Abstract
The t(5;12) translocation identified in patients with chronic myelomonocytic leukemia (CMML) encodes a TEL/platelet-derived growth factor receptor β (PDGFRβ) fusion protein. A key hypothesis for how the TEL/PDGFRβ fusion protein would function as an oncogene is that it represents a constitutively active version of the normal PDGFRβ. A link between the function of the t(5;12)-encoded TEL/PDGFRβ fusion protein and Myc expression is suggested by the fact that Myc is induced by PDGF and is essential for entry of cells into the S phase of the cell cycle. We here show that the kinase activity of TEL/PDGFRβ is necessary for Ba/F3 cells to acquire interleukin-3 (IL-3) independence and that, in contrast to their untransfected counterpart, Ba/F3 cells stably transfected with TEL/PDGFRβ maintain a high level of Myc expression after removal of IL-3. Using dominant negative mutants of Myc, we show that a threshold of active Myc is essential for TEL/PDGFRβ to transform Ba/F3 and Rat-1 cells. The findings that the kinase activity of TEL/PDGFRβ and a threshold of active Myc are involved in TEL/PDGFRβ transformation may allow for the development of therapeutic strategies in patients with t(5;12)+ CMML using specific inhibitors of the PDGFRβ kinase as well as compounds designed to interfere specifically with Myc.
THE t(5;12) TRANSLOCATION identified in patients with chronic myelomonocytic leukemia fuses the TELgene, a member of the ets family of transcription factors on chromosome 12, to the intramembranous and tyrosine kinase domains of the platelet-derived growth factor receptor β (PDGFRβ) on chromosome 5.1 The TEL sequence encoded by the TEL/PDGFRβ transcript has lost the DNA binding domain but has retained the transactivation domain in which a 5′ Helix-Loop-Helix (HLH) portion is highly conserved among a subset of ETS proteins though it has only weak homology to the HLH domain of the b-HLH family of transcription factors.2 This ETS protein HLH domain is known to be essential for full transactivating function.3,4 In fact, deletion of the HLH domain of TEL inhibits constitutive activation of the PDGFRβ kinase as well as mitogenic properties of TEL/PDGFRβ.5 6
A critical step in activation of native PDGFRβ is initiated by the binding of PDGF which induces dimerization of two adjacent PDGF receptors followed by their transphosphorylation at specific tyrosine residues. These phosphorylated residues provide binding sites for specific SH2 domains of effector proteins.7,8 The binding of effector proteins to the activated PDGFR results in the activation of multiple signal transduction pathways.9 In addition to the well elucidated “ras pathway,” necessary for PDGF-stimulated DNA synthesis,10 a novel pathway has been identified which uses the nonreceptor tyrosine kinase Src and is also induced by PDGF.11 Both pathways contribute to the cascade of transcriptional responses that signal an irreversible commitment to enter the S phase of the cell cycle. Although a well-known target of the ras pathway is the fos gene,10 the target of the Src pathway is the Myc gene, which results in a Myc protein expression throughout the cell cycle, whereas c-fos is rapidly downregulated after induction.12 Because Myc expression was shown to be increased by PDGF and to be essential to induce DNA synthesis after PDGF stimulation, we investigated whether Myc was constitutively induced by TEL/PDGFRβ in Ba/F3 hematopoietic cells and rat embryo fibroblasts.
We here report that TEL/PDGFRβ protein expression results in increased levels of Myc expression in Ba/F3 cells. Moreover, a decrease of active endogenous Myc by expression of dominant negative mutants of Myc reversed the capacity of TEL/PDGFRβ to induce soft-agar colony formation by Rat-1 cells and reversed the interleukin-3 (IL-3) independence of TEL/PDGFRβ-expressing Ba/F3 cells cultured in low serum conditions.
MATERIALS AND METHODS
Vectors.
Full-length TEL/PDGFRβ and TEL/ABL cDNAs inserted in the pSRα/MSV/TK/neo retroviral expressing vector were provided by T. Golub (Harvard University). The MycRX and Max2RX mutants have already been described13 and were provided by B. Amati (ISREC, Lausanne).
Cell transfection and transformation assays.
The IL-3–dependent leukemia murine BaF3 cell line was cultivated in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and 5% WEHI-3B cell culture supernatant as a source of IL-3. Cells (2 × 107/transfection) were transfected with the different vectors (10 μg/transfection) by electroporation at 0.28 kV and 960 microfarads using a Bio-Rad Gene Pulser apparatus. Cells transfected with TEL/PDGFRβ were selected 48 hours after transfection by G418 selection (400 μg/mL) followed by IL-3 deprivation, whereas cells transfected with the control vector were only selected with G418. Cells transfected with MycRX or the control pBabe vector were selected for resistance to puromycin (12.5 μg/mL). For transformation assays, the ability of TEL/PDGFRβ-transfected cells to grow in the absence of IL-3 was assessed by counting the cells at different times after IL-3 removal using a Coulter counter. The rat fibroblasts (Rat-1) expressing dominant negative mutants of Myc (MycRX, Max2RX) were a gift from B. Amati (ISREC Lausanne). They were cotransfected with the pSRα/MSV/TK/neo vector containing the TEL/PDGFRβ cDNA by calcium phosphate precipitation and then selected with G418 (400 μg/mL) for 12 to 15 days. For transformation assays, 2 × 104G418-resistant cells were plated in soft agar and colony formation was assessed after 2 weeks of culture. TEL/PDGFRβ protein expression was analyzed in G418-resistant cells by Western blotting.
Western blotting.
Cells were lysed in 20 mmol/L Tris pH 6.7, 0.5% sodium dodecyl sulfate (SDS), boiled for 3 minutes, and treated with 1 U of Benzon nuclease for 10 minutes at room temperature. Total cell lysates were subjected to SDS-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose filters (at 50 mA for 15 hours in 20 mmol/L Tris, 150 mmol/L glycine, 20% [vol/vol] ethanol, and 2% SDS). Western blottings were performed with anti-PDGFRβ polyclonal antibody (UBI, Lake Placid, NY), anti-Myc or anti-Max polyclonal antibodies (Santa Cruz Biotechnology, Inc, Santa Cruz, CA), or antiphosphotyrosine monoclonal antibody (Transduction Laboratories, Lexington, KY). Blots were revealed with sheep anti-mouse or anti-rabbit IgG horseradish peroxidase–linked antibodies and enhanced chemiluminescence (ECL; Amersham, Little Chalfont, Bucks, UK). The nitrocellulose filters were exposed for various times to ECL film (Amersham).
RNA preparation and Northern blot analysis.
RNA was extracted using the RNA-easy Kit (Quiagen Inc, Valencia, CA). For Northern blot analysis, 15 μg of total RNA were denaturated, mixed with ethidium bromide to a final concentration of 60 μg/mL, and fractionated on a 0.9% formaldehyde agarose gel. RNA was transferred to a Hybond N nylon membrane (Amersham). After UV cross-linking, RNAs were hybridized for 18 hours at 42°C with 107 cpm of32P-labeled cDNAs in 50% formamide, 5 × SSPE (0.75 mol/L NaCl, 5 mmol/L EDTA, 50 mmol/L sodium phosphate, pH 7.4) and 5 × Denhardt's solution. Filters were then washed twice at room temperature in 2 × SSC (0.30 mol/L NaCl, 30 mmol/L sodium citrate, pH 7) and 0.1% SDS, and once in 0.1 × SSC and 0.1% SDS at 50°C for 1 hour. Kodak XAR-5 films (Eastman Kodak Co, Rochester, NY) and Philips ultraS intensifying screens (Philips, Eindhoven, The Netherlands) were used for autoradiography.
RESULTS
Transformation of Ba/F3 cells by TEL/PDGFRβ requires the kinase activity: Inhibition by a PDGFRβ selective tyrosine kinase inhibitor.
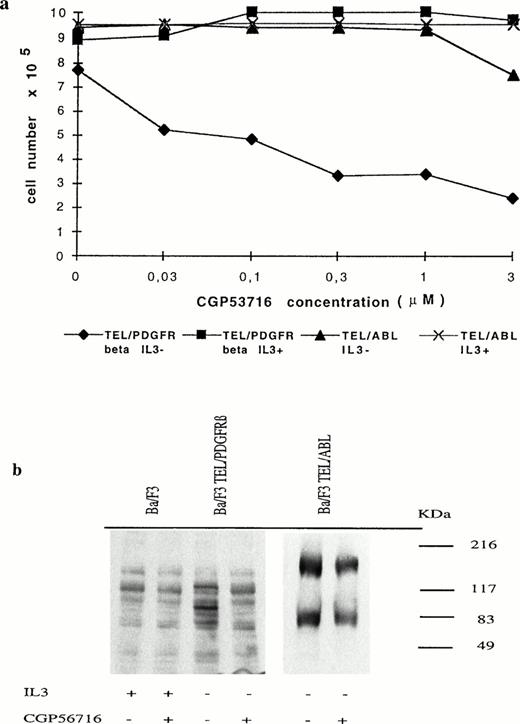
IL-3–dependent Ba/F3 cells were transfected with the pSRα/MSV/TK/neo retroviral vector containing or not the TEL/PDGFRβ full-length cDNA.1 Consistent with the results recently reported by Carroll et al,5 we found that cells expressing the TEL/PDGFRβ protein grew in the absence of IL-3 and that the fusion protein was constitutively phosphorylated (data not shown). To determine whether this phosphorylation was involved in the transforming capacity of TEL/PDGFRβ, we used a protein tyrosine kinase inhibitor (CGP53716) that shows selectivity for the PDGFR.14 As shown in Fig 1a, treatment of TEL/PDGFRβ-expressing Ba/F3 cells with 0.3 μmol/L CGP53716 completely inhibited their growth in the absence of IL-3, whereas this growth was restored by the addition of IL-3. This inhibitor did not modify the growth of TEL/ABL-expressing Ba/F3 cells even in the absence of IL-3. As expected because CGP53716 inhibits ABL tyrosine kinase at 3 μmol/L,14 a slight decrease in cell growth was observed at this concentration when TEL/ABL-expressing Ba/F3 cells were cultured in the absence of IL-3.
Growth inhibition of TEL/PDGFRβ-expressing Ba/F3 cells by CGP53716. (a) TEL/PDGFRβ- or TEL/ABL-expressing cells were seeded at a density of 3.105 cells/mL with or without IL-3 and in the presence of different concentrations of CGP53716 (provided by CIBA-GEIGY, Basel, Switzerland). Cell numbers were determined after 24 hours of culture. (⧫), TEL/PDGFRβ IL-3−; (▪), TEL/PDGFRβ IL-3+; (▴), TEL/ABL IL-3−; (X), TEL/ABL IL-3+. (b) In contrast to TEL/PDGFRβ- or TEL/ABL-expressing Ba/F3 cells, control Ba/F3 cells were maintained with IL-3. These cells were either untreated (−) or treated (+) for 18 hours with 0.3 μmol/L of CGP56713. Cell lysates were analyzed by Western blotting using an antiphosphotyrosine antibody.
Growth inhibition of TEL/PDGFRβ-expressing Ba/F3 cells by CGP53716. (a) TEL/PDGFRβ- or TEL/ABL-expressing cells were seeded at a density of 3.105 cells/mL with or without IL-3 and in the presence of different concentrations of CGP53716 (provided by CIBA-GEIGY, Basel, Switzerland). Cell numbers were determined after 24 hours of culture. (⧫), TEL/PDGFRβ IL-3−; (▪), TEL/PDGFRβ IL-3+; (▴), TEL/ABL IL-3−; (X), TEL/ABL IL-3+. (b) In contrast to TEL/PDGFRβ- or TEL/ABL-expressing Ba/F3 cells, control Ba/F3 cells were maintained with IL-3. These cells were either untreated (−) or treated (+) for 18 hours with 0.3 μmol/L of CGP56713. Cell lysates were analyzed by Western blotting using an antiphosphotyrosine antibody.
To study the PDGFRβ tyrosine kinase specificity of CGP53716 in Ba/F3 cells, Western blot analysis of cell extracts from control Ba/F3 cells maintained with IL-3 as well as from TEL/PDGFRβ- or TEL/ABL-expressing cells maintained without IL-3 and either untreated or treated for 18 hours with 0.3 μmol/L CGP53716 were performed using antiphosphotyrosine antibodies. In control Ba/F3 cells, CGP53716 did not inhibit the overall pattern of tyrosine phosphorylation (Fig 1b). In TEL/PDGFRβ-expressing cells, a band of ∼100 kD, likely to correspond to the phosphorylated fusion protein, was no longer readily detected after treatment with CGP53716. In TEL/ABL-expressing cells, an ∼180-kD protein likely to correspond to the TEL/ABL15 protein remained unchanged after treatment with CGP53716. A lower molecular weight band previously described to be highly phosphorylated in these cells14 also remained unchanged. Collectively, these results reinforced the view that CGP53716 specifically inhibited PDGFRβ tyrosine kinase activity and that the kinase activity harbored by the PDGFR part of the TEL/PDGFRβ protein was involved in the transforming activity of this fusion protein.
Myc expression is increased in TEL/PDGFRβ-expressing Ba/F3 cells.
Because the Myc oncoprotein is one of the intermediate early genes induced by PDGF in various cell lines, its expression was tested in Ba/F3 cells expressing or not the TEL/PDGFRβ protein. Because IL-3 has been shown to induce Myc,16 17 cell lysates were prepared from exponentially growing Ba/F3 cells cultured for 24 hours in the absence or in the presence of IL-3 and Myc expression was assessed by Western blotting. In the presence of IL-3, Ba/F3 cells expressed high levels of Myc independently of TEL/PDGFRβ protein expression. However, whereas Myc protein expression was maintained at high levels in cells expressing TEL/PDGFRβ protein cultured without IL-3 for 24 hours, it was dramatically decreased in cells transfected with the control vector (Fig 2a). This was correlated with Myc mRNA levels (Fig 2b).
Myc mRNA and protein expression in Ba/F3 cells expressing or not TEL/PDGFRβ. (a) Cells were cultured for 24 hours in the presence (+) or in the absence (−) of IL-3. Cell lysates were analyzed by Western blotting using an anti-Myc antibody. (b) Total RNA from Ba/F3 cells transfected with either the control vector or TEL/PDGFRβ and cultured for 18 hours without IL-3 were analyzed by Northern blotting using an Myc cDNA probe. Lower panel is ethidium bromide staining of the 28S ribosomal RNA used as a control of RNA concentration in each lane. (c) Untransfected Ba/F3 cells as well as TEL/PDGFRβ-expressing cells were cultured for 18 hours in the presence (+) or in the absence (−) of IL-3, respectively, with (+) or without (−) 0.3 μmol/L of CGP53716. Cell lysates were analyzed by Western blotting using an anti-Myc antibody.
Myc mRNA and protein expression in Ba/F3 cells expressing or not TEL/PDGFRβ. (a) Cells were cultured for 24 hours in the presence (+) or in the absence (−) of IL-3. Cell lysates were analyzed by Western blotting using an anti-Myc antibody. (b) Total RNA from Ba/F3 cells transfected with either the control vector or TEL/PDGFRβ and cultured for 18 hours without IL-3 were analyzed by Northern blotting using an Myc cDNA probe. Lower panel is ethidium bromide staining of the 28S ribosomal RNA used as a control of RNA concentration in each lane. (c) Untransfected Ba/F3 cells as well as TEL/PDGFRβ-expressing cells were cultured for 18 hours in the presence (+) or in the absence (−) of IL-3, respectively, with (+) or without (−) 0.3 μmol/L of CGP53716. Cell lysates were analyzed by Western blotting using an anti-Myc antibody.
Furthermore, CGP53716 (0.3 μmol/L) did not affect Myc protein expression induced by IL-3 in untransfected Ba/F3 cells, whereas it strongly decreased this expression in TEL/PDGFRβ-expressing Ba/F3 cells cultured without IL-3 (Fig 2c). These results provided further evidence that in TEL/PDGFRβ-expressing cells, Myc expression was a direct consequence of TEL/PDGFRβ activation.
The fact that Myc expression was increased in TEL/PDGFRβ-expressing cells and was dependent on TEL/PDGFRβ kinase activity strongly suggested that the fusion protein activated the same pathways as the PDGFRβ.
A dominant negative mutant of Myc decreases cell growth of TEL/PDGFRβ-expressing Ba/F3 cells.
To determine if Myc was essential for transformation by TEL/PDGFRβ, we overexpressed dominant negative mutants of Myc in TEL/PDGFRβ-expressing Ba/F3 and rat fibroblast (Rat-1) cells. The Myc mutants (MycRX and Max2RX) used in this study were as described.13 Briefly, Myc and Max heterodimerize and bind DNA through basic HLH leucine zipper (b-HLH-LZ) motifs. Myc mutants were generated by reciprocal exchange of b-HLH-LZ regions. MycRX contains the b-HLH-LZ region of Max and Max2RX contains the b-HLH-LZ region of Myc. MycRX sequesters Myc into inactive complexes whereas Max2RX forms stable binding complexes with wild-type Max. Because MycRX and Max2RX mutants heterodimerize, endogenous Myc activity is restored when both mutants are coexpressed in the cells.17 These Myc mutants as well as other Myc mutants18 behave as strong dominant suppressors of Myc transforming activity by decreasing endogenous active Myc protein. Because TEL/PDGFRβ-expressing Ba/F3 cells were poorly receptive to secondary transfection, Ba/F3 cells were first transfected with MycRX mutant, selected for puromycine resistance, and subsequently transfected with TEL/PDGFRβ and selected for G418 resistance.
To detect MycRX protein expression, we used an antibody directed against the carboxy terminal of Max which included the b-HLH-LZ domain. Western blot analysis indicated that an ∼67-kD protein was revealed by this antibody in Ba/F3 cells cotransfected with TEL/PDGFRβ and MycRX but not in the TEL/PDGFRβ-transfected counterparts (Fig 3a). In the absence of IL-3, MycRX and endogenous Myc expression levels were compared by Western blotting using an antibody directed against the N-terminal part of Myc. This antibody recognizes both MycRX and the endogenous Myc. Because both proteins have similar molecular weights, levels of MycRX expression were assessed by measuring an increased Myc hybridization signal. In cells cultured in medium containing 10% FCS, the level of MycRX expression was not sufficient to match the level of the endogenous Myc. Reduced endogenous Myc levels were obtained by cultivating the cells at 1% FCS concentration so that MycRX expression levels were about two times higher than endogenous Myc and likely to sequester part of the endogenous Myc (Fig 3b). Under these conditions and in contrast to the results observed in cells cultured in 10% FCS, cells coexpressing TEL/PDGFRβ and MycRX did not grow in IL-3–free medium compared with their counterparts cotransfected with TEL/PDGFRβ and the control vector (Fig 3c). Under the same conditions, Ba/F3 cells transfected with MycRX alone were able to grow in the presence of IL-3. This result suggested that TEL/PDGFRβ-expressing Ba/F3 cells were unable to grow in the absence of IL-3 when endogenous Myc was expressed at a level where MycRX exerted its dominant negative effect and thus decreased the amount of active Myc in the cell.
Myc expression and cell growth of TEL/PDGFRβ Ba/F3 cells expressing or not MycRX. (a) Protein extracts from Ba/F3 cells either transfected with TEL/PDGFRβ or cotransfected with TEL/PDGFRβ and MycRX were analyzed by Western blotting using an antibody directed against the C-terminal part of Max. (b) TEL/PDGFRβ-transfected Ba/F3 cells expressing or not expressing MycRX were cultured for 24 hours with 1% or 10% FCS, and cell lysates were analyzed by Western blotting using an Myc antibody directed against the N-terminal part of Myc. (c) TEL/PDGFRβ-transfected Ba/F3 cells expressing or not expressing MycRX were seeded at a density of 105 cells/mL in the absence of IL-3 and in the presence of either 1% or 10% FCS. Cell numbers were determined at different times of culture. Data represent the mean ± SD of three independent experiments (*P < .0001 v TEL/PDGFRβ). (▪), TEL/PDGFRβ; (•), TEL/PDGFRβ/MycRX.
Myc expression and cell growth of TEL/PDGFRβ Ba/F3 cells expressing or not MycRX. (a) Protein extracts from Ba/F3 cells either transfected with TEL/PDGFRβ or cotransfected with TEL/PDGFRβ and MycRX were analyzed by Western blotting using an antibody directed against the C-terminal part of Max. (b) TEL/PDGFRβ-transfected Ba/F3 cells expressing or not expressing MycRX were cultured for 24 hours with 1% or 10% FCS, and cell lysates were analyzed by Western blotting using an Myc antibody directed against the N-terminal part of Myc. (c) TEL/PDGFRβ-transfected Ba/F3 cells expressing or not expressing MycRX were seeded at a density of 105 cells/mL in the absence of IL-3 and in the presence of either 1% or 10% FCS. Cell numbers were determined at different times of culture. Data represent the mean ± SD of three independent experiments (*P < .0001 v TEL/PDGFRβ). (▪), TEL/PDGFRβ; (•), TEL/PDGFRβ/MycRX.
Transforming capacity of TEL/PDGFRβ is inhibited by a dominant negative mutant of Myc in Rat-1 cells.
As an additional approach to show that Myc was essential for TEL/PDGFRβ-induced transformation, we used Rat-1 cells stably expressing the Myc mutants at a level sufficient to reduce the endogenous Myc in 10% culture conditions. The Rat-1 cells used in our studies were expressing either Max2RX or MycRX/Max2RX. Because a normal activity of Myc is restored in Rat-1 cells expressing both mutants,17 these cells were used as positive controls in our experiments. We were not able to efficiently express the TEL/PDGFRβ protein in Rat-1 cells as well as in Rat-1 cells expressing MycRX. Therefore, Rat-1 cells expressing either Max2RX or MycRX/Max2RX were transfected with TEL/PDGFRβ. After 2 weeks of selection with G418, TEL/PDGFRβ expression was analyzed in the resistant cells by Western blotting. This expression was similar for both Max2RX- and MycRX/Max2RX-expressing Rat-1 cells (data not shown). Resistant cells were plated in soft agar and the number of colonies was scored after 15 to 18 days of culture. Only cells coexpressing MycRX/Max2RX and TEL/PDGFRβ were capable of forming colonies in soft agar. This indicated that expression of a dominant negative mutant of Myc could reverse the transforming potential of TEL/PDGFRβ in Rat-1 cells (Fig 4).
Expression of a dominant negative mutant of Myc impairs soft-agar colony formation by TEL/PDGFRβ. The Rat-1 expressing dominant negative mutants of Myc (MycRX, Max2RX) and transfected with the TEL/PDGFRβ vector or the control vector were plated in soft agar and the formation of colonies was assessed after 2 weeks of culture. (a) Rat-1 MycRX/Max2RX, (b) Rat-1 MycRX/Max2RX expressing TEL/PDGFRβ, (c) Rat-1 Max2RX, (d) Rat-1 Max2RX expressing TEL/PDGFRβ, (e) Number of colonies per dish. Only the colonies < 0.3 mm were counted. This is representative of three independent experiments.
Expression of a dominant negative mutant of Myc impairs soft-agar colony formation by TEL/PDGFRβ. The Rat-1 expressing dominant negative mutants of Myc (MycRX, Max2RX) and transfected with the TEL/PDGFRβ vector or the control vector were plated in soft agar and the formation of colonies was assessed after 2 weeks of culture. (a) Rat-1 MycRX/Max2RX, (b) Rat-1 MycRX/Max2RX expressing TEL/PDGFRβ, (c) Rat-1 Max2RX, (d) Rat-1 Max2RX expressing TEL/PDGFRβ, (e) Number of colonies per dish. Only the colonies < 0.3 mm were counted. This is representative of three independent experiments.
DISCUSSION
Our results indicate that the pathway leading to Myc expression is activated by TEL/PDGFRβ in Ba/F3 cells. Activation of the PDGFR by its ligand initiates several signal transduction cascades of which at least one leads to the transcriptional activation of the Myc oncogene. An active src kinase is necessary for this activation and for PDGF to induce DNA synthesis.11 Using mutants from the src family kinase, we are currently investigating whether the same processes are involved in the activation of Myc by PDGFRβ and TEL/PDGFRβ. Alternatively, overexpression of Myc in TEL/PDGFRβ-expressing Ba/F3 cells may be mediated by cytokines induced by the fusion protein. It has been reported that overexpression of PDGF B profoundly perturbs hematopoiesis in vivo producing a myeloproliferative syndrome19 and that this effect of PDGF was mediated by the induction of IL-1β.20 However, this is unlikely in the case of TEL/PDGFRβ because culture supernatants of TEL/PDGFRβ-transfected BaF3 cells did not provoke the growth of untransfected BaF3 cells (data not shown).
Using a dominant negative mutant of Myc, we showed that increased Myc expression was involved in TEL/PDGFRβ transformation and that a threshold of active Myc was essential for TEL/PDGFRβ to transform Ba/F3 and Rat-1 cells. Because Myc is known to induce cell entry into the S phase of the cell cycle, it would be of interest to investigate the cell cycle stages of TEL/PDGFRβ-transformed cells expressing or not dominant negative mutants of Myc. Previous studies have indicated that Myc is essential for transformation by BCR/ABL.21 The discovery that Myc is also essential in TEL/PDGFRβ transformation extends the repertoire of Myc action in human leukemia.
The implication of the Myc pathway in the transformation of Rat-1 cells by TEL/PDGFRβ is unlikely to reflect the whole transformation process because overexpression of Myc alone does not transform fibroblasts.22 Carroll et al5 have indicated that other PDGFRβ kinase-dependent signaling pathways are also activated by TEL/PDGFRβ. Additionally, we have found that TEL/PDGFRβ induces an increased binding of nuclear factors to the AP1 site (data not shown). However, so far, none of these pathways have been identified as being involved in the TEL/PDGFRβ transforming capacity.
Recent results have shown that TEL-induced oligomerization is essential for the activation of the tyrosine kinase activity and mitogenic properties of TEL/PDGFRβ.5,6 We have shown that CGP53716, a specific inhibitor of the PDGFRβ kinase, reverses IL-3 independence in TEL/PDGFRβ-expressing Ba/F3 cells. This reinforces the view that constitutive activation of the PDGFRβ is involved in the transforming potential of the TEL/PDGFRβ fusion protein. It has been reported that CGP56716 has an antitumor activity using v-sis overexpressing 3T3 cells, showing that it is selective for inhibition of PDGF-driven tumor growth in vivo.13 It would therefore be of interest to develop animal models to explore the effect of this inhibitor on TEL/PDGFRβ-driven tumor growth.
The findings that the kinase activity of TEL/PDGFRβ and a threshold of active Myc are involved in TEL/PDGFRβ transformation may allow for the development of therapeutic strategies using specific inhibitors of the PDGFRβ kinase as well as compounds designed to interfere specifically with Myc.
ACKNOWLEDGMENT
We thank Dr T. Golub for TEL/PDGFRβ and TEL/ABL cDNAs and Dr B. Amati for MycRX and Max2RX cDNAs and for the infected Rat-1 cells. We also thank Drs Golub and Amati for their critical reading of the manuscript and Dr E. Buchdunger (CIBA-GEIGY, Basel) for the gift of CGP53716.
Supported by INSERM and by Grant No. CA43225-10 from the National Institutes of Health (Y.E.C.). A-S.D. is a fellow of the Association pour la Recherche Contre le Cancer (ARC).
Address reprint requests to Yvon E. Cayre, U417 INSERM, Hôpital Saint-Antoine, 184 Rue du Faubourg Saint-Antoine, 75012 Paris, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.