The aim of this study was to identify the molecular mechanisms involved in neutrophil adhesion to immobilized platelets with particular focus on the possible existence of a juxtacrine system for neutrophil-platelet interactions. Platelets were immobilized onto collagen (type I)-coated coverslips that were placed in a flow chamber and neutrophils were perfused across these confluent monolayers at a shear stress of 1 to 4 dynes/cm2. Neutrophils rolled, and a significant proportion (25% to 50%) adhered to platelet monolayers. P-selectin was expressed in very large quantities on the surface of platelets and mediated all of the rolling, whereas the β2-integrin mediated firm adhesion. An activation mechanism for adhesion was necessary inasmuch as fixed neutrophils continued to roll on immobilized platelets, but did not adhere. Platelets adherent to collagen produced significant levels of platelet-activating factor (PAF). Accordingly, the firm adhesion of neutrophils to platelets was significantly inhibited by a PAF receptor antagonist (WEB 2086). Treatment of only the platelets with acetylhydrolase, which converts membrane-associated PAF to lyso-PAF, prevented 60% of the adhesion. These data suggest that PAF, on the surface of platelets, mediated a significant portion of the adhesive interaction. Addition of some selectin-binding carbohydrates (fucoidan or soluble SLEx analogs but not dextran sulfate) to the platelets caused rolling neutrophils to immediately adhere, an event that was not observed on histamine or thrombin-treated endothelium or P-selectin transfectants. These data support the view that a juxtacrine activation process exists on immobilized platelets for neutrophils. This process can be greatly enhanced on platelets and may involve a signaling mechanism through P-selectin.
THE RECRUITMENT OF neutrophils from the mainstream of the vasculature to the extravascular space is an early and essential requisite in acute inflammation. This is a multistep cascade of events in which the initial interaction is mediated by P-selectin and results in the rolling of neutrophils along the length of the endothelium.1,2 Pro-inflammatory molecules such as platelet-activating factor (PAF) are rapidly synthesized and coexpressed with P-selectin on the surface of the endothelium in response to such mediators as histamine or thrombin.3,4 PAF interacts with its receptor on the surface of neutrophils and functions to activate the CD11/CD18 glycoprotein complex.5 This adhesion molecule then supports the stable or firm adhesion of neutrophils to counterreceptors on the surface of endothelium. The tethering of neutrophils by P-selectin to the surface of endothelium is thought to be essential in allowing PAF to interact with its receptor and thereby induce neutrophil activation/adhesion. This cascade of events has been termed the juxtacrine system for neutrophil adhesion.5
In addition to endothelium, neutrophils interact with other cell types including platelets. Platelets stimulated with soluble stimuli, such as thrombin, will also express P-selectin and use this adhesion molecule to adhere to neutrophils.6 This close cell-cell interaction permits platelets to activate neutrophils and also permits for transcellular biosynthesis of certain mediators (eg, leukotrienes) that would not be produced in significant quantities by either cell alone.7 When thrombin-activated platelets are immobilized to glass, under flow conditions, these cells can also tether neutrophils to their surface via P-selectin and perhaps through L-selectin.8,9 This interaction has been described in vivo for atherosclerosis, thrombosis, and inflammation and is likely to occur when platelets adhere to either injured endothelium or exposed extracellular matrix. The platelet-induced neutrophil recruitment may be particularly important in high shear situations in which leukocytes may tether to platelets but not to endothelium.9 In addition to rolling along platelets, some investigators have reported that rolling neutrophils will adhere particularly if an exogenous chemotactic stimulus, such as N-formyl-methionyl-leucyl phenylalanine (fMLP) or a phorbol ester, phorbol myristate acetate (PMA), was applied.9-11 The fMLP- or PMA-stimulated neutrophils adhere to platelets via CD11b/CD18, suggesting that there are as yet unidentified ligands on platelets for theB2-integrin.9-11 The spontaneous conversion from rolling to adhesion of neutrophils on platelets, in the absence of exogenous stimuli, has been observed.8 10However, the endogenous activating mechanism(s) involved is entirely unknown.
The first objective of this study was to establish that the collagen-induced contact-activation of platelets was sufficient (in the absence of exogenous stimuli) to recruit neutrophils to roll and firmly adhere to the platelet monolayer. The second objective was to identify the molecular mechanisms underlying the neutrophil recruitment, with particular emphasis on the possibility that a juxtacrine system for neutrophil adhesion exists on platelets to cause rolling neutrophils to adhere. We observed that adherent platelets possessed similar characteristics to that described by Zimmerman et al4 for activated endothelium; P-selectin–dependent neutrophil rolling was spontaneously converted to CD18-dependent adhesion via endogenous PAF production. However, we also identified a critical difference, ie, administration of selectin-binding carbohydrates to the platelets caused rolling neutrophils to immediately adhere, an event not observed on histamine or thrombin-treated endothelium or P-selectin transfectants. This latter point may suggest that binding of selectin ligands to platelet P-selectin directly enhances neutrophil adhesion to this substratum.
MATERIALS AND METHODS
Neutrophil isolation.
Human neutrophils were harvested from acetate-citrate-dextrose–anticoagulated (ACD) venous blood collected from healthy donors, as previously described,12 with minor modification. All isolation steps were performed at room temperature. Briefly, neutrophils were purified by dextran sedimentation (Dextran 250 000; Spectrum Chemicals, Gardena, CA) followed by centrifugation through a density gradient (6.07% Ficoll Type 400; Sigma, St Louis, MO) with 10% hypaque sodium (Winthrop-Breon, Markham, Ontario, Canada). Isolated neutrophils were resuspended in Hanks' balanced salt solution (HBSS; with Ca2+ and Mg2+) at a concentration of 1 × 106 neutrophils/mL. This yielded neutrophils that were 97% pure and 95% viable.
Platelet isolation and monolayer preparation.
Platelets were isolated from donors by spinning the whole blood at 900 RPM for 10 minutes. The supernatant (approximately 10 mL from 30 mL whole blood) was reconstituted to 50 mL with 2 mL of ACD anti-coagulant and phosphate-buffered saline and spun for 10 minutes at 2,100 RPM. The supernatant was discarded and the pellet resuspended in 5 mL of HBSS. The platelets were diluted to a final concentration of 2 × 108 platelets/mL with HBSS, placed on ice, and used within 60 minutes of isolation.
Glass coverslips were incubated with collagen type-I for 1 hour at 37°C. One milliliter of the platelet suspension was then placed on the collagen-treated coverslips and allowed to settle for 1 hour at 37°C. This approach generated confluent monolayers of platelets. The coverslips were then gently rinsed in HBSS and incorporated into the laminar plate flow chamber.
Protocol.
A Perspex parallel-plate flow chamber similar to the one described by Lawrence et al13 was used. The platelet monolayers were used as the chamber's bottom plate. The chamber was placed in a thermoregulated plexiglass box that was maintained at 37°C by an electric heating element. The neutrophil suspension was placed in a 37°C water bath 5 minutes before perfusion and was drawn through polypropylene tubing into the chamber via a syringe pump. The experiment was visualized by an inverted phase contrast microscope, which allowed the neutrophils to be seen without fluorescent labeling. A camera was attached to the microscope and its output was directed through a VCR to a television monitor. The experiments were recorded for playback analysis. Neutrophils were perfused over the platelet monolayers and the neutrophil-platelet interactions were documented over 20 minutes. As well, neutrophils were perfused at 1, 2, and 4 dynes/cm2 and, based on these results, all subsequent experiments were performed at 2 dynes/cm2.
Molecular mechanisms underlying the neutrophil-platelet interactions were assessed. The monoclonal antibody (MoAb) G1 (generously provided by Dr R.P. McEver, University of Oklahoma, Oklahoma City, OK) directed against P-selectin was used at a concentration of 2 μg/mL. Fucoidan, a selectin-binding carbohydrate known to inhibit P-selectin function, was used at a concentration of 100 μg/mL (higher concentrations activated neutrophils). In fucoidan posttreatment experiments, untreated neutrophils were allowed to interact with the monolayer for 10 minutes. After 10 minutes, fucoidan was added to the neutrophil suspension and perfused across the platelet monolayers. Commercially available fucoidan contained high levels of endotoxin, and so fucoidan was cleaned before use. Briefly, the fucoidan (250 mg) was dissolved in 12.5 mL of distilled water and the pH was adjusted to 7.0 with 0.1 mol/L NaOH and an equal volume of 1 mol/L NaCl was added. The fucoidan salt solution (10 mg/mL fucoidan in 0.5 mol/L NaCl) was treated with 0.5 g of activated charcoal and filtered using Whatman no. 1 paper (Whatman, Maidstone, UK) to remove the activated charcoal. The activated charcoal treatment/filtering was repeated twice more. This solution was filtered through a 0.2-μm cellulose acetate filter to remove residual activated charcoal. The fucoidan solution was exhaustively dialyzed against water, lyophilized, and dissolved at 10 mg/mL in distilled water. The final solution was filtered through a 0.2-μm cellulose membrane and then lyophilized. This procedure generated 175 mg of product, which had less than 10 endotoxin units/mg. This method is based on methods to remove endotoxin from commercial heparin. Polymixin B was not used because it did not remove endotoxin from the fucoidan in part because of the highly negatively charged properties of the carbohydrate.
In other experiments, we used SLEx with an aliphatic aglycone attatched in β-glycosidic linkage to the reducing sugar (500 μmol/L; Glycomed Inc, Alameda, CA), as previously described.14 To assess the firm adhesion, MoAb IB4 (anti-CD18 MoAb) was used at a concentration of 20 μg/mL and was incubated with the neutrophils at 37°C for 10 minutes before perfusion. In additional experiments, an RGD peptide that binds to CD41 (GP IIb/IIIa) was used at 100 μmol/L (RGDS; Peninsula Laboratories Inc, Belmont, CA). In a complementary experiment, platelets from a Glanzmann's thrombasthenia patient (devoid of GP IIb/IIIa) were used to assess the importance of GP IIb/IIIa (CD41) in the neutrophil-platelet interaction.
To determine whether PAF or interleukin-8 (IL-8) were inducing the rolling neutrophils to adhere, the PAF receptor antagonist, WEB 2086 (20 μg/mL; Boehringer, Indianapolis, IN) or the MoAb against IL-8, MoAb 208 (10 μg/mL; R&D, Minneapolis, MN) were used. WEB 2086 or MoAb 208 was incubated with the neutrophil suspension for 5 minutes at 37°C before perfusion. Additionally, recombinant PAF acetylhydrolase (kindly provided by Dr G.M. Peterman, ICOS Corp, Seattle, WA), which de-acetylates PAF to lyso-PAF, was used (6 μg/mL). Platelet monolayers were pretreated with PAF-AH for 30 minutes at 37°C and washed, and then neutrophils were perfused over the platelet monolayer as previously described.
To compare neutrophil rolling and adhesion on platelet monolayers to neutrophil-endothelial interactions, some experiments were performed on histamine-treated human umbilical vein endothelium (HUVEC) and on P-selectin-transfected CHO cells (generously provided by Dr R.P. McEver). HUVEC were isolated as previously described, and only primary or first-passaged endothelium was used as further passage failed to express P-selectin.15 Histamine (25 μmol/L; Sigma) was added to induce rolling on HUVEC, and fucoidan was administered as previously described for platelet monolayers. To determine whether more effective conversion from rolling to adhesion could be induced on endothelium with fucoidan, in some experiments lower shear stress (1 dyne/cm2) was also used.
In some experiments, neutrophils were fixed by exposing the neutrophil suspension to a 1% formalin solution for 15 minutes at 4°C. The cells were then spun at 1,100 RPM for 5 minutes and resuspended in HBSS. In other experiments, the platelets were fixed with 1% formalin.
Analysis of neutrophil interactions.
The number of rolling neutrophils was determined by counting the number of neutrophils per minute that moved slower than free-flowing neutrophils across a predetermined length of monolayer. An adherent neutrophil was defined as one that stayed stationary on the monolayer for 10 seconds or more. The last 10 seconds of a given minute were analyzed for adherent neutrophils. It should be noted that neutrophils that adhered for 10 seconds on platelet monolayers generally remained adherent for the next 10 minutes. Experiments were recorded at 200× magnification and the field of view was calculated to be 0.11 mm2.
Extraction, separation, and quantification of radiolabeled PAF.
The lipids were extracted from platelets, which were layered on different substrata, using a modification15 of the method of Bligh and Dyer.16 The platelets were first laid down on different substrata (ie, fibrinogen, gelatin, or collagen) and allowed to incubate for 1 hour. They were then scraped and resuspended before the extraction step. Because endogenous PAF is rapidly degraded by acetyl hydrolase, in some experiments we used phenylmethanesulphonyl fluoride (PMSF), a serine hydrolase inhibitor, to inactivate the intracellular acetyl hydrolase activity.17 In these experiments, platelets were incubated with 2 mmol/L PMSF for 15 minutes at 37°C. In flow chamber experiments, PMSF was not used because platelets were not lysed. The scraped platelets in buffer were mixed with the extraction solvent (as previously described). To this combination, monophase sodium acetate and chloroform were added, creating a biphasic mixture. The lower chloroform layer was washed with an aqueous wash to remove any unreacted acetate. PAF, in the chloroform phase, was then separated from other lipids on thin-layer chromatography (TLC) plates, using a solvent system as described by Mueller et al.18 The samples were then scraped from the TLC plates in a narrow zone based on their comigration with authentic PAF. The radioactivity of the samples from the appropriate zone of the TLC plate was quantified by liquid scintillation counting, with a counting efficiency of approximately 0.45.
Enzyme-linked immunosorbent assay (ELISA) for P-selectin expression.
Briefly, platelets were incubated in collagen-coated well plates, fixed, and blocked with 1% bovine serum albumin. They were then labeled with 2 μg/mL S12 (a nonblocking P-selectin MoAb; generously donated by Dr R.P. McEver) by incubating for 30 minutes at 37°C and washed, and then a peroxidase-labeled goat antimouse IgG (1 μg/mL; Kirkegaard and Perry Laboratories, Gaithersburg, MD) was added for 30 minutes at 37°C. The platelets were then washed and color-developed with a TMB one-step substrate (Dako, Carpinteria, CA) system. The color reaction was stopped with 0.18 mol/L H2SO4, and color was read on a plate reader at 450 nm.
Statistics.
All flow chamber data are reported as the mean ± SEM run 3 to 8 separate times in duplicate. Means were compared using the Mann-Whitney U-test. Statistical significance was set at P < .05.
RESULTS
Neutrophils roll and adhere under flow conditions to immobilized platelets via P-selectin.
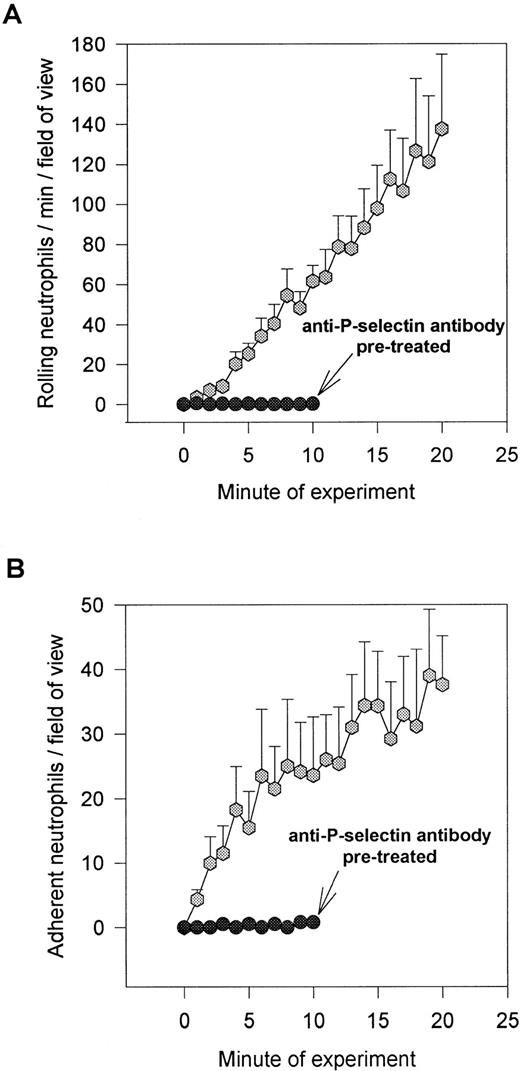
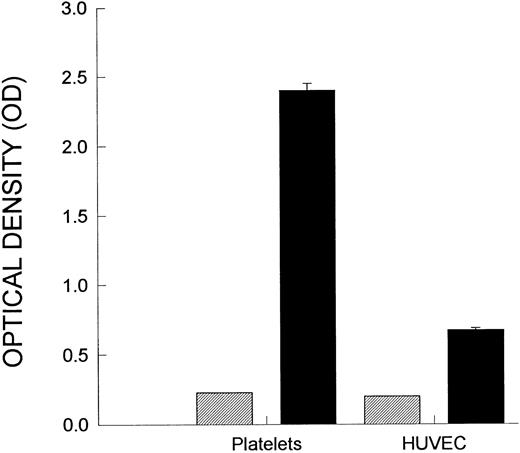
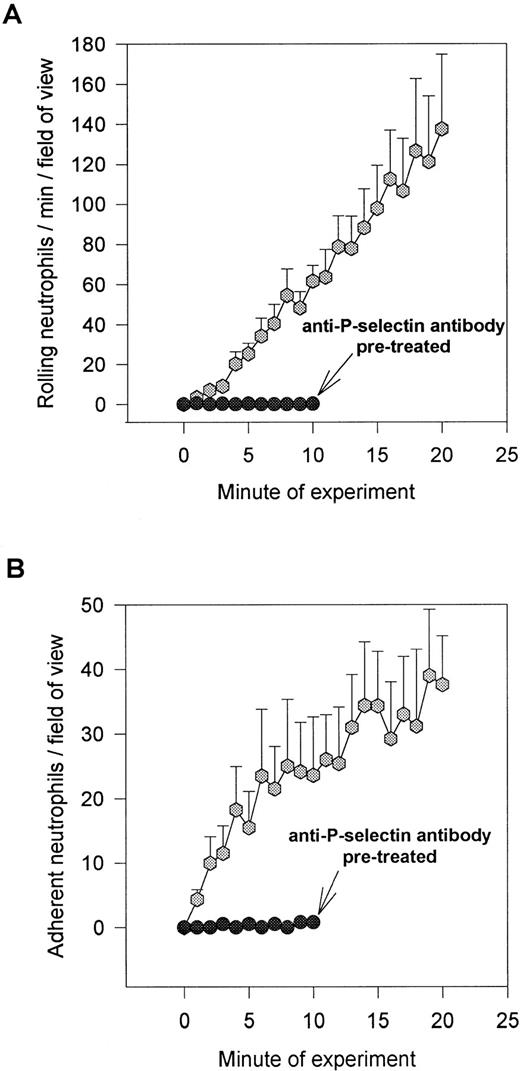
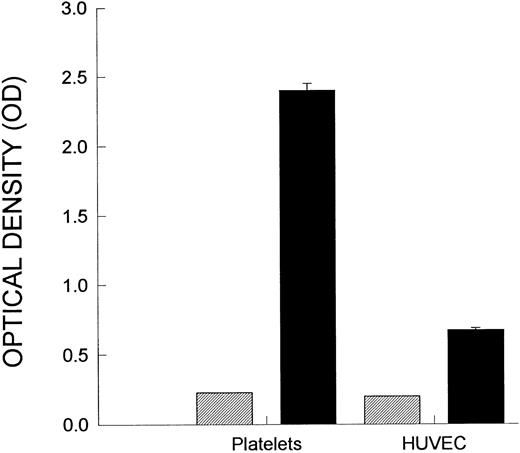
Figure 1A demonstrates a continuous increase in the number of rolling neutrophils on platelet monolayers with time of perfusion. Initial tethering interactions of neutrophils to platelet monolayers were followed by a rolling response, with the neutrophils maintaining their rounded appearance. However, with time, a significant number of the rolling cells stopped (varied from 25% to 50% of rolling cells), shape-changed, and spread on the monolayer surface (Fig 1B). The firm adhesion increased rapidly over the first 5 to 10 minutes and then the number of adhering cells seemed to plateau. Based on this pattern, experiments were generally run for 10 to 20 minutes. All of the experiments in Fig 1 and in the other figures were performed at 2 dynes/cm2. This was based on the fact that this shear promoted both neutrophil rolling and adhesion, whereas, at 1 dyne/cm2, only a few cells could be seen rolling (the majority of cells were adherent), and at 4 dynes/cm2, no neutrophil-platelet interactions were noted (data not shown). Pretreatment of the platelet monolayers with G1 (2 μg/mL), an anti–P-selectin antibody, completely prevented the neutrophil rolling (Fig 1A). Subsequent adhesion was also entirely abolished (Fig 1B), consistent with the view that rolling is a prerequisite for adhesion. There was a large amount of P-selectin expression on the cell surface of platelets immobilized on collagen relative to histamine- (Fig 2) or thrombin-treated endothelium (data not shown).
Accumulation of neutrophils over 20 minutes. Shown is the number of rolling (A) and adhering (B) neutrophils on collagen (type I) immobilized platelets. Over the first 20 minutes, approximately 25% to 50% of the rolling cells adhered at 2 dyn/cm2. Pretreatment of platelet monolayers with the anti–P-selectin antibody (G1; 2 μg/mL) prevented any neutrophil-platelet interactions. An isotype-matched control antibody did not affect neutrophil rolling or adhesion (data not shown).
Accumulation of neutrophils over 20 minutes. Shown is the number of rolling (A) and adhering (B) neutrophils on collagen (type I) immobilized platelets. Over the first 20 minutes, approximately 25% to 50% of the rolling cells adhered at 2 dyn/cm2. Pretreatment of platelet monolayers with the anti–P-selectin antibody (G1; 2 μg/mL) prevented any neutrophil-platelet interactions. An isotype-matched control antibody did not affect neutrophil rolling or adhesion (data not shown).
P-selectin expression. Shown is P-selectin expression on platelets relative to P-selectin on HUVEC. Platelets were incubated on collagen, as described in the Materials and Methods, and an ELISA was performed to measure cell-surface P-selectin expression. HUVEC were stimulated with histamine (25 μmol/L) for 10 minutes, and then the P-selectin expression was measured. (▨) Nonspecific binding; (▪) P-selectin expression.
P-selectin expression. Shown is P-selectin expression on platelets relative to P-selectin on HUVEC. Platelets were incubated on collagen, as described in the Materials and Methods, and an ELISA was performed to measure cell-surface P-selectin expression. HUVEC were stimulated with histamine (25 μmol/L) for 10 minutes, and then the P-selectin expression was measured. (▨) Nonspecific binding; (▪) P-selectin expression.
Neutrophils adhere via CD18 but not CD41 on platelet monolayers.
Despite the high density of P-selectin expression on the platelet surface, it is unlikely to be sufficient to support firm neutrophil adhesion. An antibody directed against the CD18-glycoprotein (IB4; 20 μg/mL) significantly reduced the firm adhesion of neutrophils to platelet monolayers (Table 1). The GP IIb/IIIa integrin (CD41) on platelets was not involved in mediating adhesion. An RGDS peptide did not reduce the adhesion and platelets from a Glanzmann's thrombasthenia patient, which lacked CD41 maintained adhesive interactions comparable to a normal control (Table 1). It should be noted that this experiment was only run once in duplicate due to very limited access to the patient. None of the manipulations in Table 1affected leukocyte rolling (data not shown).
PAF contributes to the neutrophil adhesion on platelet monolayers.
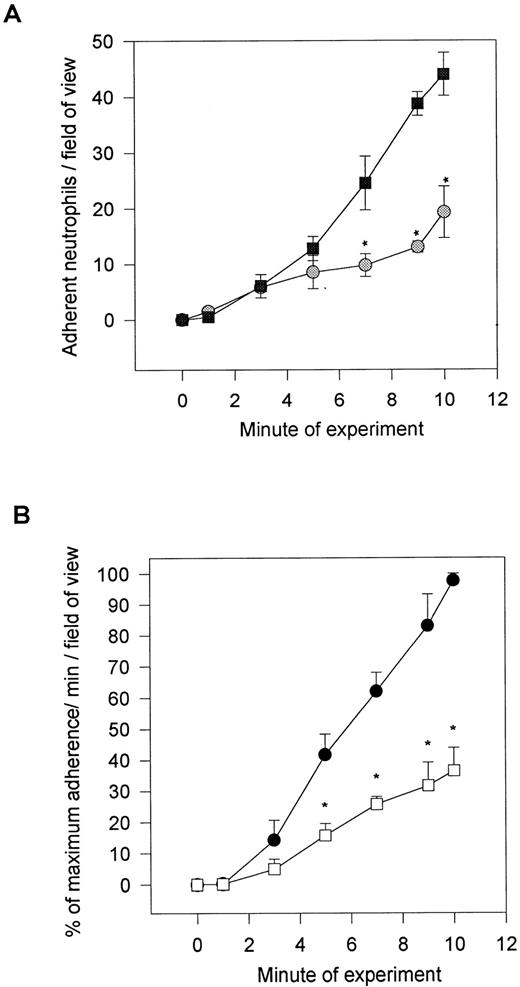
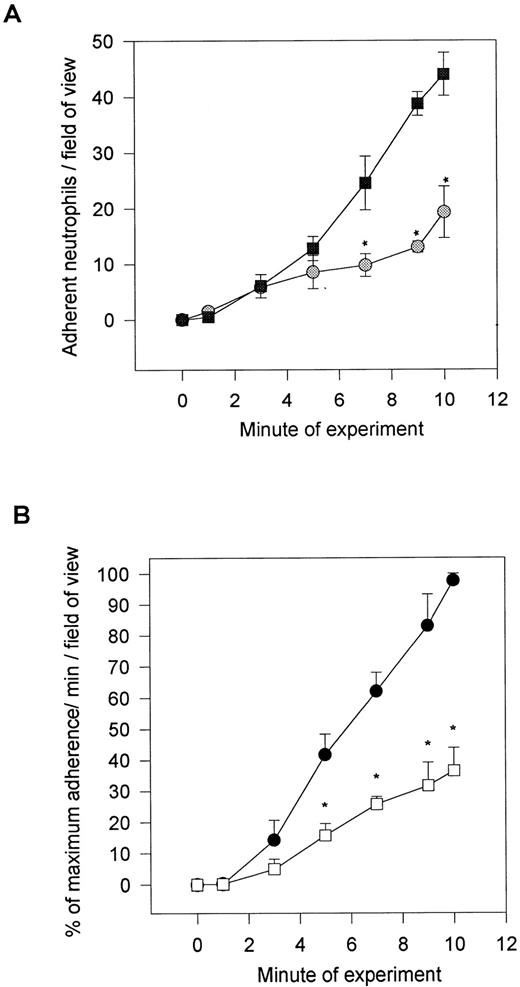
After the induction of neutrophil rolling on platelet monolayers, the neutrophils began to change shape and subsequently firmly adhere. This is not observed when neutrophils roll on P-selectin–transfected CHO cells or on immobilized P-selectin (personal observations).19 Additionally, fixed neutrophils perfused across platelet monolayers rolled at control levels; however, the adhesion was entirely eliminated (Table 1). All of these observations suggest that the neutrophils are being activated after contact with the platelet monolayer. To test whether PAF was involved, we incubated neutrophils with the PAF receptor antagonist WEB 2086 (20 μg/mL). As seen in Fig 3A, WEB 2086 reduced neutrophil adhesion to platelet monolayers by 60% (from 45 to 18 cells/field of view). This was specific for adhesion, because very significant numbers of neutrophils continued to roll on the platelet monolayer, often exceeding the number of untreated rolling cells (data not shown). The platelet surface was the likely source of PAF, because selective treatment of the platelet monolayers with PAF-AH also inhibited neutrophil adhesion by approximately 60%, whereas the placebo (solvent in which PAF-AH was dissolved) had no effect on the platelet-neutrophil interactions (Fig 3B). Additionally, fixation of the platelet monolayer, which inhibits further synthesis or release of PAF but does not affect membrane incorporated PAF, did not prevent adhesion (data not shown). However, some reduction in adhesion did occur, suggesting either that PAF synthesis/degradation was an ongoing process or that fixation blocked the synthesis and release of some other pro-adhesive factors. Indeed, supernatants from platelet monolayers had pro-adhesive activity that could not be inhibited by the PAF receptor antagonist (data not shown).
Neutrophil adhesion in the presence of (top) a PAF receptor antagonist (WEB 2086; 20 μg/mL) or after (bottom) platelet monolayer treatment either with PAF-acetylhydrolase or the solvent (placebo). The placebo and acetylhydrolase group were compared with an untreated monolayer, and the data are presented as a percentage of adhesion relative to the 10-minute value for untreated platelet monolayers. *P < .05 relative to placebo value. (A) (◍) WEB 2086-treated neutrophils; (▧) untreated neutrophils. (B) (•) Placebo-treated monolayers; (□) PAF-acetylhydrolase–treated monolayers.
Neutrophil adhesion in the presence of (top) a PAF receptor antagonist (WEB 2086; 20 μg/mL) or after (bottom) platelet monolayer treatment either with PAF-acetylhydrolase or the solvent (placebo). The placebo and acetylhydrolase group were compared with an untreated monolayer, and the data are presented as a percentage of adhesion relative to the 10-minute value for untreated platelet monolayers. *P < .05 relative to placebo value. (A) (◍) WEB 2086-treated neutrophils; (▧) untreated neutrophils. (B) (•) Placebo-treated monolayers; (□) PAF-acetylhydrolase–treated monolayers.
Because 40% of the adhesion could not be prevented by WEB 2086 and IL-8 has been reported to be released by platelets,20 we tested the possibility that this cytokine also contributed to neutrophil adhesion. An anti–IL-8 MoAb (10 μg/mL; R&D), at concentrations that prevent IL-8–induced neutrophil adhesion to endothelium,21 had no effect on neutrophil adhesion to platelet monolayers (Table 1).
Platelets produce PAF when immobilized on collagen.
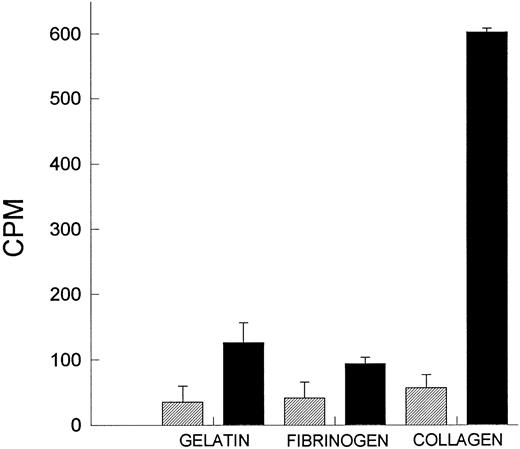
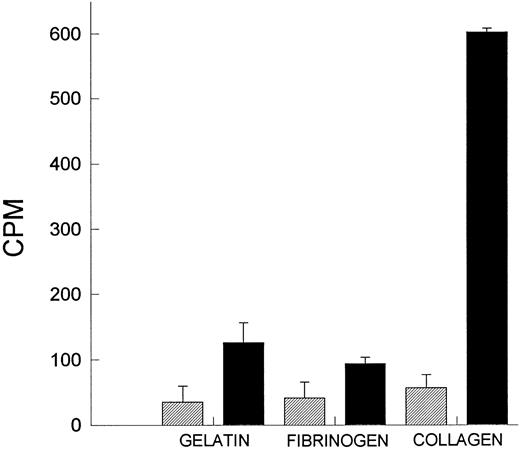
All of the data obtained thus far suggests that the platelets are the likely source of PAF. Indeed, we measured significant amounts of PAF on platelets immobilized on collagen (Fig 4). Interestingly, other substrata did not produce PAF to the same degree. PAF-AH had to be blocked by PMSF; otherwise, during the scraping and lysing step, PAF would be deacetylated by the liberated stores of PAF-AH. This serves as further evidence that we were indeed studying PAF. It is of further interest that platelets could be stimulated to produce greater amounts of PAF when additionally stimulated with a calcium ionophore (A23187; data not shown).
PAF production by platelets. Platelets were layered on gelatin, fibrinogen, and collagen, and the amount of PAF produced was measured after 1 hour of incubation. Platelets were either incubated in buffer or with 2 mmol/L PMSF for 15 minutes at 37°C to degrade the endogenous acetyl hydrolase activity. (▨) Untreated platelets; (▪) PMSF-treated platelets.
PAF production by platelets. Platelets were layered on gelatin, fibrinogen, and collagen, and the amount of PAF produced was measured after 1 hour of incubation. Platelets were either incubated in buffer or with 2 mmol/L PMSF for 15 minutes at 37°C to degrade the endogenous acetyl hydrolase activity. (▨) Untreated platelets; (▪) PMSF-treated platelets.
Signaling via soluble selectin ligands enhances adhesion on platelets but not on P-selectin–expressing endothelium.
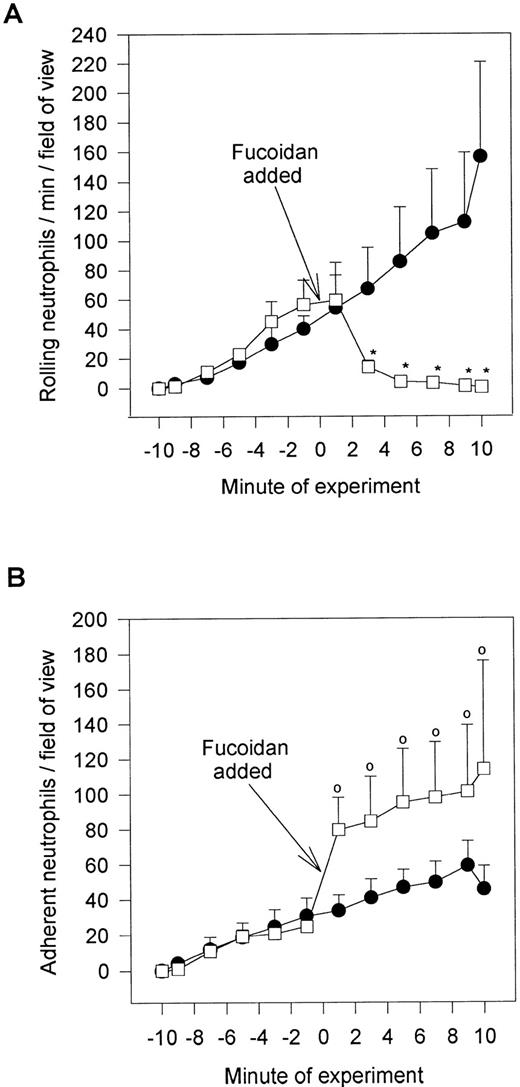
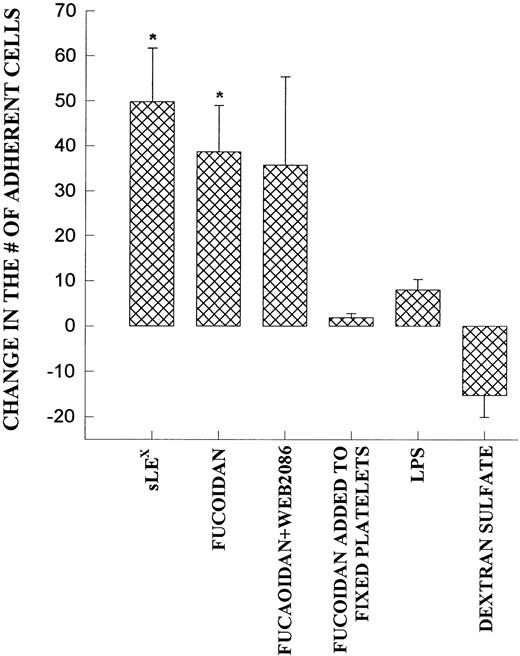
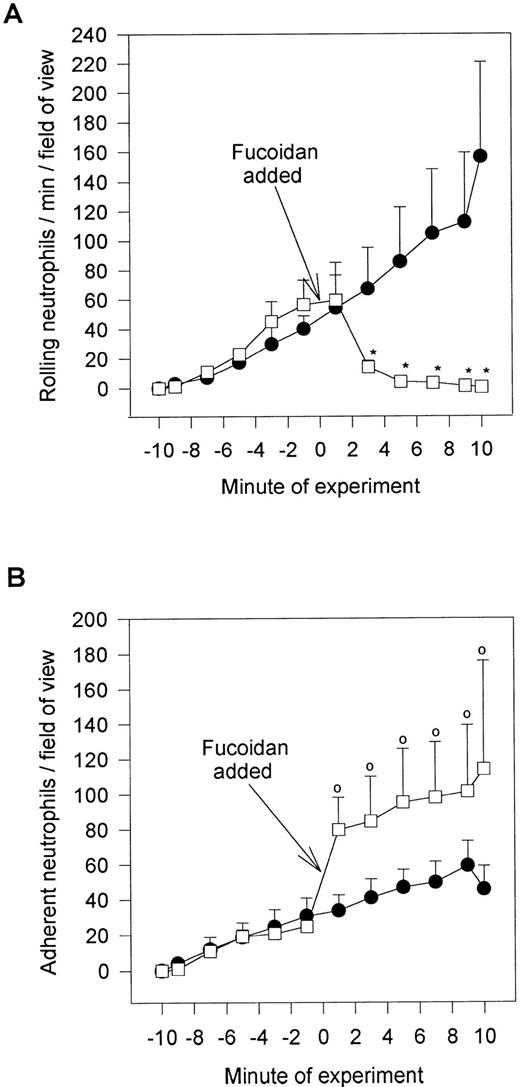
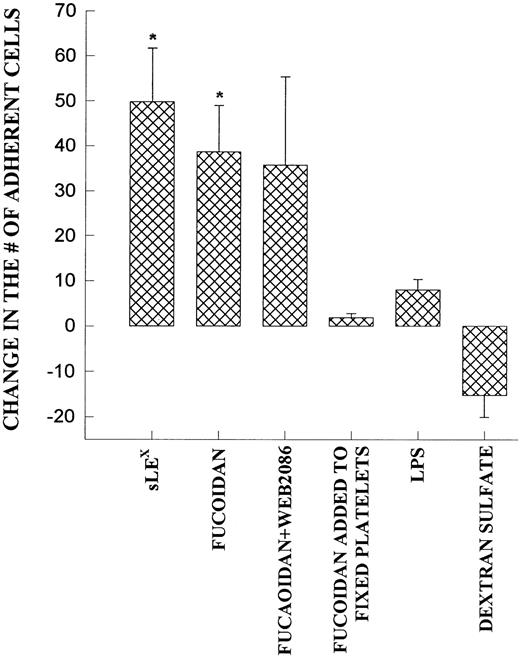
Fucoidan, a selectin-binding carbohydrate, rapidly reduced neutrophil rolling and prevented further neutrophil rolling from being initiated (Fig 5A). However, rather than detaching the majority of rolling cells, essentially all of the rolling cells adhered firmly immediately upon fucoidan administration (Fig 5B). This increased adhesion required platelet activation, because fixation of platelets completely inhibited the fucoidan-induced adhesion. Neutrophils were capable of adhering to fixed platelets in the absence of fucoidan (7.0 ± 2.9 neutrophils/min/field of view), suggesting that fixation did not remove surface adhesive molecules. We do not believe that endotoxin was the mechanism of action for the following reasons. First, the endotoxin-free fucoidan (<10 EU/mg; as described in the Materials and Methods) inhibited rolling and induced neutrophil adhesion to the same degree as endotoxin-contaminated fucoidan. Second, endotoxin (10 EU/mL; Escherichia coli 0127:B8; Sigma) alone did not induce the adhesive response (Fig 6). Third, when an SLEx analog, with undetectable levels of endotoxin was administered to rolling neutrophils, a similar pattern of conversion from rolling to adhesion was noted (Fig 6). Finally, uncleaned dextran-sulfate, at a concentration that completely inhibited rolling, did not induce an increase in adhesion (Fig 6).
Fucoidan posttreatment. Shown is the effect of the selectin-binding carbohydrate, fucoidan, on rolling neutrophils (A) and adhering neutrophils (B). Fucoidan (100 μg/mL) was administered after 10 minutes of neutrophil perfusion (time = 0), because this was the time when neutrophil adhesion plateaued. *P < .05 relative to untreated neutrophils. 0P < .05 relative to time 0 value. (•) Untreated neutrophils; (□) fucoidan posttreated neutrophils.
Fucoidan posttreatment. Shown is the effect of the selectin-binding carbohydrate, fucoidan, on rolling neutrophils (A) and adhering neutrophils (B). Fucoidan (100 μg/mL) was administered after 10 minutes of neutrophil perfusion (time = 0), because this was the time when neutrophil adhesion plateaued. *P < .05 relative to untreated neutrophils. 0P < .05 relative to time 0 value. (•) Untreated neutrophils; (□) fucoidan posttreated neutrophils.
Demonstrates the increase in neutrophil adhesion after the addition of SLEX, fucoidan, fucoidan and WEB 2086, lipopolysaccharide (LPS), or dextran sulfate. In each of these experiments, rolling and adhesion was observed for 10 minutes before the administration of the reagent and then for an additional 10 minutes. The experiment was also performed on fixed platelets. *P < .05 relative to untreated value. 0P< .05 relative to time 0 value.
Demonstrates the increase in neutrophil adhesion after the addition of SLEX, fucoidan, fucoidan and WEB 2086, lipopolysaccharide (LPS), or dextran sulfate. In each of these experiments, rolling and adhesion was observed for 10 minutes before the administration of the reagent and then for an additional 10 minutes. The experiment was also performed on fixed platelets. *P < .05 relative to untreated value. 0P< .05 relative to time 0 value.
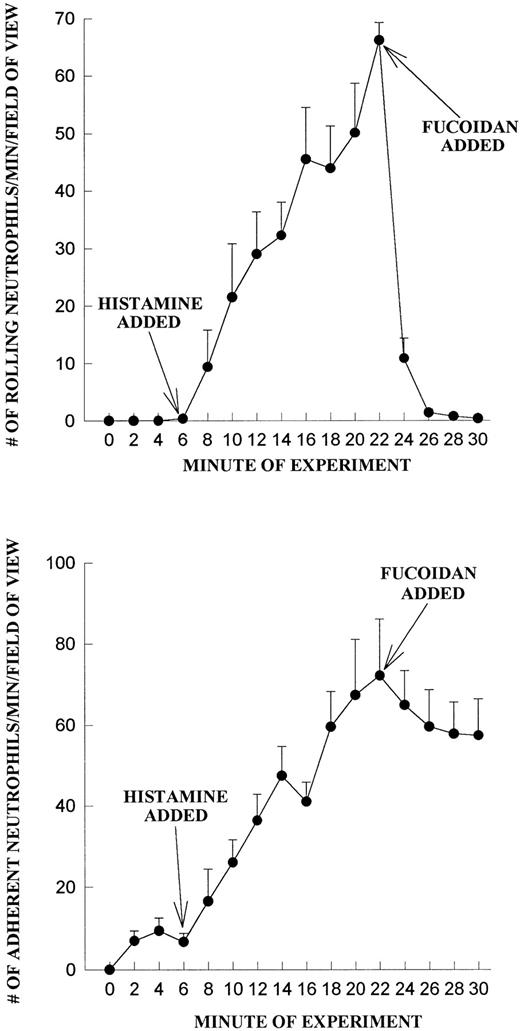
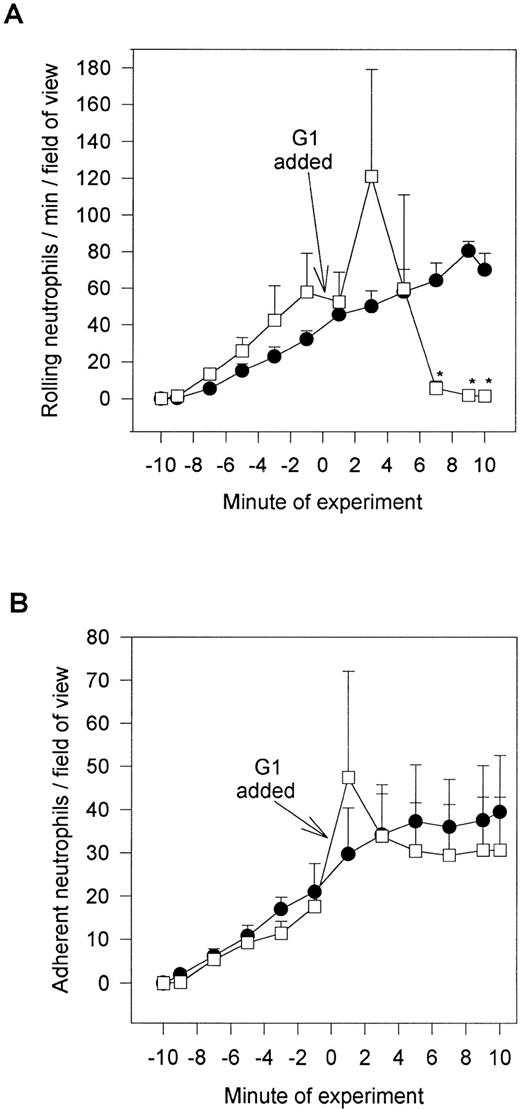
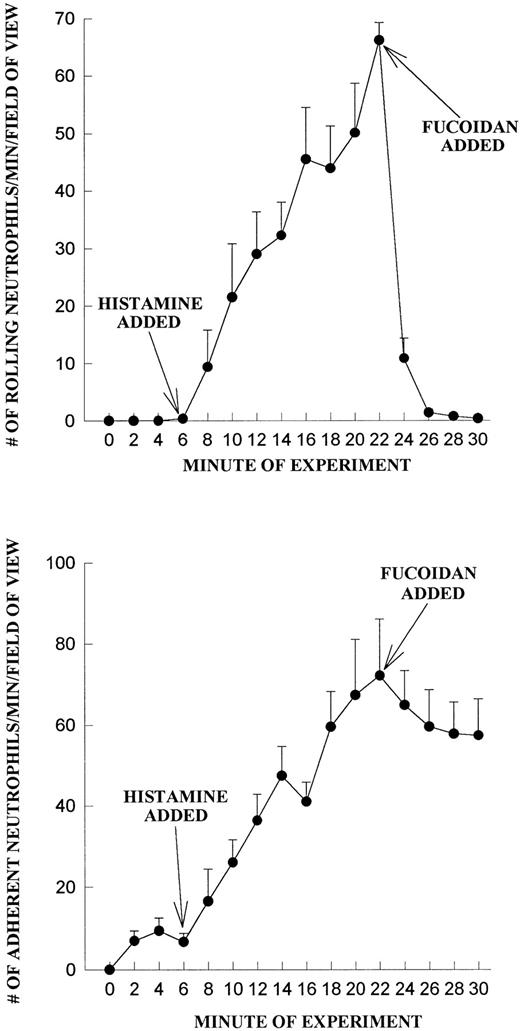
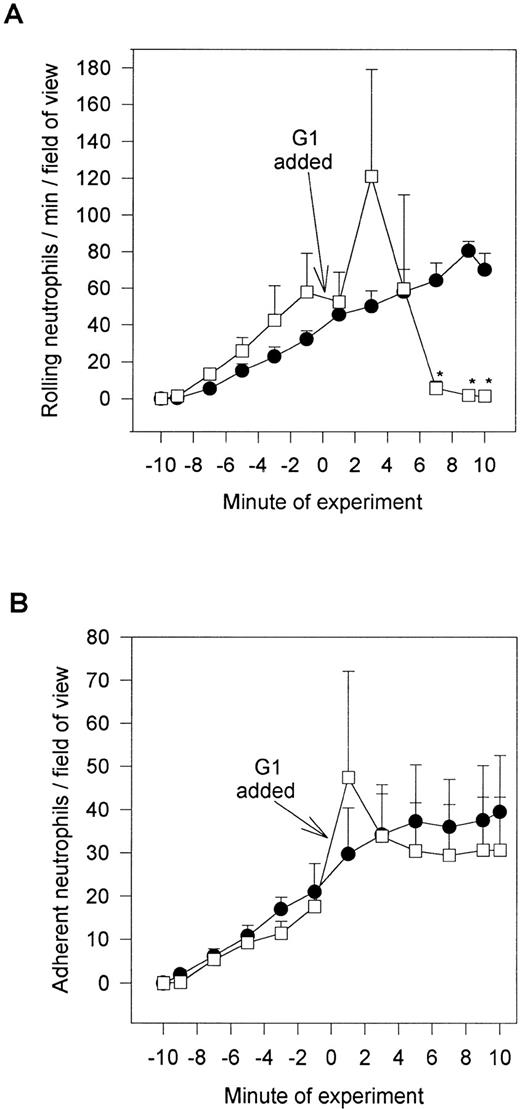
We do not believe that the fucoidan was activating the neutrophils directly. When platelets were fixed and fucoidan was added to the neutrophils, fucoidan detached rolling cells but did not increase neutrophil adhesion, suggesting that activation of platelets was a prerequisite (Fig 6). Additionally, when fucoidan was administered to neutrophils rolling on thrombin (not shown) or histamine-stimulated human umbilical vein endothelium (a P-selectin–dependent event), the rolling was completely abolished due to neutrophil detachment; adhesion was not increased (Fig 7). Because optimal adhesive interactions occur at 1 dyne/cm2 on HUVEC, we also examined whether fucoidan would increase adhesion of neutrophils to endothelium under this condition. The addition of fucoidan to endothelium even at 1 dyne/cm2 still failed to induce the rapid increase in adhesion that was observed on platelets (adhesion increased by 14% on endothelium v more than 100% on platelets). Interestingly, posttreatment with the P-selectin antibody (G1; 2 μg/mL) rapidly detached the rolling neutrophils from platelets and there was no detectable conversion of neutrophil rolling to adhesion (Fig 8). Therefore, the rapid adhesion of rolling neutrophils to the platelet monolayer, seen after fucoidan or SLEx administration, is unique to these molecules.
Fucoidan does not increase neutrophil adhesion on histamine-treated HUVEC. Endothelium was treated at 6 minutes with histamine (25 μmol/L) and neutrophil rolling (A) and neutrophil adhesion (B) were determined before and after fucoidan administration (added at 20 minutes).
Fucoidan does not increase neutrophil adhesion on histamine-treated HUVEC. Endothelium was treated at 6 minutes with histamine (25 μmol/L) and neutrophil rolling (A) and neutrophil adhesion (B) were determined before and after fucoidan administration (added at 20 minutes).
G1 posttreatment of platelet monolayers. After 10 minutes of neutrophil-platelet interaction, the anti–P-selectin antibody (G1; 2 μg/mL) was administered, and neutrophil rolling (A) and neutrophil adhesion (B) were assessed. *P < .05 relative to time 0 value. (•) Untreated neutrophils; (□) G1 posttreated neutrophils.
G1 posttreatment of platelet monolayers. After 10 minutes of neutrophil-platelet interaction, the anti–P-selectin antibody (G1; 2 μg/mL) was administered, and neutrophil rolling (A) and neutrophil adhesion (B) were assessed. *P < .05 relative to time 0 value. (•) Untreated neutrophils; (□) G1 posttreated neutrophils.
DISCUSSION
It is now well documented that neutrophils tether, roll, and adhere on activated endothelium and that the key event in the translation between rolling and adhesion is dependent on critically localized chemotactic agents produced directly on the surface of endothelium to act as a juxtacrine activating process.4 In this study, we show that contact-activated platelets, in the absence of any exogenous pro-inflammatory stimuli, can support an identical multistep cascade of adhesive interactions. This suggests that platelets not only produce adhesion molecules that can capture (tether) neutrophils to initiate rolling, but also produce chemoattractants to stage the critical conversion from rolling to firm adhesion. Consistent with this view are the data provided by us and others8-10 that the initial rolling is P-selectin–dependent and the firm adhesion is dependent on CD11b/CD18, because neutrophils in the presence of anti-CD18 or anti-CD11b antibodies or neutrophils deficient in CD18 (LAD-1 cells) were unable to firmly adhere to platelet monolayers. In the present study, we have further extended the understanding of the cascade of events underlying the platelet-neutrophil interactions by identifying platelet-derived PAF as an important molecule functioning to activate and cause neutrophils to firmly adhere to the platelet monolayer surface. However, we also highlight, for the first time, a critical difference between the multistep process on endothelium and platelets; soluble ligands for selectins can rapidly and greatly enhance the efficiency of the transition from rolling to firm adhesion but only on platelets.
In accordance with the view that PAF was a significant signal for neutrophil adhesion was the observation that the PAF receptor antagonist (WEB 2086), at a concentration that inhibits 90% to 100% of PAF-induced neutrophil adhesion to the endothelium,22,23prevented 60% of rolling cells from adhering to the platelet monolayers. Further support for the importance of PAF is highlighted by the fact that 60% of the adhesion could be inhibited with PAF-acetylhydrolase (PAF-AH), an enzyme specific for deacetylation of PAF to inactive lyso-PAF. The PAF-AH experiments provide additional insight into the source and location of the PAF. First, only the platelet monolayers were exposed to PAF-AH, suggesting that the source of PAF is likely the platelet. Second, exogenous PAF-AH is not internalized and, therefore, degradation of PAF indicates that the PAF activity is on the outside of the membrane. This strategy has been used previously to determine the sidedness of PAF in endothelial monolayers.24 Finally, we were able to detect PAF activity from platelets that had adhered to collagen, but not other substrata. Based on these results, and borrowing from the insights provided by Lorant et al5 in their study on neutrophil adhesion to thrombin-treated endothelium via PAF, we propose the following scenario. P-selectin functions to tether neutrophils to a monolayer of platelets and, once in close proximity, PAF located on the platelet surface ligates its receptor on neutrophils and mediates juxtacrine activation of the PMN to cause firm adhesion via CD18.
Although this juxtacrine activation is reminiscent of the chemoattractant-induced adhesion of neutrophils to endothelium, there are a number of critical differences. There were greater levels of P-selectin on platelets than on endothelium, but greater levels of PAF on endothelium (data not shown) than platelets. However, the end result was similar amounts of adhesion on both substrata, perhaps because the increased amount of P-selectin may slow rolling cells so that they adhere in the presence of lower concentrations of PAF. Indeed, in vivo work has demonstrated that slow rolling cells (induced with LTC4) responded to low concentrations of exogenous PAF by adhering, whereas fast rolling cells (induced by histamine) were unresponsive to the same concentration of PAF.25 An alternative explanation may be that contact-activated platelets express other pro-adhesive molecules than just PAF. Indeed, WEB 2086 and PAF-AH only inhibited 60% of the adhesion. Finally, it is possible that ligation of P-selectin on platelets causes these cells to express additional activation mechanisms to signal neutrophils to adhere.
The latter contention deserves some attention in light of the striking response of rolling neutrophils to immediately adhere on platelet monolayers exposed to two soluble P-selectin ligands, an SLEx analog or fucoidan, a fucosylated, sialylated carbohydrate polymer. Although contaminants such as endotoxin within the fucoidan or SLEx analog preparations could conceivably activate neutrophils to adhere, the adhesion consistently occurred on platelet monolayers but not on histamine-treated endothelium or P-selectin transfectants. Moreover, fucoidan at 100 μg/mL did not cause any detectable shape-change in neutrophils and did not enhance adhesion on endothelium under static conditions in the presence or absence of activating agents.26 Furthermore, we did not see differences between endotoxin contaminated (>300 EU/mg) and endotoxin-free (<10 EU/mg) fucoidan, and endotoxin per se failed to cause neutrophils to adhere on platelet monolayers. The only obvious difference between the experiments on endothelial and platelet monolayers is the substratum, suggesting that soluble selectin binding molecules activate platelets to either increase the production of additional chemotactic agents or increase adhesive ligands for CD18. In this study, we can rule out increased production of PAF as the mediator responsible for fucoidan-induced adhesion due to lack of effect of WEB 2086. However, the possibility that increased expression of ligands for CD18 (fibrinogen, ICAM-2, etc) cannot be dismissed.
Previous work has shown that selectins binding to their ligands can activate target cells or alter their functional states. For example, both membrane associated and soluble E-selectin can activate neutrophils as well as endothelium.27 Cross-linking of L-selectin has been demonstrated to be sufficient to enhance the activation of neutrophils.28,29 These data suggest that selectins, including L-selectin and E-selectin, do have the capacity to activate neutrophils. Our study, for the first time, raises the possibility that binding of selectin ligands to P-selectin on platelets, but not to P-selectin on endothelium, activates the target cell to increase its adhesivity for rolling neutrophils. It is intriguing that SLEx analog and fucoidan but not P-selectin increased adhesion on platelets. The lack of increased adhesive response to the P-selectin antibody may be related to the need to cross-link the antibody and P-selectin before this adhesive response may occur. Further definition of the mechanism will require identification of the CD18 ligand on the platelet. We now know that the CD18 ligand on platelets does not involve ICAM-210 or CD41 (this study), but may involve fibrinogen, which is secreted along with PAF and P-selectin upon platelet activation.30 Whether fibrinogen is responsible for the increased adhesion is clearly an important future venue.
P.K. is supported by a grant from the Medical Research Council (MRC) and is an Alberta Heritage Foundation for Medical Research (AHFMR) senior scholar and MRC scientist. G.A.Z. is supported by Grant No. R01 HL44525 from the National Institutes of Health (NIH). D.E.L. is supported by the Physician Scientists Award HL 02726 from the NIH.
Address reprint requests to Paul Kubes, PhD, Immunology Research Group, Dept. of Medical Physiology, Faculty of Medicine, University of Calgary, Calgary, Alberta, T2N 4N1 Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.