Ceramide has been suggested as the secondary messenger mediating the apoptotic signal for Fas engagement. By using different inhibitors, we demonstrated here that ceramide is unlikely a mediator of Fas-initiated apoptosis. First, cAMP prevented cell death induced by ceramide but not by Fas. Second, ceramide-triggered, but not Fas-triggered, apoptosis was antagonized by the free radical scavenger C60. Third, the metal chelator pyrrolidinedithiocarbamate suppressed ceramide-initiated DNA fragmentation but had no effect on the Fas-induced cell death. Fourth, the SAPK/ERK kinase dominant negative mutant, which attenuated ceramide-induced cell death, did not prevent Fas-induced apoptosis. Finally, activation of NF-κB inhibited ceramide-induced but not Fas-initiated apoptosis. The fact that many antagonists of ceramide-induced apoptosis could not suppress Fas-mediated cell death clearly indicates that ceramide is not the mediator for Fas-initiated apoptotic signal.
Fas (APO-1) IS A 45-kD membrane protein when engaged by anti-Fas antibody or Fas ligand triggers programmed cell death (for review, see Nagata1). The death pathway initiated from Fas activation involves a series of death-induced molecules.1 FADD (Fas-associating protein with death domain) or MORT1 is recruited to Fas upon its engagement.2,3 FADD then binds FLICE (FADD-like ICE) or MACH (MORT-1–associated CED-3 homologue).4,5 The association with the Fas death-inducing signaling complex activates FLICE,6 followed by eventual activation of ICE and CPP32.7
The activation of acidic sphingomyelinase that leads to the hydrolysis of sphingomyelin and generation of ceramide has also been suggested as the apoptotic pathway downstream of Fas ligation.8-12Ceramide is reported as a common intermediator for stimulation by tumor necrosis factor (TNF), interleukin-1, nerve growth factor, lipopolysaccharide, ionizing radiation, serum withdrawal, and daunorubicin.12-19 The role of ceramide in the apoptosis induced by some of these stimuli is illustrated by the ability of membrane-permeable ceramide to trigger cell death.13,14,19In addition, defects in the sphingomyelinase/ceramide pathway confers the resistance to radiation-, TNF-α–, and UV-induced apoptosis,20-22 supporting the role of ceramide in these types of death induction. Ceramide-induced cell death involves the activation of c-Jun N-terminal kinase (JNK).23
In this study, we compared the sensitivity of Fas and ceramide to various regulatory reagents. We showed that ceramide-induced apoptosis was antagonized by cAMP, C60, and pyrrolidinedithiocarbamate (PDTC). However, Fas-triggered cell death proceeded in the presence of these inhibitors. In addition, the negative mutant of SAPK/ERK kinase (SEK) suppressed ceramide-triggered death but did not prevent Fas-induced apoptosis. Furthermore, activation of NF-κB antagonized ceramide but not Fas-initiated apoptosis. These observations are not consistent with the model that ceramide acts downstream of Fas signaling. Together with recent observations,24 25 our results argue against a role of ceramide in Fas-mediated apoptosis.
MATERIALS AND METHODS
Reagents and cell lines.
12-O-tetradecanoylphorbol 13-acetate (TPA), A23187, N6, 2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (Bt2cAMP), forskolin, and PDTC were purchased from Sigma Chemical Co (St Louis, MO). Anti-Fas antibody CH-1126 was purchased from Upstate Biotech Inc (Lake Placid, NY). C2-ceramide and C6-ceramide were obtained from Biomol (Plymouth Meeting, PA). ICE inhibitor z-VAD-FK was purchased from Kamiya (Thousand Oaks, CA). The two regioisomers with C3 or D3 symmetry of water-soluble carboxylic acid C60derivatives (carboxylfullerenes) were synthesized as previously described.27 Both C60 (C3) and C60(D3) are effective scavengers of oxgen radicals, with complete elimination of hydroxyl radicals and superoxide radicals at concentrations of 5 to 50 μmol/L, and are potent inhibitors of neuronal apoptosis, which is associated with increased intracellular free radical production.27 H-89 and H-85 were purchased from Seikagaku (Tokyo, Japan). Human T lymphoblastomas CEM (ATCC CCL 119) and human T-cell leukemia Jurkat (ATCC TIB 152) were obtained from American Type Culture Collection (Rockville, MD). Recombinant human TNF-α was purchased from R&D (Minneapolis, MN).
Plasmids.
CMV-RelA(p65) and κB-TATA-CAT28 were kind gifts of Dr Warren C. Greene (University of California, San Francisco, CA). HA-JNK129 was obtained from Michael Karin (University of California, San Diego, CA). SEK-negative mutants SEK1(K→R) and SEK1(A→L)30 were obtained from Dr Leonard I. Zon (Harvard Medical School, Boston, MA). Green fluorescence protein expression vector pEGFP-N1 was obtained from Clontech (Palo Alto, CA).
Transfection.
T cells (1.6 × 107) were washed once with STBS (25 mmol/L Tris-HCl, pH 7.4, 137 mmol/L NaCl, 5 mmol/L KCl, 0.6 mmol/L Na2HPO4, 0.7 mmol/L CaCl2, 0.5 mmol/L MgCl2) and incubated with a total of 10 μg DNA in 1.2 mL STBS containing 0.5 mg/mL diethylaminoethyl (DEAE)-dextran for 20 minutes at room temperature. T cells were then treated with 15% dimethylsulfoxide for 3 minutes and washed once with STBS.31 32 After 24 to 48 hours, transfected cells were then stimulated with anti-Fas or ceramide, and the cell death was quantitated.
JNK activity assay.
The inhibition of JNK by the dominant negative form of SEK was performed with cotransfection of HA-JNK1. T cells were activated as indicated, washed twice with phosphate-buffered saline (PBS), and lysed in ice-cold lysis buffer (20 mmol/L Tris-HCl, pH 8.0, 1% Triton X-100, 10% glycerol, 137 mmol/L NaCl, 1.5 mmol/L MgCl2, 1 mmol/L EDTA, 50 mmol/L NaF, 1 mmol/L Na3VO4, and 1 mmol/L phenylmethylsulfonyl fluoride). Detergent-insoluble material was removed by centrifugation at 14,000g for 10 minutes at 4°C. For each immuno-precipitation, 200 μg of cell lysate was mixed with anti-HA 12CA5 (Boehring Mannheim, Mannheim, Germany) and incubated at 4°C for 2 hours. Twenty microliters of protein A-sepharose (Pharmacia, Uppsala, Sweden) was then added and incubated for an additional 2 hours at 4°C. Immune complexes were washed three times with lysis buffer and once with kinase buffer (30 mmol/L Tris-HCl, pH 8.0, 20 mmol/L MgCl2, 2 mmol/L MnCl2). Immune complexes were then incubated in kinase buffer (30 μL) containing 2 mmol/L ATP, GST-c-Jun(1-79), and 5 μCi of (γ-32P)ATP for 30 minutes at 30°C. Incubations were terminated by adding 15 μL of 3× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and boiling for 3 minutes. The reaction products were resolved on 15% SDS-PAGE followed by autoradiography and quantitated by Phosphorimager (Molecular Dynamics, Sunnyvale, CA).33
Cell death measurement.
All cultures (except those treated with ceramide) were performed in RPMI with 10% fetal calf serum (both from GIBCO, Grand Island, NY), 10 mmol/L glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 × 10−5 mol/L 2-ME.34 For apoptosis induced with ceramide, serum free-medium were used throughout the experiment. The extent of apoptosis was determined by propidium iodide (PI) staining or annexin V staining. Cells were treated with different inducers and/or inhibitors, washed with PBS, and fixed with ethanol. DNA content was determined by staining with 20 μg/mL PI and analyzed by FACScan (Becton Dickinson, Mountain View, CA). The fraction of cells with sub-G1 DNA content was assessed with the CELLFIT program (Becton Dickinson). For annexin V staining, the treated cells were washed, resuspended in annexin V-fluorescein isothiocyanate (FITC) (1 μg/mL; Clontech), incubated at room temperature for 15 minutes in the dark, and analyzed on FACScan. For the ceramide/Fas sensitivity of cell transiently transfected with CMV-p65 or pEBG-SEK(A→L), survival was monitored with green fluorescence protein expression vector pEGFP-N1. Treated cells were examined using a Nikon Diaphot 2000 fluorescence microscope (Tokyo, Japan) 48 hours after transfection.
RESULTS AND DISCUSSION
cAMP prevented ceramide-induced cell death but had no effect on Fas-initiated apoptosis.
Ceramide is a potent inducer of cell death in T-lymphoma cells Jurkat and CEM (not shown for CEM). Cell death was assessed by DNA fragmentation as represented by sub-G1 fraction (Fig 1B). DNA fragmentation induced by ceramide never exceeded 45%, despite extensive cell death. A similar observation was also earlier reported.35 We first identified a few reagents that could block ceramide-initiated cell death. cAMP was an effective antagonist of ceramide-induced cell death (Fig 1C). Forskolin (10 μmol/L) suppressed DNA fragmentation initiated by ceramide by at least 50%. A similar extent of inhibition was observed with dibutyryl cAMP (Bt2cAMP) at 1 mmol/L (Fig 2A). There was a dose-dependent inhibition of cAMP on ceramide-triggered cell death. The inhibition of ceramide-induced cell death could be detected with Bt2cAMP as low as 100 μmol/L. In contrast, apoptosis triggered by anti-Fas antibody CH-11 was resistant to cAMP. Treatment with forskolin up to 50 μmol/L or Bt2cAMP up to 2.5 mmol/L did not reduce the apoptosis triggered by Fas (Fig 2B). cAMP agonists by itself did not induce any T-cell death at the highest concentrations used here.
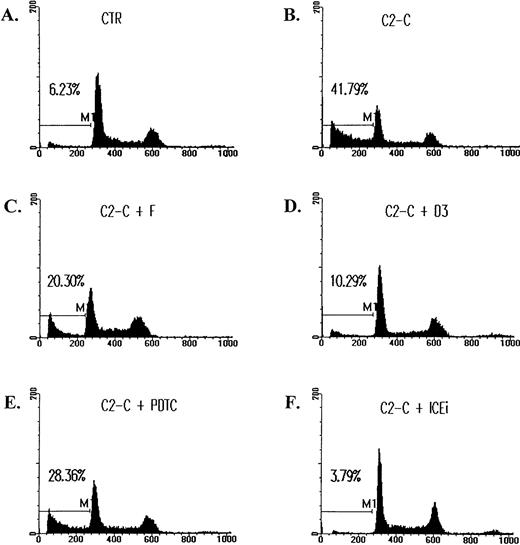
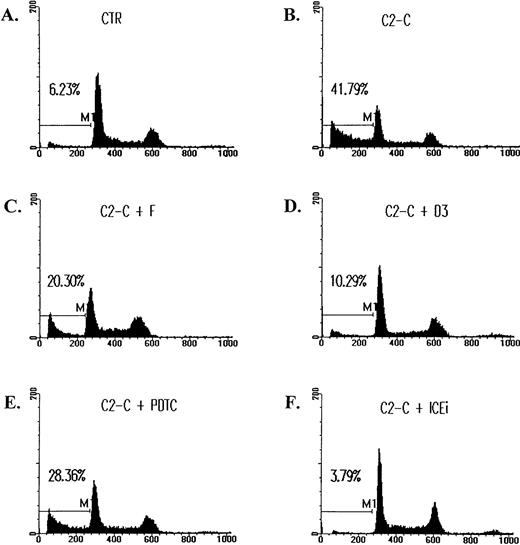
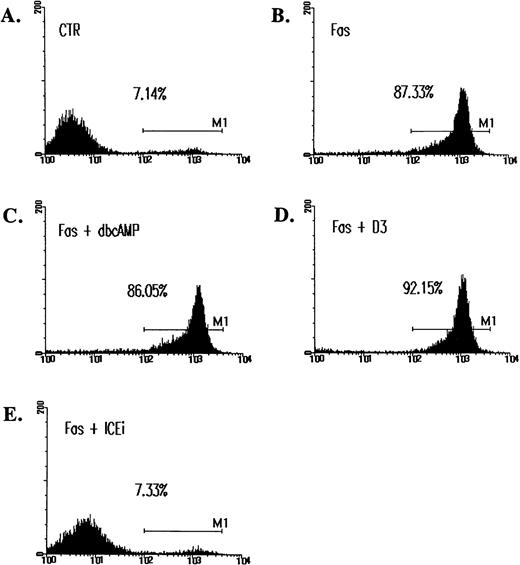
The inhibition of ceramide-induced cell DNA fragmentation. Jurkat T cells were treated with 5 μmol/L of C2-ceramide (C2-C) in the absence or presence of various antagonists for 4 hours, washed with PBS, and fixed with ethanol. DNA content was determined by staining with 20 μg/mL PI and analyzed by FACScan (Becton Dickinson). Fraction of cells with sub-G1DNA content (M1 fraction in the diagram) were assessed with CELLFIT program (Becton Dickinson). CTR, untreated cell control. The inhibitors used were as follows: forskolin (F), 10 μmol/L; C60 D3 (D3), 100 μmol/L; PDTC, 200 μmol/L; Z-VAD-FK (ICEi), 300 μmol/L. The sub-G1 fractions were less than 6% for cells treated with inhibitors only. The exception was PDTC, in which a slightly elevated background death (8%) was observed.
The inhibition of ceramide-induced cell DNA fragmentation. Jurkat T cells were treated with 5 μmol/L of C2-ceramide (C2-C) in the absence or presence of various antagonists for 4 hours, washed with PBS, and fixed with ethanol. DNA content was determined by staining with 20 μg/mL PI and analyzed by FACScan (Becton Dickinson). Fraction of cells with sub-G1DNA content (M1 fraction in the diagram) were assessed with CELLFIT program (Becton Dickinson). CTR, untreated cell control. The inhibitors used were as follows: forskolin (F), 10 μmol/L; C60 D3 (D3), 100 μmol/L; PDTC, 200 μmol/L; Z-VAD-FK (ICEi), 300 μmol/L. The sub-G1 fractions were less than 6% for cells treated with inhibitors only. The exception was PDTC, in which a slightly elevated background death (8%) was observed.
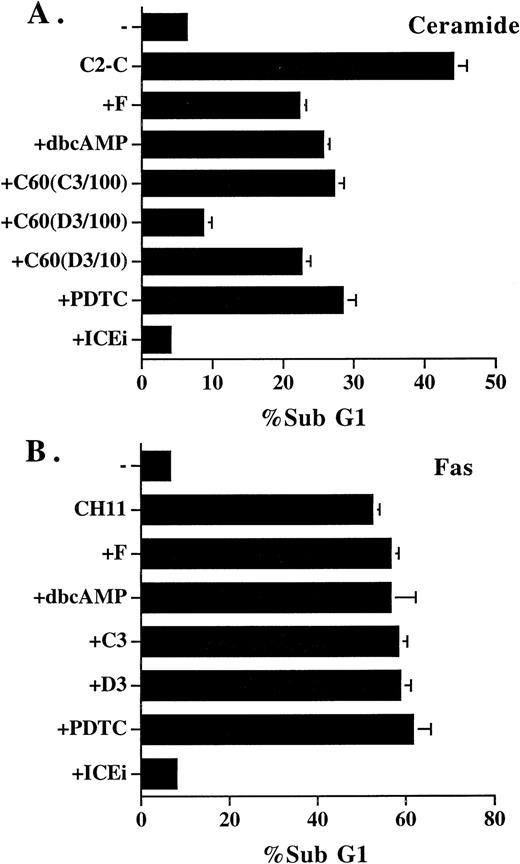
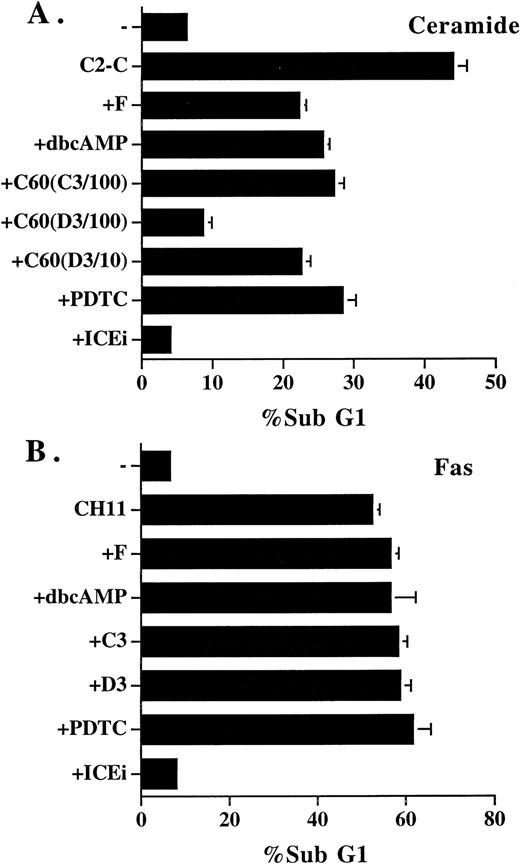
Inhibitors that blocked ceramide-induced DNA fragmentation did not prevent Fas-induced DNA fragmentation. (A) Jurkat cells were treated with ceramide and various inhibitors for 4 hours. The sub-G1 DNA content was quantitated as described in Fig1. Additional inhibitors used were as follows: dbcAMP, 1 mmol/L; C60 (C3/100), 100 μmol/L; C60 (D3/100), 100 μmol/L; C60 (D3/10), 10 μmol/L. (B) Jurkat cells were stimulated with anti-Fas antibody CH11 (125 ng/mL) in the absence or presence of the inhibitors for 14 hours, and the sub-G1 DNA content was determined. The inhibitors used were as follows: forskolin, 50 μmol/L; dbcAMP, 2.5 mmol/L; C60 (C3), 100 μmol/L; C60 (D3), 100 μmol/L; PDTC, 200 μmol/L; Z-VAD-FK (ICEi), 300 μmol/L. None of the inhibitors alone induced DNA fragmentation at the concentrations used, except PDTC, in which an 1% to 2% increase over control was observed. The result is the average of duplicates, with standard deviation shown as an error bar. Those not shown are too small in scale. Experiments were repeated three times with the same results.
Inhibitors that blocked ceramide-induced DNA fragmentation did not prevent Fas-induced DNA fragmentation. (A) Jurkat cells were treated with ceramide and various inhibitors for 4 hours. The sub-G1 DNA content was quantitated as described in Fig1. Additional inhibitors used were as follows: dbcAMP, 1 mmol/L; C60 (C3/100), 100 μmol/L; C60 (D3/100), 100 μmol/L; C60 (D3/10), 10 μmol/L. (B) Jurkat cells were stimulated with anti-Fas antibody CH11 (125 ng/mL) in the absence or presence of the inhibitors for 14 hours, and the sub-G1 DNA content was determined. The inhibitors used were as follows: forskolin, 50 μmol/L; dbcAMP, 2.5 mmol/L; C60 (C3), 100 μmol/L; C60 (D3), 100 μmol/L; PDTC, 200 μmol/L; Z-VAD-FK (ICEi), 300 μmol/L. None of the inhibitors alone induced DNA fragmentation at the concentrations used, except PDTC, in which an 1% to 2% increase over control was observed. The result is the average of duplicates, with standard deviation shown as an error bar. Those not shown are too small in scale. Experiments were repeated three times with the same results.
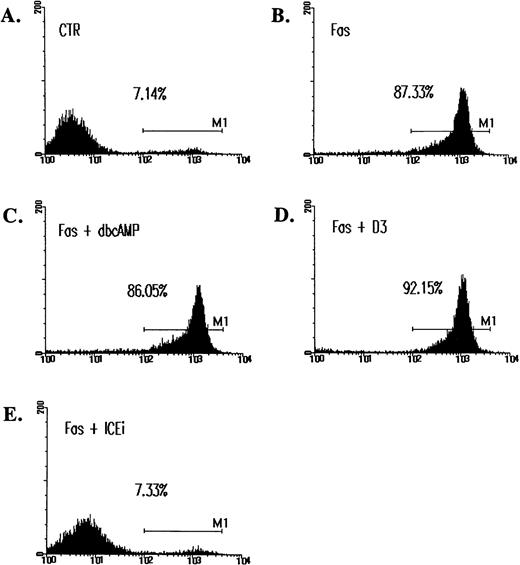
The observation that Fas-initiated cell death cannot be prevented by cAMP is consistent with an earlier report,36 yet is in direct contrast to the prominent inhibitory effect of cAMP recently documented.37 Because DNA fragmentation may not well represent actual apoptosis,38 39 we also quantitated cell death by annexin V staining. Figure 3 shows that Fas-induced apoptosis was associated with an extensive phosphatidylserine translocation, which was clearly not inhibited by cAMP. cAMP is an antagonist for ceramide-induced cell death but not for Fas-initiated apoptosis.
Inability of cAMP and C60(D3) to prevent Fas-induced apoptosis as assessed by annexin V binding. Jurkat cells were treated with CH-11 and inhibitors for 14 hours as described in Fig 2. Cells were washed, resuspended in annexin V-FITC (1 μg/mL; Clontech), and analyzed on FACScan. M1 is designated as the fraction of apoptotic cells. In data not shown, cells treated with ceramide were similarly assayed. cAMP, (C60)D3, and ICEi were used at the same concentration as in Fig 2B.
Inability of cAMP and C60(D3) to prevent Fas-induced apoptosis as assessed by annexin V binding. Jurkat cells were treated with CH-11 and inhibitors for 14 hours as described in Fig 2. Cells were washed, resuspended in annexin V-FITC (1 μg/mL; Clontech), and analyzed on FACScan. M1 is designated as the fraction of apoptotic cells. In data not shown, cells treated with ceramide were similarly assayed. cAMP, (C60)D3, and ICEi were used at the same concentration as in Fig 2B.
C60 and PDTC prevented ceramide-induced cell death but had no effect on Fas-initiated apoptosis.
We have futher observed that two malonic acid derivatives of C60 (carboxyfullerens)27 were antagonist of ceramide-induced cell death (Figs 1D and 2A). The pure carbon sphere of C60 (buckminsterfullerene) is known for its avid reactivity with free radicals, yet the usage is limited by its water insolublility. The two regioisoforms with C3 or D3 symmetry of water-soluble C60 derivatives remain as potent free radical scavengers and are effective antagonists of apoptotic neronal death induced by serum deprivation, glutamate receptor agonists, and amyloid peptide.27 Ceramide-triggered apoptosis was suppressed by 50% in the presence of 10 μmol/L C60 (D3) and was inhibited by 90% with 100 μmol/L C60 (D3). We have repeatedly observed that C60 (D3) was more effective than C60 (C3) in the prevention of ceramide-induced cell death (Fig 2A). In contrast, C60 (D3) and C60 (C3) (at 100 μmol/L each) had no preventive effect on Fas-triggered cell death as analyzed by both sub-G1 fraction and annexin V binding (Figs 2B and 3D). The same contrast was found with metal chelator PDTC. High concentration of PDTC antagonized ceramide-induced apoptosis (Figs 1E and 2A), but did not inhibit Fas-initiated cell death (Fig 2B). Of all the inhibitors analyzed in this study, PDTC was the only reagent that by itself increased spontaneous cell death at the concentration used (1% to 3% over control, data not shown). Because such increase was minute, the differential effect of PDTC on ceramide- and Fas-initiated apoptosis was still prominent. As a control, the potent ICE proteases inhibitor z-VAD-FK completely abolished cell death induced by either ceramide or Fas (Figs 1, 2, and 3), consistent with the notion that both ceramide- and Fas-induced cell death are known to involve caspases such as ICE.7 40
Therefore, we have identified three types of reagents that antagonized ceramide-induced cell death but did not prevent Fas-induced cell death. Between 50% and 90% of ceramide-triggered cell death was suppressed by these inhibitors. If ceramide is the major apoptotic messenger downstream of Fas, a lesser but still prominent inhibition on Fas-induced cell death by these inhibitors should be detectable. The distinct effect on Fas- and ceramide-induced apoptosis suggest that ceramide is dissociated from Fas-induced death cascade.
SEK dominant negative mutant did not interfere Fas-induced cell death.
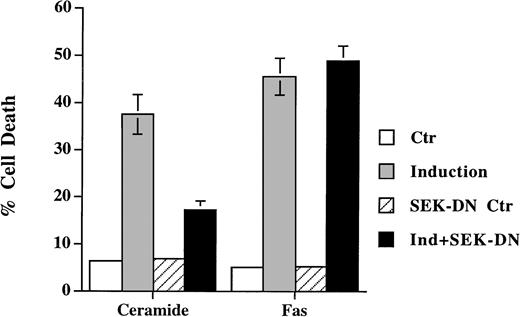
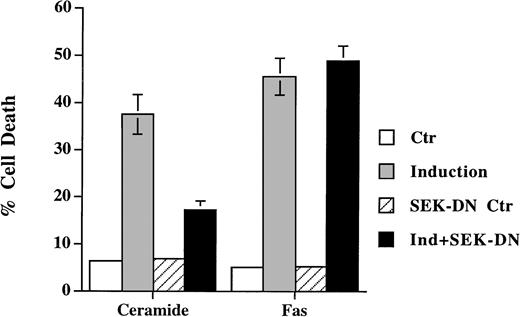
SEK/JNK activation is shown to be essential for ceramide-initiated apoptosis.23 Despite the fact that JNK is activated after Fas engagement, its role in apoptosis is less certain. Lenczowski et al41 demonstrated that the activation of SEK/JNK is downstream of ICE protease and is not required for Fas-induced apoptosis. On the contrary, the prevention of JNK activation by SEK dominant negative mutant is shown to block Fas-induced cell death in L929 and 293 cells.42 We have thus studied the effect of the dominant negative mutant of SEK30 on the same cell when treated with ceramide and anti-Fas. A transient transfection analysis was used to assess the effect on cell death in which SEK mutant was cotransfected with green fluorescence protein. Expression of the dominant negative mutants of SEK blocked the activation of the cotransfected HA-JNK1.32 Ceramide triggered cell death was suppressed by coexpression of SEK (A→L) in Jurkat cells (Fig 4), but the inhibition was less with SEK (K→R) mutant (data not shown). This is in contrast to the inability of SEK (A→L) to interfere with Fas-induced apoptosis (Fig 4). In both Jurkat and CEM cells (not shown for CEM), the inhibition of SEK activation prevented ceramide- but not Fas-induced apoptosis. JNK activation was not essential for Fas-mediated apoptosis at least in these two T-lymphoma cells.
Dominant negative mutant of SEK prevented ceramide- but not Fas-induced apoptosis. Jurkat cells were transfected with 5 μg of either SEK1(A→L) or pEBG vector, together with 5 μg of pEGFP-N1 (Clontech). Thirty-six hours later, cells were untreated (Ctr) or treated with either C2-ceramide (5 μmol/L) or anti-Fas antibody CH11 (100 ng/mL) (Induction). Ceramide-induced cell death was analyzed 6 hours later, whereas Fas-induced cell death were quantitated 12 hours later. Data are the average of duplicates.
Dominant negative mutant of SEK prevented ceramide- but not Fas-induced apoptosis. Jurkat cells were transfected with 5 μg of either SEK1(A→L) or pEBG vector, together with 5 μg of pEGFP-N1 (Clontech). Thirty-six hours later, cells were untreated (Ctr) or treated with either C2-ceramide (5 μmol/L) or anti-Fas antibody CH11 (100 ng/mL) (Induction). Ceramide-induced cell death was analyzed 6 hours later, whereas Fas-induced cell death were quantitated 12 hours later. Data are the average of duplicates.
Activation of NF-κB did not interfere Fas-induced cell death.
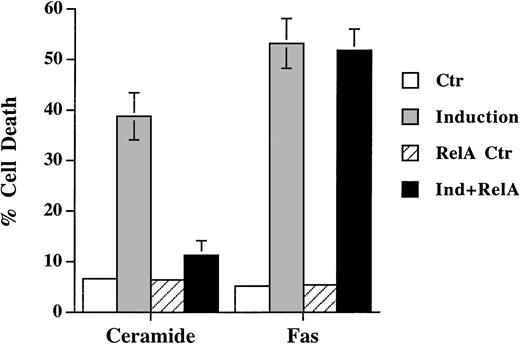
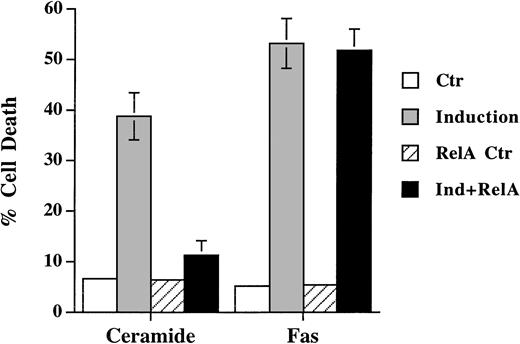
In accordance with the recent finding that activation of NF-κB prevents the apoptotic signal of TNF-α, we also found that NF-κB activation antagonized ceramide-induced DNA fragmentation (Fig 5). Transient expression of NF-κB p65, which led to activation of κB-CAT (not shown), prevented ceramide-induced apoptosis. On the contrary, Fas-induced cell death was not affected by the activation of NF-κB in Jurkat cells. These results further showed that ceramide is not a mediator of Fas-induced apoptosis, for otherwise Fas would be equally sensitive to the inhibition by NF-κB.
Differential sensitivity of ceramide- and Fas-triggered cell death to activation of NF-κB. Jurkat cells were transfected with 5 μg of either CMV-RelA or CMV vector, together with 5 μg of pEGFP-N1 (Clontech). Thirty-six hours later, cells were untreated or treated with either C2-ceramide (5 μmol/L) or anti-Fas antibody CH11 (100 ng/mL). Ceramide-induced cell death was analyzed 6 hours later, whereas Fas-induced cell death were quantitated 14 hours later. Data are the average of duplicates.
Differential sensitivity of ceramide- and Fas-triggered cell death to activation of NF-κB. Jurkat cells were transfected with 5 μg of either CMV-RelA or CMV vector, together with 5 μg of pEGFP-N1 (Clontech). Thirty-six hours later, cells were untreated or treated with either C2-ceramide (5 μmol/L) or anti-Fas antibody CH11 (100 ng/mL). Ceramide-induced cell death was analyzed 6 hours later, whereas Fas-induced cell death were quantitated 14 hours later. Data are the average of duplicates.
In summary, we have presented evidence that ceramide is not essential for Fas-induced cell death. Ceramide-triggered cell death was sensitive to the inhibition of cAMP, yet its blockage did not prevent Fas-initiated apoptosis. Two other antagonists, C60 and PDTC, also demonstrate such selectivity in suppressing ceramide-induced apoptosis. We have further shown that blocking SEK activation diminished ceramide-induced cell death in T lymphomas Jurkat and CEM, yet had no effect on Fas-triggered apoptosis on the same cells (Fig 4). A similar distinction was also found with NF-κB activation (Fig 5). Therefore, a large fraction of Fas-triggered death pathways must be ceramide-independent. Even though ceramide has been suggested as the mediator for Fas-initiated apoptosis, the linkage between ceramide and Fas-induced cell death is less than confirmative. Recent biochemical analysis have questioned the role of ceramide in Fas-induced apoptosis. In one study, the direct measurement of sphingosine-based ceramide failed to detect induction of ceramide up to 2 hours after Fas triggering.25 In another study, ceramide increase was found to be slower than Fas-induced cell death and can be inhibited by Z-VAD-FMK.24 Our results are fully in support of these observations and suggest that ceramide is not the mediator of Fas-initiated apoptosis.
Mixed effect of cAMP and C60 on TNF-α–induced apoptosis.
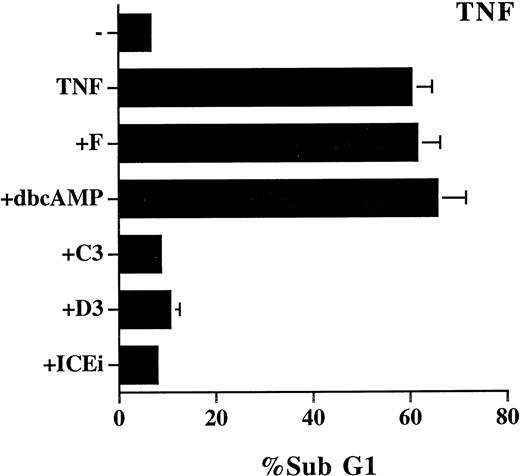
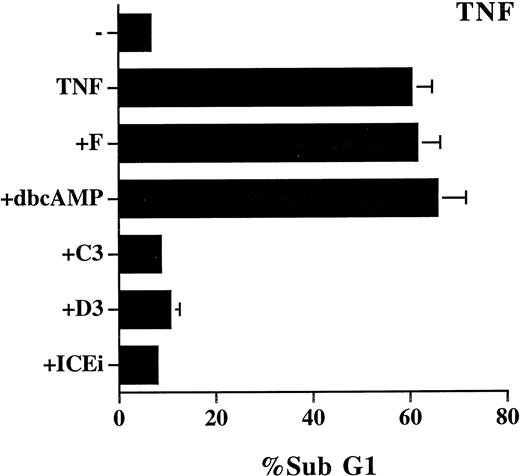
We also examined the effect of cAMP, C60, and PDTC on TNF-α–induced apoptosis, which is reported to be mediated by ceramide.14,16 The study was performed on L929 cells because TNF-α alone does not induce cell death in Jurkat cells. The effect of these inhibitors on TNF-α–induced cell death was not as unequivocal as on Fas-mediated apoptosis. PDTC (5 μmol/L) induced spontaneous cell death (70%) in L929 cells, and its inhibitory effect on TNF-α–induced cell death cannot assessed. TNF-α–triggered apoptosis was completely prevented by 50 μmol/L of C60(C3) and C60 (D3), yet was resistant to treatment with forskolin and Bt2cAMP (Fig 6). In contrast to the NF-κB–independent Fas-mediated apoptosis (Fig 5), TNF-α–induced cell death is also known to be suppressed by the activation of NF-κB.43-45 Some of the discrepancy may be attributed to the difference in apoptotic signaling between Fas and TNF-α previously reported.36 For example, the different sensitivity to C60 (Figs 2 and 6) is well correlated with the involvement of reactive oxgen radicals in TNF-α–induced, but not in Fas-induced, cell death.36 It may also be noted that the mixed effect of the inhibitors on TNF-α–mediated apoptosis is consistent with the observation that ceramide contributes to a fraction, but not all, of TNF-α–induced cell death.35
Mixed effect of cAMP and C60 on TNF-α–induced apoptosis. L929 cells were treated with recombinant TNF-α (250 μg/mL) in the absence or presence of the indicated inhibitors for 18 hours. The sub-G1 DNA content was quantitated as described in Fig 1. The concentration of the inhibitors used were as follows: forskolin, 10 μmol/L; dbcAMP, 1.0 mmol/L; C60 (C3), 50 μmol/L; C60 (D3), 50 μmol/L; Z-VAD-FK (ICEi), 100 μmol/L.
Mixed effect of cAMP and C60 on TNF-α–induced apoptosis. L929 cells were treated with recombinant TNF-α (250 μg/mL) in the absence or presence of the indicated inhibitors for 18 hours. The sub-G1 DNA content was quantitated as described in Fig 1. The concentration of the inhibitors used were as follows: forskolin, 10 μmol/L; dbcAMP, 1.0 mmol/L; C60 (C3), 50 μmol/L; C60 (D3), 50 μmol/L; Z-VAD-FK (ICEi), 100 μmol/L.
The distinction between the Fas-induced apoptotic cascade and ceramide-induced death pathway shows that death processes induced by different stimulation can be clearly independent of each other. The combination of different stimulations may hence be more effective in the induction of apoptosis, especially in tumor cells for therapeutic purposes, notably, ceramide-mediated cell death initiated by TNF-α, daunorubicin, and γ-irradiation, which are reagents widely used in tumor treatment.20-22 The dissociation of Fas-induced death signals from ceramide may indicate an additional dimension in manipulation of apoptosis.
ACKNOWLEDGMENT
The authors thank Dr Leonard Zon for SEK(A→L) and SEK(K→R), Dr Warren C. Greene for κB-TATA-CAT, and Dr Michael Karin for HA-JNK1. We also thank Douglas Platt for editorial correction of the manuscript.
Supported by Grant No. DOH86-HR-508 from the Department of Health, by Grant No. NSC 85-2331-B001-050 M30 from National Science Council, and by a grant from Academia Sinica, Taiwan, Republic of China.
Address reprint requests to Ming-Zong Lai, PhD, Institute of Molecular Biology, Academia Sinica, Nankang, Taipei 11529, Taiwan, Republic of China.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.