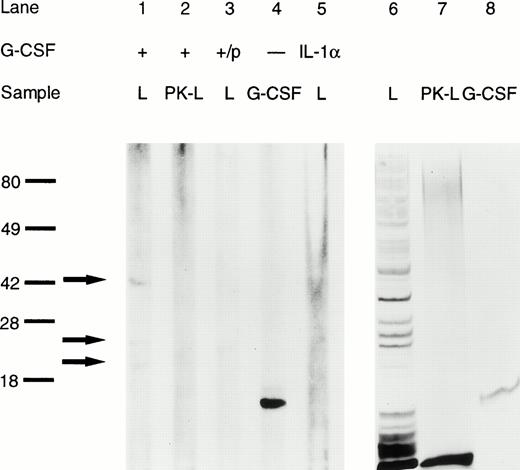
If G-CSF altered the virulence properties of K pneumoniae, it should bind to the bacteria via a specific binding site. The question of a possible binding of G-CSF to K pneumoniae was investigated by two independent methods. First, immunoblotting of whole bacterial cell lysates showed three binding sites with a molecular weight of 41, 25, and 21 kD, respectively (Fig 8, lane 1). The absence of binding to lysates pretreated with proteinase K indicates that the binding sites were, at least in part, of protein nature (Fig 8, lane 2). Controls included incubation of the blotted lysate with G-CSF preincubated with rabbit anti–G-CSF–IgG, rmIL-1α (thus omitting G-CSF), and incubation with mouse IgG1, all of which showed no binding (Fig 8).
Binding of G-CSF to K pneumoniae B5055 whole cell lysates as assessed by immunoblotting. L, bacterial cell lysate; PK-L, bacterial cell lysate pretreated with proteinase K; +/p, G-CSF preabsorbed with rabbit anti-human G-CSF-IgG. Lanes 1 through 5 are from a representative blot. Lanes 6 through 8 are from a silver-stained identical replica of the gel. Binding of G-CSF to specific bands is indicated by arrows; molecular weight (in kD) is indicated on the left.
Binding of G-CSF to K pneumoniae B5055 whole cell lysates as assessed by immunoblotting. L, bacterial cell lysate; PK-L, bacterial cell lysate pretreated with proteinase K; +/p, G-CSF preabsorbed with rabbit anti-human G-CSF-IgG. Lanes 1 through 5 are from a representative blot. Lanes 6 through 8 are from a silver-stained identical replica of the gel. Binding of G-CSF to specific bands is indicated by arrows; molecular weight (in kD) is indicated on the left.
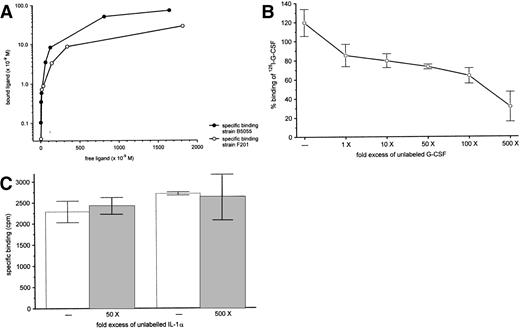
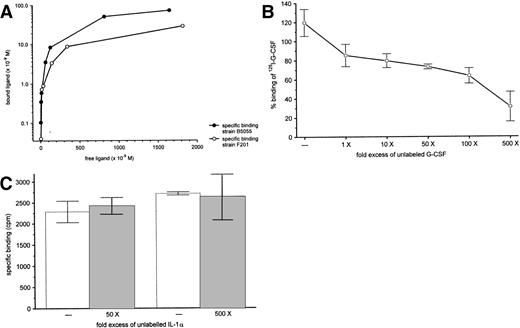
We also analyzed specific binding using 125I-labeled G-CSF. As shown in Fig 9A, binding of125I–G-CSF to the virulent strain B5055 was dose dependent and saturable. 125I–G-CSF also bound to the avirulent strain F201, although to a lesser extend. Specific binding could be inhibited in a dose-dependent manner by increasing amounts of competing unlabeled G-CSF suggesting the presence of specific binding sites for G-CSF (Fig 9B). Competition with a different cytokine, IL-1α, did not displace specific binding of 125I–G-CSF (Fig 9C).
Binding of 125I-G-CSF to K pneumoniaeB5055. (A) Binding curve of 125I-G-CSF after 40 minutes at 4°C with K pneumoniae B5055 or K pneumoniae F201. Results are representative of at least three independent experiments. (B) Competition of G-CSF with 125I-G-CSF for binding toK pneumoniae B5055. 125I-G-CSF (50 ng) was incubated (4°C, 40 minutes) with K pneumoniae, along with various concentrations of unlabeled G-CSF. The results, plotted as specific binding relative to results of an assay with no added competitor, are from four independent experiments, each done in duplicate and are shown as mean ± standard deviation. (C) Competition of IL-1α with 125I-G-CSF for binding to K pneumoniae B5055. 125I-G-CSF (50 ng) was incubated (4°C, 40 minutes) with K pneumoniae, along with various concentrations of unlabeled IL-1α. The results, plotted as specific binding, are from four independent experiments, each performed in duplicate, and are shown as mean ± standard deviation.
Binding of 125I-G-CSF to K pneumoniaeB5055. (A) Binding curve of 125I-G-CSF after 40 minutes at 4°C with K pneumoniae B5055 or K pneumoniae F201. Results are representative of at least three independent experiments. (B) Competition of G-CSF with 125I-G-CSF for binding toK pneumoniae B5055. 125I-G-CSF (50 ng) was incubated (4°C, 40 minutes) with K pneumoniae, along with various concentrations of unlabeled G-CSF. The results, plotted as specific binding relative to results of an assay with no added competitor, are from four independent experiments, each done in duplicate and are shown as mean ± standard deviation. (C) Competition of IL-1α with 125I-G-CSF for binding to K pneumoniae B5055. 125I-G-CSF (50 ng) was incubated (4°C, 40 minutes) with K pneumoniae, along with various concentrations of unlabeled IL-1α. The results, plotted as specific binding, are from four independent experiments, each performed in duplicate, and are shown as mean ± standard deviation.
DISCUSSION
In this study, we show that the administration of G-CSF to a non-neutropenic host may worsen a subsequent pulmonary infection byK pneumoniae because of a direct effect of the cytokine on the pathogen. Although some studies point to an effect of cytokines from eukaryotes on procaryotic cells in vitro,29-32 to our knowledge there are no reports showing the potential in vivo significance of this observation. Here, we report for the first time that G-CSF not only binds to and modifies bacterial cells in vitro, but that these effects have fatal consequences in a relevant model of bacterial pneumonia and sepsis.
The deleterious effect on mortality from experimental pneumonia was observed when G-CSF was administered before the induction of pneumonia (Fig 1). Bacterial load in lungs, livers, and spleens of pretreated animals indicated that, in this situation, bacteria spread more easily from the lung to distant organs and that there was a decreased eradication of the bacteria from these secondarily infected organs (Fig2, Table 2). Histological analysis showed the development of severe necroses and abscesses within liver and spleen (Fig 4B and C). When treatment with G-CSF was started 24 hours after bacterial challenge, we observed a mixed picture: although mortality and histological changes in the lungs were not different from control animals, we found the same type and size of abscesses and confluent hepatic necrosis in the liver as in mice pretreated with G-CSF. A recent study using a highly virulent strain of Pasteurella multocida to induce gram-negative bacterial pneumonia and sepsis in rabbits showed a similar histology: treatment with G-CSF led to significantly increased inflammation in liver and spleen compared with the placebo group.33 In addition, rats pretreated with G-CSF showed a significant decrease in survival when challenged subsequently withE coli.34 However, the basis for this adverse outcome was not investigated.34 A history of treatment with G-CSF was also a highly significant risk factor for the development of subsequent disseminated Mycobacterium aviumcomplex infection in patients with acquired immunodeficiency syndrome.35
The impaired uptake and killing of the bacteria observed here was surprising, because G-CSF has been shown to promote phagocytosis and subsequent elimination of microbial organisms.36 We therefore hypothesized that G-CSF might have a direct action on the bacteria resulting in increased bacterial virulence, which outweighs the well-known effects of G-CSF on host defenses. To investigate this question, we asked whether G-CSF binds to K pneumoniae. Using two independent methods, we could show that G-CSF binds to at least three binding sites (Fig 8). These binding sites are, in part, of protein nature because pretreatment of the bacterial cell lysates with proteinase K abolished binding (Fig 8). Studies with125I–G-CSF showed dose dependency and saturability of binding of G-CSF to K pneumoniae (Fig 9A). Also, specificity could be shown by competition of unlabeled G-CSF with125I–G-CSF for the binding to K pneumoniae, again in a dose-dependent manner (Fig 9B). Finally, another cytokine, IL-1, which binds to E coli,29 did not replace bound 125I–G-CSF from its receptor(s), even when present in a 500-fold excess (Fig 9C).
Having established the binding of an eukaryote growth factor to prokaryote cells, the next question to be asked was whether this binding of G-CSF had any consequences on the virulence of K pneumoniae. Earlier reports of effects of cytokines on bacteria showed mainly an effect on the growth, but those differences were at most 1 log only in vitro29 and are thus questionable as to whether they play a role in vivo. No other experiments were performed to investigate whether the binding of a cytokine to a bacterium had any consequences with regard to bacterial virulence or outcome from infection.29 Only one paper showed enhancement of invasiveness of Shigella flexneri to HeLa cells when the bacteria were coincubated with TNF-α,31providing some functional data in terms of virulence, but there was no identification of possible virulence factors being altered because of the presence of the cytokine.31 Hence, we first looked at the growth of K pneumoniae in the presence or absence of G-CSF. For these and subsequent experiments, we chose a concentration of G-CSF shown to be within therapeutically achievable concentrations (10 ng/mL).27 We could not show any differences in the growth rate of the bacteria (Fig 5). However, the fact that the virulence ofKlebsiella strains in humans and animals is strongly correlated with the degree of encapsulation is well established.28,37-40 We therefore investigated whether the coincubation with G-CSF of the strain used in this study, K pneumoniae B5055, would result in an altered production of cell-bound CPS. Indeed, K pneumoniae B5055 produced significantly more cell-bound CPS when grown in the presence of G-CSF than when grown in media alone (Fig 6). To confirm the functional importance of this finding, we subjected the bacteria grown either in the presence or absence of G-CSF to a PMN killing assay in which killing of the bacteria by neutrophils in vitro depends on phagocytosis. As expected of bacteria with increased amounts of cell-bound CPS, bacteria coincubated with G-CSF were significantly more resistant to killing by neutrophils than untreated controls (Fig 7). In separate assays, we could show that this enhanced resistance was caused by decreased phagocytosis of the bacteria, which can be explained most easily by the increased production of cell-bound CPS.17 28
Because other cytokines like IL-1 have been described to increase virulence of gram-negative bacteria29 and because this cytokine plays a major role in gram-negative bacterial infection and sepsis,41 we investigated the possibility that it may affect the virulence of K pneumoniae as well. Interestingly, a 500-fold excess of IL-1α did not compete with binding of125I–G-CSF to K pneumoniae (Fig 9C), whereas unlabeled G-CSF did in a dose-dependent manner (Fig 9B). Accordingly, the coincubation of the bacteria with IL-1α did not lead to increased bacterial growth or enhanced production of cell-bound CPS (Figs 5 and6).
Thus, our in vitro studies correlated well with our in vivo results which show enhanced bacterial spread to distant sites only when G-CSF was present before infection. Also, the data obtained using the MoAb against K2-CPS, MoAb III/5-1, lent further support to our hypothesis, because its administration in an optimal dose17 18 together with G-CSF not only reversed the increased mortality observed with pretreatment with G-CSF alone (Fig 1), but also ameliorated the massive necrotic changes observed in animals receiving only G-CSF. The MoAb against K2-CPS, III/5-1, presumably directly counteracted the effect of G-CSF on K pneumoniae, which resulted in the enhanced production of CPS. In fact, those animals receiving both antimicrobial therapy (MoAb III/5-1) and therapy to enhance host defenses (G-CSF) showed the best outcome with respect to survival, body weight, bacterial colony counts, and histology (Figs 1 and 2).
At first glance, these observations seem to contrast with a prior study which showed a beneficial effect of G-CSF (administered at the same dose, ie, 50 μg/kg) on survival in a similar pneumonia model using rats instead of mice, but also with K pneumoniae.3However, there are marked differences between this study and our observations. Most importantly, the bacterial strain used by Nelson et al3 seems to be rather avirulent because approximately 5 × 107 CFU were needed to elicit a lethal pneumonia. Consequently, mortality induced by this level of bacterial challenge may have been caused by lethal intoxication, perhaps by LPS, and not by replicating and disseminating bacteria, attributable to the CPS. In contrast, the strain used in our study is even more virulent based on differences in CPS production17 than a strain used in one of our previous reports (K pneumoniaeCaroli)17,18 in which a 1000-fold fewer amount of CFU (3 × 104) was able to provoke a fulminant pneumonia in rats.18 To test this hypothesis, we incubated an avirulent strain of K pneumoniae (F201, producing only minimal amounts of K2-CPS) with G-CSF under the same conditions as the virulent strain (B5055). Although G-CSF bound to this avirulent strain F201 (Fig9A), there was no increase in cell-bound CPS production (Fig 6, inset). When administered intranasally into mice, no lethality was observed with K pneumoniae F201 (up to a dose of 5 × 107CFU) in contrast to K pneumoniae B5055 (LD50 = 1.3 × 103 CFU; T.K. Held, M. Trautmann, and A.S. Cross, manuscript in preparation). Such differences in the response of various bacterial strains have also been shown in other studies: IL-1, IL-2, and GM-CSF enhanced growth only of virulent strains of E coli, but not of avirulent strains.29,30 Indeed, in all reports showing a beneficial effect of treatment of bacterial infections with G-CSF, a relatively high number of organisms had to be administered to elicit a lethal effect: 5 × 106 CFU ofP aeruginosa42; 2 × 107 CFU of P aeruginosa6; 5.9 × 106 CFU ofP aeruginosa7; 5 × 1010 CFU of E coli43; 1 × 106 CFU of S pneumoniae4; 4.5 × 107 CFU of S aureus; 1.2 × 106 CFU of E coli; or 1 × 106 CFU of S marcescens8; or a fecal inoculum containing up to 42 different bacterial species.44
G-CSF enhances the number and function of neutrophils36,45-48 and is recently considered as a prophylactic agent against sepsis, even in non-neutropenic patients.49-52 However, we here show that G-CSF, in addition to its known effects on the host, may also act directly on the invading bacteria by increasing the virulence of K pneumoniae.Our results show that this dual action under some conditions may actually shift the balance in favor of the bacteria and cause deleterious effects. The potential of this latter outcome should be considered, especially in situations in which G-CSF is administered without concomittant antimicrobial therapy such as antiinfective agents or protective antibodies.
ACKNOWLEDGMENT
We are grateful to Drs H. Hahn and R. Chang for critical discussion. The invaluable help of Dr Stephanie Treiber-Held is deeply appreciated. We also thank Ed Shatzer and Dr P. Stevens for generously supplying cytokines and various antibodies.
Supported in part by a grant from AMGEN GmbH, München, Germany.
Presented in part at the 96th General Meeting of the American Society for Microbiology, New Orleans, LA, May 19-23, 1996 (Abstract E-18).
Address reprint requests to Thomas K. Held, MD, Abteilung für Innere Medizin m.S. Hämatologie und Onkologie, Virchow-Klinikum der Humboldt-Universität, Augustenburger Platz 1, 13353 Berlin, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.