Chronic lymphocytic leukemia (CLL) is characterized by a clonal expansion of CD5+ B cells in the peripheral blood. Associated immune aberrations include abnormal Th-cell function and pathogenic autoantibodies. Under most circumstances, CLL B cells do not proliferate in culture and express a limited repertoire of surface antigens, including CD19, CD20, CD23, CD27, CD40, and CD70. In this report, we demonstrate that freshly isolated B cells from a subset of CLL cases constitutively express CD40 ligand (CD40L, CD154), a member of the tumor necrosis factor family which is normally expressed by activated CD4+ T cells and mediates T-cell–dependent B-cell proliferation and antibody production. The degree of CD40L expression varied considerably among the CLL cases examined. CD40L was detected in purified CLL B cells by immunofluorescence flow cytometry, by RT-PCR, and by immunoprecipitation. To demonstrate that CD40L in the CLL B cells is functional, we used irradiated CLL cells to stimulate IgG production by target, nonmalignant B cells in coculture. The CLL B cells induced IgG production by normal B cells to a similar degree as did purified T cells in a process which was partially inhibited by monoclonal antibody to CD40L. This is one of the first reports of CD40L expression in a B-cell tumor. The data suggest that CD40L in the tumor cells may be a factor in the generation of pathologic antibodies by normal B cells in some patients with CLL.
CHRONIC LYMPHOCYTIC leukemia (CLL) is the most frequent adult leukemia in the United States and is usually noted due to an excess of well-differentiated B lymphocytes in the peripheral blood. The diagnosis is confirmed by fluorescence flow cytometry of the lymphocytes, which characteristically express cell surface antigens CD5, CD19, and CD23.1 Although the prognosis for early stage CLL is excellent, many patients with only a lymphocytosis require treatment for and suffer complications from immune manifestations of this disease.2-4 Autoimmune sequelae of CLL include both Coomb's positive hemolytic anemia and thrombocytopenia.5,6Independent of treatment, patients with CLL are vulnerable to infectious pathogens due to impaired humoral immunity characterized by hypogammaglobulinemia.7-10 A variety of T-cell derangements have also been observed, which include a T-cell lymphocytosis, an inverted ratio of CD4+ to CD8+ T cells,11 deficient T-cell help,12,13 and reduced capacity to secrete cytokines such as interleukin-2 (IL-2).14
CD40 ligand (CD40L; CD154, gp39, T-BAM)15 is mainly expressed in activated, CD4+ T cells and, along with CD28, is one of the main costimulatory signals by which specific, antigen-reactive T cells offer help to B cells presenting that antigen in the context of MHC-II.16-19 The receptor on the B cell for CD40L is CD40, a member of the tumor necrosis factor (TNF) receptor superfamily that is expressed in almost all B lymphocytes as well as in macrophage and endothelial cells.20 Ligation of CD40 by its ligand, CD40L, in the germinal center B cell results in inhibition of apoptosis,21 proliferation, differentiation with Ig isotype switching,22-24 and expression of activation antigens, including CD2317,25 and Fas.26,27 As in most B-cell tumors, CLL B cells express CD40,28-31 and several groups have observed that CLL B cells can be induced to differentiate into Ig-secreting cells.32-34
Normal T lymphocytes express CD40L for only a few hours after activation. In T cells derived from patients with systemic lupus erythematosus (SLE), CD40L expression is increased and prolonged.35,36 Several groups have described CD40L expression in pathologic samples from certain T-cell malignancies and also in human T-cell line cells.37,38 Whether human B cells express CD40L is less established. Grammer et al39demonstrated that human peripheral blood B cells could be induced to express CD40L subsequent to stimulation with calcium ionophore and phorbol ester, and that Epstein-Barr virus (EBV)-transformed lymphoblastoid cell line cells (LCLs) express functional CD40L after exposure to B-cell mitogens.39 Additional reports indicate that CD40L is expressed in certain human B-cell tumors40and in B cells from patients with SLE.36
In this work, we analyzed freshly purified CLL B cells and found that, in a subset of CLL patient samples, there was significant CD40L expression in the unstimulated malignant cells. CD40L in the CLL B cells was functional in a costimulatory capacity, in that the CLL cells induced antibody production by nonmalignant, human B cells. These results provide evidence for a possible mechanism of paracrine tumor stimulation in CLL and for excess autoantibody production due to inappropriate B-cell “help.”
MATERIALS AND METHODS
Patient samples.
Approval for the research protocol was obtained from the internal review boards of The New York Hospital and The Hospital for Special Surgery, and verbal, informed consent was obtained before phlebotomy. In each case, the diagnosis of CLL was established by the coexpression of CD5 and CD19 in an expanded, clonal population of peripheral blood lymphocytes. Mononuclear cells were isolated from 10 to 30 mL of fresh, heparinized peripheral blood by Ficoll-Hypaque centrifugation. For most of the experiments, T and B lymphocytes were separated by rosetting with sheep red blood cells according to standard techniques, and the degree of separation was checked by immunofluorescence flow cytometry.
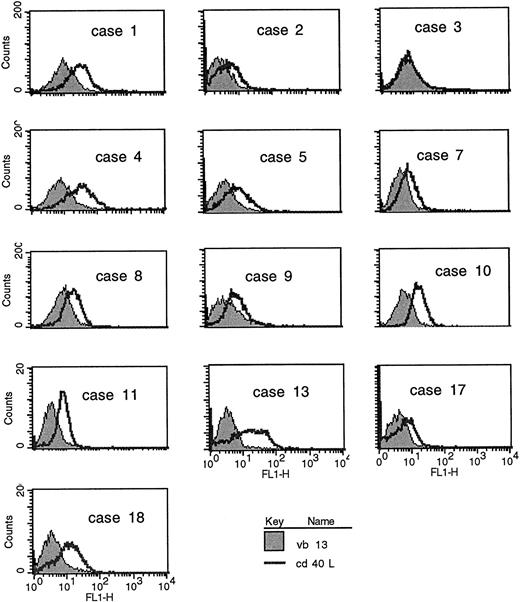
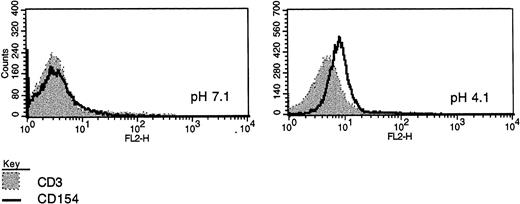
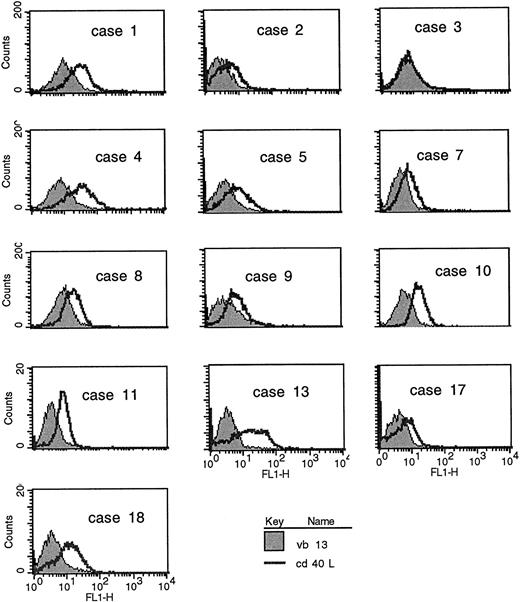
CD40L expression in freshly purified CLL B cells. (A) Peripheral blood mononuclear cells from patients with CLL (all untreated for at least 4 months) were purified by Ficoll-hypaque centrifugation and depleted of T cells by rosetting with sheep red blood cells. Shown are the results of immunofluorescence flow cytometry in consecutive, unselected cases for which the cells could be stained immediately after purification. In each case, greater than 96% of the cells expressed high levels of CD19, and in the majority greater than 99% of the purified cells expressed CD19. As shown, each histogram except for the first represents the fluorescence intensity after incubation with an irrelevant, isotype-matched control antibody (murine IgG1 antihuman TcRVβ13, shaded histograms) or with anti-CD40L (open histograms), followed by goat antimouse-FITC. In the first case, the shaded histogram indicates CD3 expression. (B) Peripheral blood mononuclear cells were washed briefly in neutral (pH 7.1) or acidic PBS (pH 4.1) to remove soluble CD40 from the surface39 before exposure to MoAbs for immunofluorescence cytometry. After washing in neutral PBS, cells from each sample were examined with CD19-FITC and CD3-FITC concomitantly with either CD40L-PE (clone 89-76) or with a negative, control PE-conjugated antibody. In each plot, the linear histogram reflects PE fluorescence intensity after exposure to CD40L-PE and the shaded histogram reflects fluorescence intensity after exposure to the control antibody, in each case analyzed among the gated, CD19-FITC–positive cells.
CD40L expression in freshly purified CLL B cells. (A) Peripheral blood mononuclear cells from patients with CLL (all untreated for at least 4 months) were purified by Ficoll-hypaque centrifugation and depleted of T cells by rosetting with sheep red blood cells. Shown are the results of immunofluorescence flow cytometry in consecutive, unselected cases for which the cells could be stained immediately after purification. In each case, greater than 96% of the cells expressed high levels of CD19, and in the majority greater than 99% of the purified cells expressed CD19. As shown, each histogram except for the first represents the fluorescence intensity after incubation with an irrelevant, isotype-matched control antibody (murine IgG1 antihuman TcRVβ13, shaded histograms) or with anti-CD40L (open histograms), followed by goat antimouse-FITC. In the first case, the shaded histogram indicates CD3 expression. (B) Peripheral blood mononuclear cells were washed briefly in neutral (pH 7.1) or acidic PBS (pH 4.1) to remove soluble CD40 from the surface39 before exposure to MoAbs for immunofluorescence cytometry. After washing in neutral PBS, cells from each sample were examined with CD19-FITC and CD3-FITC concomitantly with either CD40L-PE (clone 89-76) or with a negative, control PE-conjugated antibody. In each plot, the linear histogram reflects PE fluorescence intensity after exposure to CD40L-PE and the shaded histogram reflects fluorescence intensity after exposure to the control antibody, in each case analyzed among the gated, CD19-FITC–positive cells.
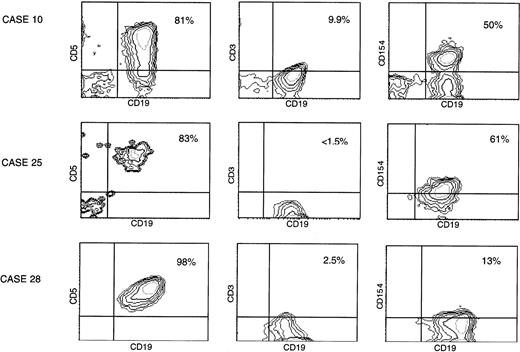
Two-color immunofluorescence demonstrates B-cell expression of CD40L. (A) Unfractionated, peripheral blood cells from patients were analyzed by two-color immunofluorescence flow cytometry immediately after isolation. Shown are three examples including the best case (no. 10), an intermediate case (no. 25), and a low CD40L (CD154) case (no. 28). Each contour plot reflects expression of CD19 on the x-axis, demonstrated with a directly conjugated anti-CD19-FITC MoAb. The plots on the left demonstrate the results after exposure also to a PE-conjugated antibody to CD5, those in the center after exposure to a PE-conjugated antibody to CD3, and those on the right after exposure to a PE-conjugated antibody to CD40L (TRAP clone). The percentage of double-positive cells for each sample is indicated.
Two-color immunofluorescence demonstrates B-cell expression of CD40L. (A) Unfractionated, peripheral blood cells from patients were analyzed by two-color immunofluorescence flow cytometry immediately after isolation. Shown are three examples including the best case (no. 10), an intermediate case (no. 25), and a low CD40L (CD154) case (no. 28). Each contour plot reflects expression of CD19 on the x-axis, demonstrated with a directly conjugated anti-CD19-FITC MoAb. The plots on the left demonstrate the results after exposure also to a PE-conjugated antibody to CD5, those in the center after exposure to a PE-conjugated antibody to CD3, and those on the right after exposure to a PE-conjugated antibody to CD40L (TRAP clone). The percentage of double-positive cells for each sample is indicated.
Cell culture.
Unless otherwise indicated, cells were analyzed immediately subsequent to their purification. For some experiments, as indicated, cells were cultured in C50 media (RPMI supplemented with penicillin-streptomycin with L-glutamine and 10% fetal calf serum [FCS]) only or with IL-2 at 40 U/mL (Hemagen Diagnostics, Waltham, MA) and a 1:60,000 dilution of formalinized Staphylococcus Aureus Cowan 1 (SAC; Pansorbin; Calbiochem, San Diego, CA), recombinant IL-4 at 10 ng/mL (Genzyme Diagnostics, Cambridge, MA), monoclonal antibody (MoAb) to CD40 at 1 μg/mL (IgGκ1; Genzyme), an F(ab′)2preparation of MoAb to human IgM at 0.5 μg/mL (Jackson Immunoresearch Laboratories Inc, West Grove, PA), phorbol myristate acetate (PMA) at 10 ng/mL (Sigma) and ionomycin at 1.25 μg/mL (Calbiochem), or pokeweed mitogen (PWM; Life Technologies, Gaithersburg, MD). For cDNA preparation, Jurkat mutants that constitutively express CD40L (clone D1.1) were used as a source of CD40L.41 These mutant Jurkat cell lines were the kind gift of Dr Seth Lederman (Columbia University, New York, NY). The Ramos Burkitt's lymphoma cell line was obtained from American Type Culture Collection (ATCC; Rockville, MD). For immunoprecipitation experiments, in addition to the CLL samples, we used purified CD4+ polyclonal T-cell lines derived from patients with B-CLL that respond to the toxic shock syndrome toxin-1 (TSST-1; Toxin Technology, Sarasota, FL) superantigen. These cells were maintained in culture with IL-2 at 40 U/mL and were periodically triggered with TSST-1 (used at 10 ng/mL) in the presence of allogeneic antigen-presenting cells, as described previously.27 42
Flow cytometry and immunofluorescence analysis.
Cells were washed in cold Hank's Buffered Saline Solution (HBSS) and incubated with antibodies according to standard techniques. Cell fluorescence was measured using a Becton Dickinson FACScan (Becton Dickinson, San Jose, CA). Cells were assessed for forward and side scatter, and events in the viable cell gate were analyzed for fluorescence intensity using the CellQuest program (Becton Dickinson). For acid washing of cells before analysis, the cells were exposed to acidic PBS (pH 4.1) for 3 minutes at room temperature and then washed twice with normal pH before exposure to MoAbs, as indicated, similar to the technique reported by Grammer et al.39
MoAbs.
Sterile antibodies used in cell culture experiments included anti-CD40 (murine IgG1; Genzyme), anti-CD40L (murine IgG1,, clone M-90; Genzyme), and anti-CD23 (EBVCS2; ATCC). In the blocking coculture experiments, the dose of each antibody used was 5 μg/mL. For single-color fluorescence flow cytometry, the antibodies used were anti-CD3 (OKT3; ATCC), anti-Vβ13 (murine IgGκ1), anti-CD19 (Immunotech, Westbrook, ME), anti-CD40 (Genzyme), and anti-CD40L (clone M-90, murine IgGκ1; Genzyme), in each case followed by goat antimouse Ig-fluorescein isothiocyanate (FITC). Antibodies used for two-color analyses included anti-CD3-FITC (Immunotech), anti-CD40-FITC (Biosource, Camarillo, CA), anti-CD19-FITC (Immunotech), anti-CD3-PE (Biosource), anti-CD5PE (Biosource), anti-CD40L-PE (TRAP clone; PharMingen, San Diego, CA), and anti-CD40L-PE (clone 89-76; Becton Dickinson).
Reverse transcriptase-polymerase chain reaction (RT-PCR).
RNAs were isolated from approximately 1 × 107 cells using the TRIzol reagent (Life Technologies/GIBCO BRL, Grand Island, NY), according to the manufacturer's instructions, and the yield in each case was measured by spectroscopy. For each sample, cDNAs were prepared using approximately 1 μg of RNA, using a reverse transcriptase kit (GIBCO). For CD40L, the primers used were 5′-GGCCATTATGCACAGGTTGAAT-3′ (1100-1122) and 5′-GGGGAGGGAAGAGACTGACAAA-3′ (1469-1489),43which yielded a single amplified CD40L fragment of 396 bp. The quality of each cDNA preparation was checked by amplification of GAPDH sequences using primers 5′-GAAGATGGTATCACCTGGAC-3′ and 5′-GAAGAAGCTGCTGGTGTAGT-3′ (Stratagene, La Jolla, CA), which yield a single, amplified fragment size of 600 bp.
Immunoprecipitation.
Freshly isolated purified CLL B cells were placed in culture under a variety of circumstances. After 36 or 60 hours as indicated, the cells were washed and cell surface proteins biotinylated using Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL).44 Lysates were then subjected to immunoprecipitation overnight at 4°C using 3 μg of antibody to CD40L (TRAP clone; PharMingen) or with 3 μg of a control, isotype-matched antibody to IL-4 (Genzyme), in each case conjugated to protein A Sepharose 4 Fast Flow beads (Pharmacia Biotech, Piscataway, NJ). Precipitated materials were boiled for 4 minutes before sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene difluoride (PVDF) membrane, exposed to horseradish peroxidase (HRP)-conjugated streptavidin (Pierce) for 90 minutes, and then imaged using a chemiluminescence substrate (Amersham, Buckinghamshire, UK).
Ig measurement.
IgG and IgA secreted into the supernatants was measured by a standard enzyme-linked immunoabsorbance assay (ELISA) protocol. In each experiment, standard curves for the particular isotype were determined, and the samples were diluted such that the absorbance readings fell in the linear range of detection. For each circumstance and in each experiment, antibody measurements were determined in triplicate. Results were discounted if the standard deviation among the three triplicate readings represented greater than 15% of the mean or if the result for the diluted sample did not fall within the linear range of the standard curve. IgG production, as shown in Fig 6, reflects the results of single-case analyses, with error bars indicating the standard error for measurements of each experimental circumstance.
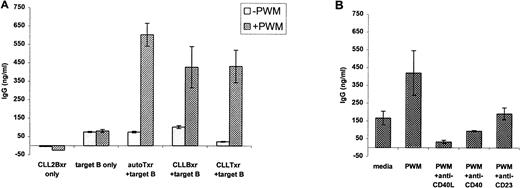
CLL B cells offer T-cell costimulatory type help to target, nonmalignant B cells. (A) CLL B cells induce normal, target B cells to produce antibodies in the presence of PWM. A total of 5 × 104 autologous T cells (AutoTxr) from the normal target B-cell donor, CLL B cells (CLLBxr), or T cells derived from the CLL patient (CLLTxr) were irradiated (2,000 rad) and placed in culture with 5 × 104 target, purified B cells from the normal donor, in the absence or presence of PWM. Control circumstances included 5 × 104 target, unirradiated B cells alone, with and without PWM, and 5 × 104 irradiated CLL B cells, in the absence of target B cells. IgG production was assayed by ELISA from supernatants obtained after 7 days of coculture. (B) CLL B-cell–induced, PWM-dependent IgG production by normal B cells is inhibited by antibody to CD40L. The experiment shown is typical of a series in which 8 × 104irradiated CLL B cells were exposed to MoAbs (final concentration, 5 μg/mL) before coculture with 4 × 104 normal, unirradiated B cells and assayed for IgG production. In this case, CLL B cells from case no. 13 induced greater than 400 ng/mL of IgG in the supernatant at 7 days, in an effect that was reduced in the presence of antibodies including antibody to CD40L.
CLL B cells offer T-cell costimulatory type help to target, nonmalignant B cells. (A) CLL B cells induce normal, target B cells to produce antibodies in the presence of PWM. A total of 5 × 104 autologous T cells (AutoTxr) from the normal target B-cell donor, CLL B cells (CLLBxr), or T cells derived from the CLL patient (CLLTxr) were irradiated (2,000 rad) and placed in culture with 5 × 104 target, purified B cells from the normal donor, in the absence or presence of PWM. Control circumstances included 5 × 104 target, unirradiated B cells alone, with and without PWM, and 5 × 104 irradiated CLL B cells, in the absence of target B cells. IgG production was assayed by ELISA from supernatants obtained after 7 days of coculture. (B) CLL B-cell–induced, PWM-dependent IgG production by normal B cells is inhibited by antibody to CD40L. The experiment shown is typical of a series in which 8 × 104irradiated CLL B cells were exposed to MoAbs (final concentration, 5 μg/mL) before coculture with 4 × 104 normal, unirradiated B cells and assayed for IgG production. In this case, CLL B cells from case no. 13 induced greater than 400 ng/mL of IgG in the supernatant at 7 days, in an effect that was reduced in the presence of antibodies including antibody to CD40L.
RESULTS
CD40L expression is evident in some CLL B cells by immunofluorescence flow cytometry.
CLL B cells from patients with untreated CLL were purified from heparinized peripheral blood samples by Ficoll hypaque centrifugation and separated from the T lymphocytes by rosetting with sheep red blood cells. Of the non-T cells obtained, greater than 96% of the cells in each case expressed CD19. In the majority of cases, greater than 99% of the purified cells were B cells. These cells were analyzed directly, without stimulation, by immunofluorescence flow cytometry for B- and T-cell antigens, including CD40L. In the first series (Fig 1A), B cells from consecutive, unselected cases were purified and examined using an MoAb to CD40L (clone M-90) or, as a negative control, an isotype-matched control antibody to a T-cell receptor (TCR) β chain (anti-Vβ13). As shown, the level of CD40L expression varied considerably among the CLL samples but significant antibody binding was evident in 6 (cases no. 1, 4, 10, 11, 13, and 18) of these first 13 cases analyzed.
To demonstrate definitively that the CD40L signal was not due to a small proportion of T cells in the samples, two-color immunofluorescence flow cytometry was performed using a directly conjugated fluorescent antibody (TRAP clone) on additional unselected samples, also immediately after purification of the cells. As shown in Fig 2, in some cases, the CD19+ (B) cells demonstrated CD40L expression above background, as determined with a PE-conjugated antibody to CD3. Using this technique, the molecule was detected in approximately one fourth of the cases analyzed. In 1 case (no. 10) for which we had demonstrated fairly strong expression in initial analyses (Fig 1), we were able again to obtain fresh cells 6 months later. This two-color immunofluorescence pattern in the upper panel of Fig 2 represents the best example of the two-color stains we have performed. More typical were intermediate results, as shown in the middle panel of Fig 2 (6 cases), or low-level expression, as in the bottom panel (15 cases). Of note, in two-color FACS analyses of greater than 20 fresh specimens from patients with CLL, we have never observed significant CD40L expression in the unstimulated T cells.
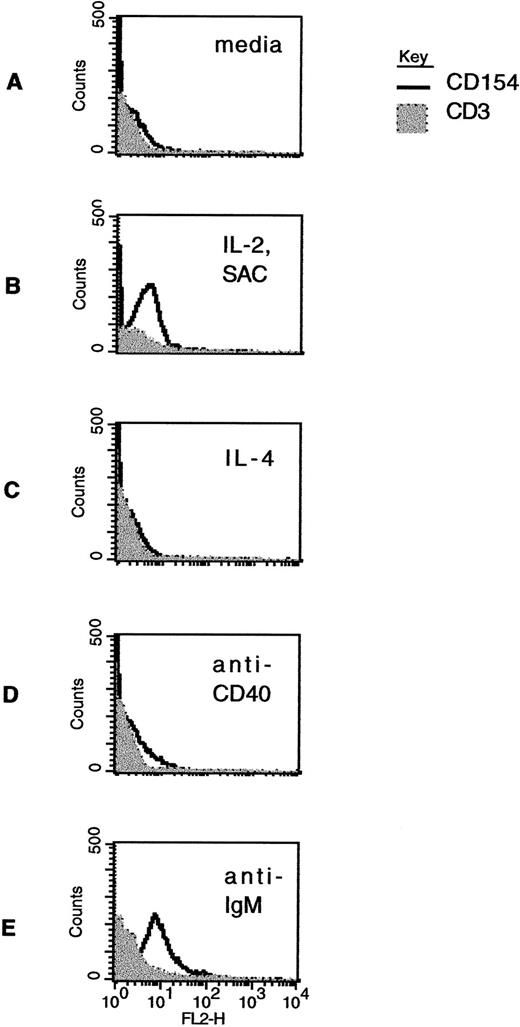
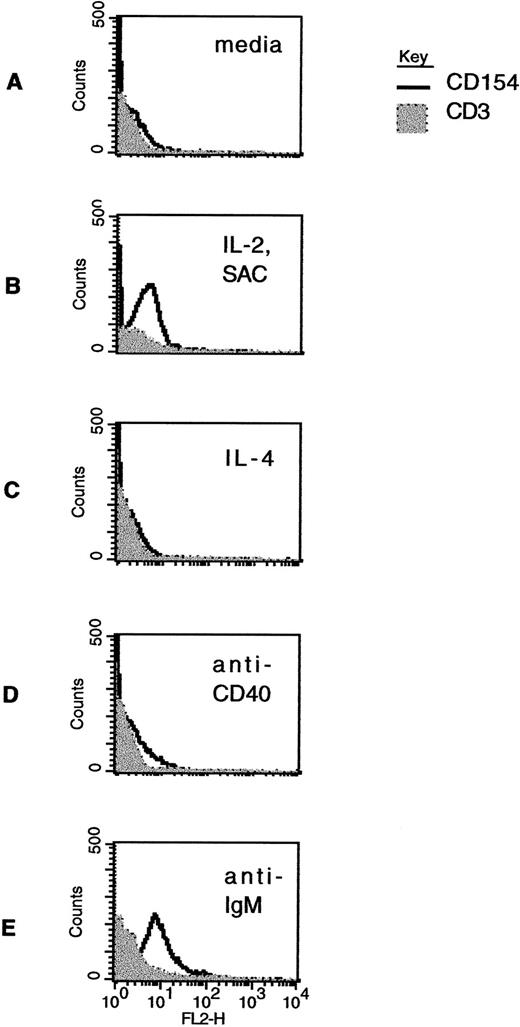
Modulation of CD40L expression in CLL B cells by stimulation in vitro. Freshly isolated CLL B cells were exposed to media alone (A); to the combination of IL-2 (40 U/mL) and SAC (1:60,000 dilution) (B); to IL-4 (10 ng/mL) (C); to a murine MoAb to CD40 (1 μg/mL) (D); or to an F(ab′)2 preparation of antihuman IgM (0.5 μg/mL) (E). After 60 hours in culture, the cells were washed and then analyzed by two-color immunofluorescence cytometry using CD19-FITC and CD3-FITC, together with CD40L-PE (clone 89-76) or with a negative, control PE-conjugated antibody. The linear histograms reflect PE fluorescence intensity after exposure to CD40L-PE and the shaded, background histograms reflect PE fluorescence intensity after exposure to a control antibody, in each case analyzed among the gated, CD19-FITC–positive cells.
Modulation of CD40L expression in CLL B cells by stimulation in vitro. Freshly isolated CLL B cells were exposed to media alone (A); to the combination of IL-2 (40 U/mL) and SAC (1:60,000 dilution) (B); to IL-4 (10 ng/mL) (C); to a murine MoAb to CD40 (1 μg/mL) (D); or to an F(ab′)2 preparation of antihuman IgM (0.5 μg/mL) (E). After 60 hours in culture, the cells were washed and then analyzed by two-color immunofluorescence cytometry using CD19-FITC and CD3-FITC, together with CD40L-PE (clone 89-76) or with a negative, control PE-conjugated antibody. The linear histograms reflect PE fluorescence intensity after exposure to CD40L-PE and the shaded, background histograms reflect PE fluorescence intensity after exposure to a control antibody, in each case analyzed among the gated, CD19-FITC–positive cells.
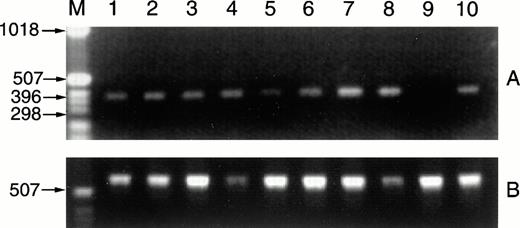
CD40L message is detected in RNAs prepared from CLL samples. Fresh CLL B cells (1 × 107) purified from the peripheral blood of 2 patients were exposed overnight in culture to media alone, to SAC and IL-2 (1:60,000 and 40 U/mL), to PMA and ionomycin (10 ng/mL and 1.25 μg/mL), or to IL-4 (10 ng/mL). After 18 hours, RNAs were isolated, cDNAs were prepared by reverse transcriptase, and CD40L sequences were amplified (A). The RNAs analyzed are as follows: lane 1, CLL case no. 15 B cells with media; lane 2, with IL-2 and SAC; lane 3, with PMA and ionomycin; lane 4, with IL-4; lane 5, CLL case no. 16 B cells with media; lane 6, with IL-2 and SAC; lane 7, with PMA and ionomycin; lane 8, with IL-4. Positive and negative controls for detection of CD40L RNA sequences included RNAs from 1 × 107 D1.1 cells (lane 10, Jurkat mutants that constitutively express CD40L) and Ramos Burkitt's lymphoma B cells (lane 9, these do not express CD40L). The cDNA preparations were evaluated by amplification of GAPDH sequences (B).
CD40L message is detected in RNAs prepared from CLL samples. Fresh CLL B cells (1 × 107) purified from the peripheral blood of 2 patients were exposed overnight in culture to media alone, to SAC and IL-2 (1:60,000 and 40 U/mL), to PMA and ionomycin (10 ng/mL and 1.25 μg/mL), or to IL-4 (10 ng/mL). After 18 hours, RNAs were isolated, cDNAs were prepared by reverse transcriptase, and CD40L sequences were amplified (A). The RNAs analyzed are as follows: lane 1, CLL case no. 15 B cells with media; lane 2, with IL-2 and SAC; lane 3, with PMA and ionomycin; lane 4, with IL-4; lane 5, CLL case no. 16 B cells with media; lane 6, with IL-2 and SAC; lane 7, with PMA and ionomycin; lane 8, with IL-4. Positive and negative controls for detection of CD40L RNA sequences included RNAs from 1 × 107 D1.1 cells (lane 10, Jurkat mutants that constitutively express CD40L) and Ramos Burkitt's lymphoma B cells (lane 9, these do not express CD40L). The cDNA preparations were evaluated by amplification of GAPDH sequences (B).
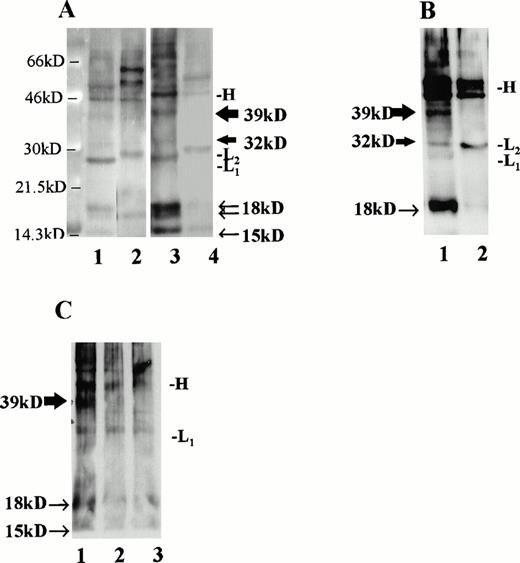
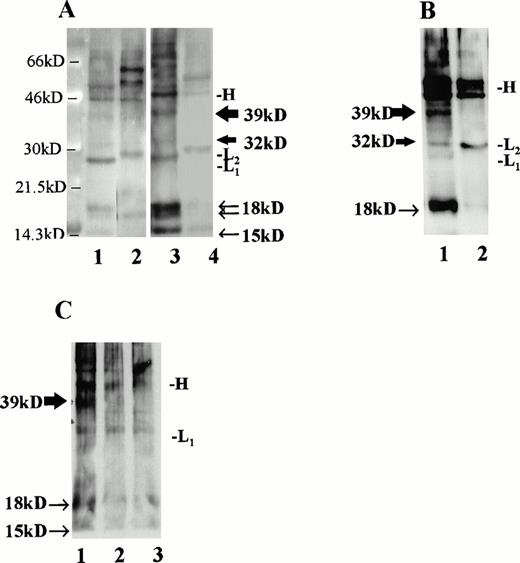
Immunoprecipitation of CLL B-cell lysates with MoAb to CD40L. (A) CLL B cells were cultured with media alone (lanes 1 and 2) or IL-4 at 10 ng/mL (lanes 3 and 4) and, after 36 hours, cell surface proteins were biotinylated and lysates were prepared. For each circumstance, lysates from 6 × 106 cells were subjected to immunoprecipitation using MoAb to CD40L (TRAP clone, lanes 1 and 3) or MoAb to IL-4 (lanes 2 and 4). As shown, the precipitated monoclonal Ig heavy chain (46 kD, designated “H”) and light chains (∼29 kD, designated “L1” or “L2” for the κ chains of the antibody to CD40L and to IL-4, respectively) are evident in addition to the CD40L-specific bands. The 39- and 32-kD bands, corresponding to the two transmembrane forms of CD40L, are indicated with thick arrows, and the soluble forms of CD40L, which include a doublet at 18 kD and an additional, single band at 15 kD, are indicated with narrow arrows. (B) CLL B cells were stimulated for 60 hours in culture with IL-2 and SAC, after which cell surface proteins were biotinylated and lysates prepared. In this experiment, 210 μg of protein were used in each immunoprecipitation, either with MoAb to CD40L (lane 1, TRAP clone) or to IL-4 (lane 2). (C) Lysates from cultured, superantigen-reactive, CD4+ T cells derived from a CLL patient were used for immunoprecipitation of CD40L after biotinylation of cell surface proteins. Lane 1 shows a 39-kD band after lysates from 1 × 106 T cells were used for immunoprecipitation. In lanes 2 (5 × 105 T cells) and 3 (2.5 × 105 T cells), the band is not evident.
Immunoprecipitation of CLL B-cell lysates with MoAb to CD40L. (A) CLL B cells were cultured with media alone (lanes 1 and 2) or IL-4 at 10 ng/mL (lanes 3 and 4) and, after 36 hours, cell surface proteins were biotinylated and lysates were prepared. For each circumstance, lysates from 6 × 106 cells were subjected to immunoprecipitation using MoAb to CD40L (TRAP clone, lanes 1 and 3) or MoAb to IL-4 (lanes 2 and 4). As shown, the precipitated monoclonal Ig heavy chain (46 kD, designated “H”) and light chains (∼29 kD, designated “L1” or “L2” for the κ chains of the antibody to CD40L and to IL-4, respectively) are evident in addition to the CD40L-specific bands. The 39- and 32-kD bands, corresponding to the two transmembrane forms of CD40L, are indicated with thick arrows, and the soluble forms of CD40L, which include a doublet at 18 kD and an additional, single band at 15 kD, are indicated with narrow arrows. (B) CLL B cells were stimulated for 60 hours in culture with IL-2 and SAC, after which cell surface proteins were biotinylated and lysates prepared. In this experiment, 210 μg of protein were used in each immunoprecipitation, either with MoAb to CD40L (lane 1, TRAP clone) or to IL-4 (lane 2). (C) Lysates from cultured, superantigen-reactive, CD4+ T cells derived from a CLL patient were used for immunoprecipitation of CD40L after biotinylation of cell surface proteins. Lane 1 shows a 39-kD band after lysates from 1 × 106 T cells were used for immunoprecipitation. In lanes 2 (5 × 105 T cells) and 3 (2.5 × 105 T cells), the band is not evident.
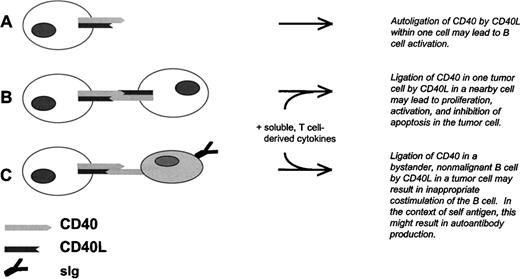
Model for CLL B-cell tumor growth and antibody production by nonmalignant B cells due to aberrant expression of CD40L in CD40+ CLL B cells. (A) Autoligation of CD40 by CD40L at the CLL B-cell surface, or possibly secreted by the same cell, results in intracellular tumor cell signaling. (B) Ligation of CD40 in one CLL B cell by CD40L in a nearby CLL cell results in tumor cell activation, proliferation, and inhibition of apoptosis. This process is likely to depend also on cytokines derived from nearby T lymphocytes. (C) Ligation of CD40 in a bystander B cell by CLL B-cell–derived CD40L results in activation, differentiation, and antibody production by the nonmalignant cell. As in (B), this process is likely to depend on cytokines derived from nearby T lymphocytes, and on the presence of antigen.
Model for CLL B-cell tumor growth and antibody production by nonmalignant B cells due to aberrant expression of CD40L in CD40+ CLL B cells. (A) Autoligation of CD40 by CD40L at the CLL B-cell surface, or possibly secreted by the same cell, results in intracellular tumor cell signaling. (B) Ligation of CD40 in one CLL B cell by CD40L in a nearby CLL cell results in tumor cell activation, proliferation, and inhibition of apoptosis. This process is likely to depend also on cytokines derived from nearby T lymphocytes. (C) Ligation of CD40 in a bystander B cell by CLL B-cell–derived CD40L results in activation, differentiation, and antibody production by the nonmalignant cell. As in (B), this process is likely to depend on cytokines derived from nearby T lymphocytes, and on the presence of antigen.
Because there is some evidence that detection of CD40L by immunofluorescence flow cytometry may be dependent on the particular antibody used and that recognition of CD40L at the cell surface may be hindered by the presence of soluble CD40,45 we elected to evaluate CD40L in CLL B cells using an additional MoAb (clone 89-76). In addition, we examined the effect of acid-washing CLL B cells with PBS (pH 4.1) before exposure to MoAb to CD40L for FACS analysis, according to the technique of Grammar et al,39 to remove soluble CD40 that may be adherent to the B cell, particularly as may occur in CD40-expressing B-cell tumors such as CLL.45 Using the 89-76 clone, 3 of 6 cases analyzed were positive, including 2 that were also positive with the TRAP clone. The effect of acid-washing was similar to that reported by Grammer et al39 using lymphoblastoid B cells and is shown in Fig 1B.
CLL B-cell expression of CD40L is impacted by stimulation in vitro.
In cases that were positive, CD40L expression diminished rapidly over time in culture with media only, such that the signal was less intense 48 hours after isolating the cells, became further diminished after 72 hours, and was undetectable thereafter (data not shown). However, some stimuli did appear to augment CD40L in cases that had initially undetectable levels. Figure 3 shows CD40L expression in CD19+ cells from a single case that were treated for 48 hours after purification under a variety of experimental circumstances. As shown in Fig 3B, the combination of IL-2 and SAC in culture was associated with increased CD40L expression in the CLL B cells. This effect was observed in each of 5 cases for which we analyzed this effect. In this particular sample, IL-4 did not result in augmented CD40L expression (Fig 3C), but the effect of this cytokine has varied among the 6 cases analyzed so far. Other B-cell stimulants also appear to augment CD40L expression in the CLL cells. For example, signaling induced by MoAb to CD40 (Fig 3D) and through ligation of the B-cell surface antigen receptor (Fig 3E) both resulted in increased CD40L expression at the cell surface.
CD40L message is readily detected in purified B cells from CLL patients.
B cells were purified from 2 clinical CLL samples and placed in culture for 16 hours before RNA extraction for analysis by RT-PCR. As shown in Fig 4, CD40L message was readily detected in both of the CLL samples exposed in vitro to media alone (lanes 1 and 5). In addition, there was an increase in message in cells from the second case after exposure to the combination of IL-2 and SAC (lane 6), to PMA and ionomycin (lane 7), and to IL-4 (lanes 8), as compared with the amplified signal for the housekeeping gene GAPDH. These results correlated well with those obtained by immunofluorescence flow cytometry.
CD40L can be detected in CLL samples by immunoprecipitation.
MoAb (TRAP clone) was used to precipitate CD40L from several samples. For these experiments, lysates were prepared after biotinylation of cell surface proteins. Material from 6 × 106 cells was precipitated with either antibody to CD40L or with an isotype-matched control antibody to IL-4 (Fig 5A). As shown, we observed major bands at 39 and 32 kD corresponding to the transmembrane forms of CD40L,46,47 which in this case were most evident after exposure of the cells to IL-4 (lane 3). In addition, a doublet was evident at 18 kD and another, single band at 15kD, as have been reported48 and attributed to soluble CD40L.49 The CD40L bands were not seen after immunoprecipitation with a control antibody to human IL-4 (lanes 2 and 4). In a separate experiment, to control for the amount of protein in each sample rather than cell number, CLL cells were stimulated for 60 hours with IL-2 and SAC and then examined for CD40L using 210 μg of biotinylated protein in each sample (Fig 5B). As shown, there is a prominent 39-kD band apparent in the stimulated cell lysate precipitated with antibody to CD40L, but not with the control antibody.
Finally, to determine that the CD40L signal was not due to a small fraction of T cells in each sample, we used activated polyclonal CD4+ T cells, derived from a CLL patient and triggered periodically with TSST-1 and IL-2, for immunoprecipitation after biotinylation according to the same technique. These CLL patient-derived T cells bear an activated phenotype and express CD40L.49a As shown in Fig 5C, CD40L was not detected by immunoprecipitation in our system when 5 or 2.5 × 105 biotinylated T cells were used (lanes 2 and 3), but was readily observed after 1 × 106 T cells were used (lane 1). Thus, the absolute minimum number of T cells required to provide the signal we observed in the CLL samples would be greater than 8% of the 6 × 106 cells used in each immunoprecipitation, which was not the case. In the cells used for the experiment in Fig 5A, the proportion of B cells (CD19+, CD3−) was 97% after culture with media alone and 98% after exposure to IL-4. In Fig 5B, the proportion of B cells was greater than 99.5% at the time of lysate preparation.
CLL B cells do not proliferate spontaneously in vitro.
Other investigators have determined that ligation of CD40 results in CLL B-cell activation31,49 and proliferation.50In our laboratory, we performed thymidine incorporation assays to examine CLL B-cell proliferation in culture under a variety of circumstances. Given that the CD40/CD40L molecular pair is an important modulator of CLL B cell growth, one might anticipate that the CD40, CD40L-expressing tumor cells would proliferate spontaneously in culture. However, as has been observed by other investigators, we did not observe significant thymidine incorporation in any of the 6 cases we studied without the addition of irradiated, CD40L+ T cells (data not shown). These negative results suggest that other factors besides CD40L, such as additional signals provided by accessory molecules or T-cell–derived cytokines, are necessary for B-cell proliferation in the context of CD40 ligation.
Capacity of CLL B cells to offer help via CD40L.
Because CD40L has a central role in T-cell–induced B-cell differentiation and antibody formation, we investigated the capacity of CLL B cells to trigger antibody production by normal, bystander B cells in coculture. For these experiments, we used as targets B cells purified from the peripheral blood of healthy volunteers. Effector cells, used to stimulate antibody production by the target B cells, included CLL B cells, autologous T cells obtained from the normal donor in each experiment, and also T cells purified from the CLL patients. These effector cells were irradiated and then placed in culture with the target B cells as indicated in Fig 6. Supernatants were removed and analyzed for IgG and IgA secretion by ELISA.
The CLL B cells were variable in their capacity to stimulate antibody production, but in general the effects were most clear in the presence of B-cell mitogens such as PWM. As shown in Fig 6A, the unirradiated target B cells alone did not generate significant levels of IgG, even with the addition of PWM. This indicates that the IgG produced was not simply due to a few contaminating T cells in the target B-cell preparation. In addition, the irradiated, effector CLL B cells alone did not produce IgG in the presence of PWM. However, when irradiated effector cells were added to the target B cells, the degree of help provided by the CLL B cells was of the same order of magnitude as that provided by purified, autologous T cells. Although it is possible that some contaminating tumor-derived T cells were present among the irradiated, effector CLL B cells, those would be unlikely to account for a signal so strong as that obtained with 100% T cells derived from the patient's blood. Therefore, it is highly unlikely that this result is due to a small percentage of CLL patient-derived T cells that may have been present in the effector B-cell population.
Figure 6B shows the effects of a blocking antibody to CD40L (clone M-90) in this coculture system. Here, the irradiated CLL B cells were exposed either to antibody to CD40L, to CD40, or to CD23 before coculture with the target B cells. As shown, each of the antibodies was associated with reduced IgG production, but the result was most marked with antibody to CD40L. Similar assays for IgA demonstrated only low level secretion of IgA (on the order of 100 to 150 ng/mL), even in the presence of PWM (data not shown). This result is consistent with a complex, redundant system in which multiple factors, including CD40L, instigate antibody production and isotype switching in B cells. Our data demonstrate that under some circumstances, costimulatory function and isotype switching, normally induced by T cells, can be mediated by malignant CLL B lymphocytes.
DISCUSSION
CD40L is primarily expressed in activated T cells and has not been considered a regular feature of any B-cell tumor. We have observed that freshly purified B cells from a significant proportion of CLL cases express CD40L and can induce antibody production by target, nonmalignant B cells in a process that is partially blocked by antibody to CD40L. Although previous investigators have established that the CD40/CD40L molecular pair is an important modulator of CLL B-cell growth,28,49,50 we and other investigators have observed that CLL B cells do not proliferate spontaneously in vitro.49-52 Therefore, the extent to which CLL B-cell–derived CD40L impacts tumor growth may depend on costimulation via accessory molecules and the presence of T-lymphocyte–derived cytokines, such as IL-4.50 Taken together, the data and the literature suggest a model for CLL tumor growth and associated immune aberrations due to tumor cells that, at least in some cases, express both CD40 and CD40L (Fig 7).
In work quite similar to our findings, Nusslein et al53demonstrated that irradiated or even formaldehyde-fixed CLL B cells have the capacity to stimulate IgE production by nonmalignant B cells in the presence of IL-4. However, our data contrast with those of Brugnoni et al,54 who stained non-T cells from the peripheral blood of 11 CLL patients and did not detect CD40L expression in those cells. It is possible that the negative result found by Brugnoni et al54 was due to the heterogeneity of CD40L expression in CLL and its subtle expression in most cases. In addition, those investigators used only the TRAP clone for CD40L, which in our laboratory offered a positive signal in only one fourth of the cases. Closer to our work, Cerutti et al55 analyzed human B-1 (CD5+) B cells and noted above-background CD40L expression, at levels similar to what we have observed in the human CLL cells. Most recently, while this report was under review, Trentin et al56 described CD40L expression in some CLL cases by FACS analysis and by RT-PCR. In this report, we include data obtained using three distinct MoAbs and, in addition, demonstrate that the signal for CD40L in the B cells can be modulated by acid-washing the cells. Thus, the results may depend on the particular reagent used and also on the presence of soluble CD40 in the sample.
Ranheim et al57 reported that CLL B cells express both CD27 and its ligand, CD70, and that expression of these molecules in the CLL cells is affected by ligation of CD40. Thus, although CLL cells are considered as resting B cells, unable to proliferate in vitro without T-cell–derived signals, there may be a dynamic aspect of the tumor in vivo due to infiltrating CD4+ T cells that provide cytokines and antigen-dependent costimulatory signals. Based on the observations that CD40 ligation results in NF-κB activation58 and that constitutive, high NF-κB activity is observed in 293 cells expressing both CD40 and CD40L,59even low-level, transient expression of CD40L by the CD40-expressing tumor cells may have profound effects on tumor cell growth, differentiation, and apoptosis.
We did not have sufficient clinical information to correlate CD40L expression in the CLL B cells with clinical features of autoimmune disease in these patients. Given that the Fas antigen and its ligand have been identified as critical mediators of apoptotic deletion of peripheral, autoreactive B cells,60 we speculate that the failure to delete autoreactive B cells in patients with CLL might be due to failed Fas-mediated apoptosis in those nonmalignant cells. Specifically, CD40L-stimulated B cells induced to express the Fas antigen would be protected from Fas-mediated deletion by cross-linking of the surface antigen receptor.26 Thus, most B cells that receive inappropriate, CLL B-cell–derived help but bear Ig that is not engaged by antigen would undergo apoptosis, which might account for the hypogammaglobulinemia characteristic of this disease. However, B cells responsive to a particular viral or other infectious agent present in the host, or those reactive with autologous structures, might survive Fas ligation subsequent to CD40 engagement and differentiate into antibody-producing cells.
ACKNOWLEDGMENT
The authors thank the CLL patients and their physicians for providing clinical samples. We thank Dr Radha K. Vakkalanka for expert guidance in RT-PCR.
Supported with funds from The William H. Kearns Foundation, by The Dorothy Rodbell Cohen Foundation for Sarcoma Research, and by National Institutes of Health Grants No. R29CA71589 and P50 AR42588.
Address reprint requests to Elaine J. Schattner, MD, Room C-640, Cornell University Medical College, 1300 York Ave, New York, NY 10021.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.