Abstract
We investigated how in vivo effects of single hematopoietic cytokines change if given in combination for a prolonged time. Mice were treated with every combination of recombinant human (rh) erythropoietin (EPO), rh granulocyte colony-stimulating factor (G-CSF), recombinant rat (rr) stem cell factor (SCF), and rh interleukin (IL)-11 by continuous infusion over 7 days (full factorial design with three dose levels for each cytokine). Burst-forming unit–erythroid (BFU-E), colony-forming unit–erythroid (CFU-E), and colony-forming unit–granulocyte-macrophage (CFU-GM) were determined in bone marrow and spleen, reticulocytes, hematocrit, granulocytes, and thrombocytes in the peripheral blood. An analysis of variance (ANOVA) and multiple comparison of means was used to evaluate the data. For several cell types, cytokine effects superimposed in an additive way if combined. However, in a large number of circumstances, nonadditive pairwise interactions were found. They differed in type and magnitude involving high-dose saturation, high-dose antagonistic effects, and even effect reversals (qualitative interactions). Hence, in general, it was not possible to foresee the combination effects on the basis of existing knowledge of single effects. On the other hand, the cytokine network was robust and no system hazards were observed under multiple cytokine combinations. The results illustrate that the cytokine network has nonlinear dynamic properties in vivo with dose-response characteristics of one cytokine being continuously modified by other cytokines.
THE IN VIVO EFFECTS of exogenous administration of single cytokines, among them erythropoietin (EPO), granulocyte colony-stimulating factor (G-CSF), stem cell factor (SCF), and interleukin (IL)-11, have been extensively described. EPO is well known as a specific stimulator of erythroid cell production in vivo.1 G-CSF likewise stimulates granulopoiesis with certain side effects on erythropoiesis and the hematopoietic stem cells most likely caused by induction of migration of stem and early progenitor cells from bone marrow to the spleen.2-6 SCF is assumed to have a stimulatory effect on the stem cell compartment and therefore its exogenous application leads to a general stimulation of hematopoiesis.7-10 The same holds true for IL-11, which in addition seems to induce strong migration of stem and early progenitor cells from the bone marrow to the spleen in mice.11,12Hematopoietic growth factors have redundant properties, ie, different factors can lead to the same effect. In addition, they are pleiotropic, meaning that one cytokine is able to mediate different effects.13 For a detailed understanding of the quantitative features of the cytokine network in vivo, it is however important to understand how much and in which way single cytokine effects are modified by other cytokines and how far this is concentration-dependent.
It is the objective of this study to investigate in greater detail how growth factor effects are modified by each other if given simultaneously in vivo for a prolonged time. Simple effect superpositioning and also various kinds of synergistic or inhibitory interactions could result. To address these questions, we previously analyzed the in vivo effects of combined administration of only two growth factors such as G-CSF and EPO,14 EPO and SCF,10 EPO and IL-11.12 Other investigators have undertaken similar experiments.8 15-18
Here we report about the in vivo effects of a combined simultaneous administration, investigating all possible combinations of EPO, G-CSF, SCF, and IL-11. We used a full-factorial experimental design with three dose levels of each cytokine (placebo, medium, and high dose). This design therefore involved 34 = 81 different dose combinations. Two mice were examined at each design point. This design enabled us to detect single cytokine effects, as well as second, third, or fourth order cytokine interactions. Statistically, a second order interaction exists if the relative effects of one cytokine over its dose range depend on the level of one other cytokine. A third order interaction exists if these pairwise effects again depend on the levels of a third cytokine etc. We used an analysis of variance (ANOVA) and multiple mean comparisons (Scheffé-test) as appropriate statistical methods to evaluate the (interaction) effects.
The objective of our investigation was explorative in two respects. First, we wanted to obtain an insight into the complex interaction structure of the cytokine network in vivo, and second we wanted to see whether a factorial study approach would be an informative strategy.
MATERIALS AND METHODS
Mice
In all experiments female C57BL/6 mice, 12 to 16 weeks old, weighing 20 to 25 g were used.
Hematopoietic Growth Factors
Recombinant rat (rr) pegylated SCF and recombinant human (rh) G-CSF were donated by Amgen (Thousand Oaks, CA). rhIL-11 was a gift from Genetics Institute (Cambridge, MA). rhEPO was a donation of Boehringer Mannheim (Almere, The Netherlands).
Administration of Growth Factors
Growth factors were appropriately diluted, mixed, and administered by subcutaneously implanted osmotic mini pumps (type Alzet 1007D or 2002, Alza Corp, Palo Alto, CA). Delivery rates of these mini pumps were reported by the company to be constant over a 2-week period. This was confirmed by some pilot studies that we performed (unpublished data). To test whether the implantation of these pumps by itself affected hematologic values, we assessed the effect of a 7-day and 14-day implantation of pumps filled with saline. No changes compared with normal, untreated mice were observed (data not shown). To cover a whole dose response range for each single cytokine, we chose dose levels that were expected to give a medium and a high response. Mice were treated with 0, 20, or 80 μg/kg/d rhIL-11, with 0, 40, or 100 μg/kg/d rrSCF, with 0, 10, or 100 μg/kg/d rhG-CSF, and with 0, 2.5, or 25 U/d rhEPO, further to be called levels 0 (none), 1 (medium), and 2 (high) for each cytokine, respectively. Cytokines were administered for 7 days in all possible dose combinations. Hematologic parameters were evaluated at day 7 of treatment.
Progenitor Cell Assays and Calculation of Total Body Cell Numbers
Femur and spleen single cell suspensions were made according to standard procedures. BFU-E, CFU-E, and CFU-GM were cultured with the methylcellulose method of Iscove and Sieber.19 In addition, BFU-E and CFU-GM were stimulated with 100 ng/mL rrSCF, 10 ng/mL recombinant mouse (rm) GM-CSF (supplied by Behringwerke, Marburg, Germany) and 2 U/mL rhEPO. CFU-E were stimulated with 500 mU/mL rhEPO. These culturing conditions resulted in optimal colony growth. Total bone marrow cell numbers were calculated under the assumption that one femur contains 6% of total marrow.20 Total body cell numbers were calculated as follows: Total = Femur × 17 + spleen. Circulating cells in the blood were ignored.
Blood Values
Blood was obtained from the orbital plexus before the mice were killed. Hematocrit percentages, reticulocytes percentages, granulocytes (106/mL), and platelets (106/mL) were determined according to standard procedures.
Statistical Methods
ANOVA was used to detect significant main effects of single cytokines and interactions of cytokine combinations. All effect estimates are statistically independent of each other due to the underlying additive model of the ANOVA. We used a full factorial design with two measurements at each design point (34 × 2 = 162 mice). Due to the hidden replications in this factorial study design, highly efficient effect estimates were expected.21 However, an ANOVA is neither able to interpolate dose-response relationships nor detect the direction of effects. Cytokine doses are coded as ordered categories (dose levels 0, 1, and 2). To show significant differences of individual means, we performed multiple post hoc comparisons of means using the Scheffé-test. All hematologic parameters except hematocrit, reticulocytes, and thrombocytes were log-transformed to achieve homogeneity of variance and normal distribution. Our findings are visualized as three-dimensional bar diagrams showing mean cell counts (Z-axis) according to the dose levels of two cytokines (X- and Y-axis). It has to be emphasized that these mean cell counts are averaged over all possible levels of all other cytokines, generally involving 18 mice.
RESULTS
Table 1 summarizes the essential results of the ANOVA. Significance levels of all effects with P < .01 can be read from the table. The effect direction of significant main effects (all of them have monotonic characteristics) is given by the small arrows. Such effect directions make no sense for interaction effects.
It has to be emphasized that the main effects are not single cytokine effects in the univariate sense. Rather, they represent a cytokine effect averaged over all levels of the other three cytokines. Furthermore, nonsignificant main effects do not necessarily mean that there is no cytokine effect at all. It could be the case that this effect is only pronounced at a special level of another cytokine, but on average, is not traceable. In such a case, the corresponding effect is only detectable by looking at the interaction effects.
In the following, we specify the effects of all four cytokines under consideration on erythropoiesis, granulopoiesis and the thrombocytes and describe how these are influenced by the other cytokines. It should be noted that interactions in the statistical sense are deviations from additive effects (eg, underadditive, overadditive). If this applies to log-transformed data, this implies deviations from a multiplicative effect in terms of nontransformed data.
EPO
Erythropoiesis.
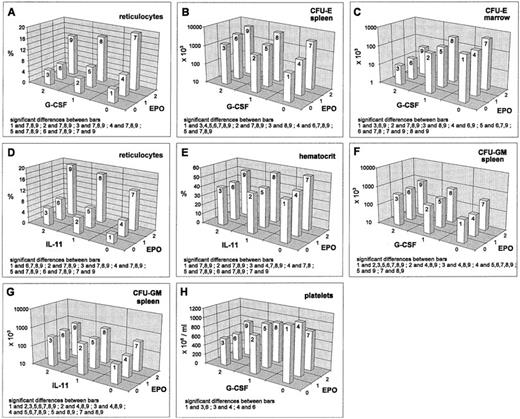
EPO increased all erythroid cell stages except the BFU-E in the bone marrow. In the reticulocyte compartment, this stimulatory effect is lessened and in the splenic CFU-E compartment, it is strengthened by G-CSF. These effects are real interactions (deviating from additivity on the log-transformed scale). For the reticulocytes, inhibition is only seen at high EPO levels (Fig 1A) and in the splenic CFU-E, we have a saturation effect at high levels of both cytokines (Fig 1B). In contrast, there are additive effects without interactions in the bone marrow CFU-E (stimulation by EPO, inhibition by G-CSF; Fig 1C). IL-11 shows an additive stimulatory effect on the reticulocytes, which leads to a maximum response for the combination high EPO and high IL-11 (Fig 1D). A similar additive pattern is seen in the splenic erythropoietic precursors (data not shown). The EPO-stimulation on the hematocrit is slightly reduced by IL-11 (Fig 1E), while IL-11 does not influence the stimulation caused by EPO in the bone marrow.
Mean values (cell count or percent values) depending on EPO/G-CSF, respectively EPO/IL-11, averaged over all other cytokine levels; significant differences of means (post-hoc Scheffé-test with simultaneous significance level P < .01) are listed in the plots.
Mean values (cell count or percent values) depending on EPO/G-CSF, respectively EPO/IL-11, averaged over all other cytokine levels; significant differences of means (post-hoc Scheffé-test with simultaneous significance level P < .01) are listed in the plots.
Granulopoiesis.
Regarding granulocytes, we found a slight stimulating effect of EPO (data not shown). Also the splenic CFU-GM were stimulated by EPO (Fig1F and G). Both effects showed additive superpositioning with the G-CSF and IL-11 effects suggesting mutual independence of the regulatoric mechanisms.
Thrombopoiesis.
Averaged over all other cytokine levels, no EPO effect on platelets was detectable. But, if looking at the interaction of EPO and G-CSF, we found that EPO, if given at high dose, modulates the G-CSF inhibition (Fig 1H).
G-CSF
Erythropoiesis.
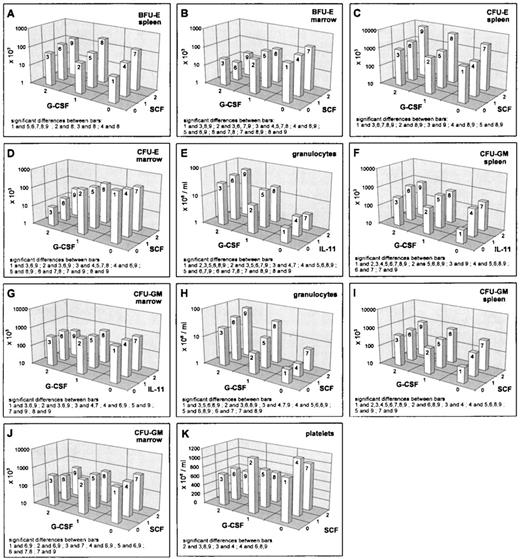
G-CSF decreased the reticulocyte numbers, as well as the erythroid progenitors in the bone marrow (Figs 1A, 2B and D). However, the hematocrit remained unchanged under G-CSF at day 7 (data not shown). Splenic erythroid progenitors were slightly stimulated (Figs 1B, 2A and C). Interactions of G-CSF with EPO have already been discussed above. No interaction effect was detected between G-CSF and IL-11. There were statistically significant interactions between G-CSF and SCF in the BFU-E. In the spleen, we noted that the stimulation by SCF disappears at high G-CSF level (Fig2A), whereas a depressive effect of SCF in the bone marrow BFU-E was only seen under additional G-CSF application (Fig 2B). In the splenic CFU-E, we found an additive superpositioning of both stimulatory effects, G-CSF and SCF (Fig 2C), whereas for the bone marrow CFU-E, the high-dose inhibition of G-CSF was weakened somewhat by high-dose SCF (Fig 2D).
Mean values (cell count) depending on G-CSF/SCF, respectively G-CSF/IL-11, averaged over all other cytokine levels; significant differences of means (post-hoc Scheffé-test with simultaneous significance level P < .01) are listed in the plots.
Mean values (cell count) depending on G-CSF/SCF, respectively G-CSF/IL-11, averaged over all other cytokine levels; significant differences of means (post-hoc Scheffé-test with simultaneous significance level P < .01) are listed in the plots.
Granulopoiesis.
Blood granulocyte counts were increased by G-CSF (Fig 2E and H). The same holds true for splenic CFU-GM (Fig 2F and I), whereas bone marrow CFU-GM numbers were decreased (Fig 2G and J). Simultaneous effects of G-CSF with IL-11 in the granulocyte compartments were shown to be of an additive kind (Fig 2E). The same holds for G-CSF and EPO on splenic CFU-GM (see above, Fig 1F). IL-11 and G-CSF showed interaction effects in the CFU-GM: in the spleen there was a superpositioning effect with saturation at high-dose levels (Fig 2F) and in the bone marrow, we could see lower suppression with a combination of high G-CSF and medium IL-11 compared with no or high IL-11 (Fig 2G). The G-CSF × SCF interaction turns out to show saturation characteristics in the granulocytes and the splenic CFU-GM (Fig 2H and I). In the bone marrow CFU-GM, a decreasing effect of G-CSF is only seen under simultaneous SCF administration (Fig 2J).
Thrombopoiesis.
Platelets were suppressed under G-CSF. This is modulated by EPO (as mentioned above, Fig 1H) and by IL-11 (data not shown). The latter effect is simply additive. A qualitative interaction was found for SCF and G-CSF with stimulation at moderate doses of each cytokine alone and depression when given jointly (Fig 2K).
IL-11
Erythropoiesis.
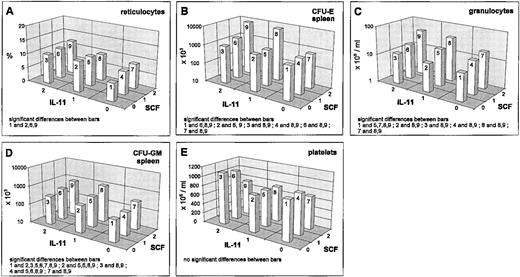
IL-11 stimulated reticulocytes and splenic progenitors (Fig 3A and B). This effect was not seen in the bone marrow. However, the hematocrit was reduced by IL-11 (Fig 1E). Interactions of IL-11 with EPO and G-CSF have been reported above. An interesting interaction was seen between IL-11 and SCF in the splenic CFU-E. There was an extreme gain if IL-11 and SCF were administered simultaneously at medium or high and high doses, respectively (Fig 3B). Although this interaction effect has been shown to be statistically significantly different at different G-CSF levels, these differences were not of a qualitative kind. The above mentioned gain was independent of the G-CSF level. G-CSF seems to induce only a slight quantitative change of the described IL-11 × SCF interaction effect (data not shown). This IL-11 × SCF interaction effect is not detectable in the bone marrow and likewise not in the reticulocytes (Fig 3A) at this time point.
Mean values (cell count or percent values) depending on SCF/IL-11, averaged over all other cytokine levels; significant differences of means (post-hoc Scheffé-test with simultaneous significance level P < .01) are listed in the plots.
Mean values (cell count or percent values) depending on SCF/IL-11, averaged over all other cytokine levels; significant differences of means (post-hoc Scheffé-test with simultaneous significance level P < .01) are listed in the plots.
Granulopoiesis.
Granulocytes are also slightly stimulated by IL-11 (Fig 3C). IL-11 reduces the CFU-GM numbers in the bone marrow slightly (Fig 2G), but increases them in the spleen (Fig 3D). Important simultaneous effects of IL-11 on granulopoiesis are detectable with G-CSF (reported above) and SCF. For granulocytes, IL-11 and SCF act as additive stimulators (Fig 3C). The same holds for splenic CFU-GM (Fig 3D).
Thrombopoiesis.
A weak stimulation of the platelets is detectable under IL-11, which is reduced under high SCF (Fig 3E). This interaction effect turns out to be not statistically significant. Likewise, no significant interaction with other cytokines is seen for these cells.
SCF
Erythropoiesis.
Granulopoiesis/thrombopoiesis.
DISCUSSION
As others have previously shown, we notice that each of the cytokines applied has specific dose-dependent hematopoietic effects. However, many of these effects are modified in the presence of other cytokines in such a way that the effect characteristics are changed in a quantitative or even qualitative way.
To further characterize these findings, we first describe the main effects of each cytokine and then discuss how these are modified by the relevant interactions.
Main effects.
EPO showed the well-expected stimulatory effects on erythropoiesis. Furthermore a weak, but significant, stimulatory effect on splenic CFU-GM and granulocyte numbers was found. We interpret this result as a consequence of an EPO-induced cell migration from the marrow to the spleen where both the erythropoietic and granulopoietic lineage find an increased amplification.22
G-CSF is confirmed to have a stimulatory effect on granulocyte production and to have the strong ability to increase BFU-E, CFU-E, and CFU-GM in the spleen accompanied by a decline in the bone marrow.5,6 We believe that a part of this phenomenon can be explained by G-CSF–induced progenitor migration from bone marrow to spleen. The G-CSF–induced shift of erythropoiesis from marrow to spleen has no effect on the hematocrit after a 7-day stimulation, which is consistent with recent findings.2,5 6
SCF is shown to have a general stimulatory effect on progenitor numbers in the spleen, but not in the bone marrow. One should consider that this could be confounded by migration effects. Altogether SCF leads to an increase of the total number of progenitor cells (BFU-E, CFU-E, CFU-GM) in the animal. As a consequence, one finds a mild increase in granulocyte numbers. In the present study, we could not see an increase in reticulocytes. However, in an independent investigation, we showed that SCF also induced a temporary reticulocytosis, which peaks at about day 10.10The decline of the thrombocytes during SCF application could not be explained by the available data set. Possibly there is a time delay because SCF supports the production of megakaryocyte precursor colonies,23 which might result in an increased platelet production at later times.
IL-11 has pronounced stimulatory effects on the total number of progenitor cells due to a splenic increase of these cells. As a consequence, one observes an increase in reticulocytes, granulocytes, and thrombocytes. The latter one is caused by IL-11–stimulated megakaryocytopoiesis.24,25 However, the hematocrit drops, which is presumably due to an expansion of the plasma volume.12 26
Interactions.
Under the assumption that two different cytokines act completely independent of each other, one expects that the shape of the dose-response characteristics of one cytokine is not changed, but only shifted up or downwards depending on the level of the second cytokine. Deviations from this parallel shift situation are called interactions. An interaction is called qualitative if it reverses the sign of the differences between the mean values. Interactions without a change of the effect direction are called quantitative. Third or fourth order interactions imply analogous dependencies of three or four cytokines.
The major novel finding from our experiments is that a large number of pairwise quantitative interactions could be revealed. Qualitative pairwise interactions, as well as third or fourth order interactions were shown to play a minor role. We only observed few qualitative relevant reversions of a cytokine effect by the additional administration of another factor. The results indicate that cytokine effects in vivo are not independent from each other. Fortunately the interactions seem to be of a “benign type” in the sense that potentially hazardous situations with severe effect reversals or even collapses of the system were not detected, indicating that the cytokine-hematopoiesis network is fairly robust.
The pairwise quantitative interactions we found can be grouped into different patterns.
The most frequent type of pairwise interaction showed a saturation characteristic at high doses and was observed especially for interactions involving G-CSF in the splenic microenvironment (Figs 1B,2A, F, and I) and in the blood neutrophils (Fig 2H). This type of interaction implies that the relative gain by adding a second cytokine is limited at higher doses. In an analogous, but reversed way, we found examples where the depressing effects of two cytokines were limited to a lower bound if given jointly (eg, EPO and G-CSF on platelets, Fig1H).
A second type of pairwise interaction has the opposite feature with an enhancing effect at high doses. We observed an enhanced stimulation for splenic and total body CFU-E for IL-11 × SCF (Fig 3B). An enhanced inhibition was found for SCF × G-CSF treatment in the marrow CFU-GM (Fig 2J).
A third type of pairwise interaction is characterized by an inhibitory effect of one cytokine if the second is given in high doses. This type of antagonistic pattern was detected in the reticulocyte compartment for G-CSF × EPO (Fig 1A). A reduction of reticulocytes by G-CSF is only observed under high EPO.
Fourth, a qualitative interaction with effect reversal was finally found in one situation where application of SCF or G-CSF alone in moderate doses was stimulatory for platelets, but inhibitory in combination (Fig 2K).
With respect to specific pairwise cytokine effects, we found the following relevant interactions indicating that effects of a single factor were able to be modified by a second factor: (1) effects of SCF are modified by IL-11 and vice versa with respect to erythroid precursor cells; (2) effects of G-CSF are modified by IL-11 and vice versa with respect to granuloid precursors; (3) effects of G-CSF are modified by SCF for all lineages including the thrombocytes; and (4) effects of EPO are modified by G-CSF and vice versa for splenic erythroid precursors, reticulocytes, and thrombocytes.
It should be emphasized that most pronounced interaction effects were detected in the spleen, which indicates that the spleen is a highly cytokine-sensitive microenvironment in the mouse. On one hand, there seems to be some special interaction of IL-11 and SCF generating the extreme synergistic stimulation of the splenic CFU-E. On the other hand, the saturation effects seen indicate a maximal carrying capacity of the spleen, which is reached by simultaneous stimulation effects, either migration, amplification, or increased input.
Several aspects and limitations have to be kept in mind when discussing the data presented.
First, the evaluation of the hematopoietic state on day 7 can only be regarded as a snap shot in the whole dynamics of the hematopoietic feedback system. Extensions to longer times might show quantitative changes in response dynamics.
Second, no pharmacogenetic measurements of the plasma levels of the cytokines were undertaken. However, we believe that the high-dose rates used are at the upper physiologic range of the system.
Third, rh and rr cytokines have been used. This was motivated by availability and the potential implications in clinical settings. It is possible that dose response characteristics of the native murine proteins differ from the recombinant factors. Generally, one expects that the recombinant factors should be less efficient in triggering response due to subpotential binding to the receptors. Nevertheless, the pattern of response should remain relatively unaffected.
Fourth, no measurements of progenitor cells in the peripheral blood were undertaken. We would, however, expect pronounced increases in all such circumstances where the spleen content of progenitors increases remarkably without being accompanied by a parallel increase in the bone marrow. Such situations are expected for combinations of G-CSF and SCF (Fig 2A, B, C, D, I, and J), G-CSF and IL-11 (Fig 2F and G) or G-CSF and EPO (Fig 1A and B). More detailed investigations are encouraged here with respect to the potential implications in optimizing peripheral stem cell harvest protocols.
Fifth, these experiments have been undertaken in normal otherwise untreated mice. With respect to a clinical setting, it would be highly relevant to investigate how prolonged application of cytokine combinations affects the recovery pattern after cytoreductive treatment. At present, we only have very limited information on dose-responses in situations of reduced cell numbers. One study that we performed investigated prolonged simultaneous exposure of etoposide with EPO and G-CSF.27 We anticipate a changing pattern of cytokine interactions under these circumstances. Such studies should have an impact on the design of more effective chemotherapies with regard to a better timing and dosing of cytokine combinations.
Sixth, one has to comment on some statistical aspects of this explorative analysis. The factorial study design enables a very powerful detection of significant effects. One should keep in mind thatP values do not serve as estimates of effect magnitudes, but rather, tell how probable it is that these effects occur by chance. Effects with P < .01 were treated as real effects. Few of these effects would turn out to be formally nonsignificant under a strict, but very conservative, adjustment of significance levels (eg, Bonferroni-Holm). Because the scope of this study was explorative, we favor this approach to minimize false-negative decisions (overlooking effects), although the chance of finding false-positive effects may increase.
The observations made strongly suggest that the effects of cytokines are not independent. Several mechanisms may contribute to this. There may be simply spatial or architectural constraints imposing a maximum to the carrying capacity of a microenvironment. There may be loss of cells during the migration process of progenitors. There may be conditional effects in the sense that a certain cytokine has to be present in a specific concentration and timing to sensitize or desensitize a cell population for the effect of a second cytokine. Furthermore, we have to acknowledge the aspect of temporal development and possibilities of feedback regulation that can change levels of endogenous cytokines and subsequently the hematopoietic status. We believe that some of this dynamic complexity can, in fact, be understood by a computer simulation model of hematopoiesis. Our group has pursued this objective over the last years and has developed models of murine erythropoiesis and granulopoiesis,20,22,28-33thrombopoiesis,34 human erythropoiesis, and human granulopoiesis,35-38 which describe single cytokine actions. It will be the objective of future developments to use the models to more clearly delineate the contributions of each of the cytokines and to analyze the mechanisms of the interactions seen in the above experiment.
In conclusion, we showed by a full factorial combination experiment that cytokine dose-response characteristics in vivo are modified in a quantitative and, in few cases, in a qualitative way by other cytokines added. This provides new insight into the dynamic functioning of the cytokine network in vivo. The experimental findings also show that combination effects are hardly predictable from the knowledge of single factor effects. Hence, similar combination studies with factorial design are needed to obtain clear insight into the human hematopoietic system.
Supported by Grant No. LO 342/5-2 from the Deutsche Forschungsgemeinschaft (Bonn, Germany) (to M.L.).
Address reprint requests to M. Loeffler, MD, Institute for Medical Informatics, Statistics and Epidemiology, University of Leipzig, Liebigstraβe 27, 04103 Leipzig, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.