Abstract
Recent studies have shown efficient gene transfer to primitive progenitors in human cord blood (CB) when the cells are incubated in retrovirus-containing supernatants on fibronectin-coated dishes. We have now used this approach to achieve efficient gene transfer to human CB cells with the capacity to regenerate lymphoid and myeloid progeny in nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) mice. CD34+ cell-enriched populations were first cultured for 3 days in serum-free medium containing interleukin-3 (IL-3), IL-6, granulocyte colony-stimulating factor, Flt3-ligand, and Steel factor followed by two 24-hour incubations with a MSCV-NEO virus-containing medium obtained under either serum-free or serum-replete conditions. The presence of serum during the latter 2 days made no consistent difference to the total number of cells, colony-forming cells (CFC), or long-term culture-initiating cells (LTC-IC) recovered at the end of the 5-day culture period, and the cells infected under either condition regenerated similar numbers of human CD34+ (myeloid) CFC and human CD19+ (B lymphoid) cells for up to 20 weeks in NOD/SCID recipients. However, the presence of serum increased the viral titer in the producer cell-conditioned medium and this was correlated with a twofold to threefold higher efficiency of gene transfer to all progenitor types. With the higher titer viral supernatant, 17% ± 3% and 17% ± 8%, G418-resistant in vivo repopulating cells and LTC-IC were obtained. As expected, the proportion of NEO + repopulating cells determined by polymerase chain reaction analysis of in vivo generated CFC was even higher (32% ± 10%). There was no correlation between the frequency of gene transfer to LTC-IC and colony-forming unit–granulocyte-macrophage (CFU-GM), or to NOD/SCID repopulating cells and CFU-GM (r2 = 0.16 and 0.17, respectively), whereas values for LTC-IC and NOD/SCID repopulating cells were highly and significantly correlated (r2 = 0.85). These findings provide further evidence of a close relationship between human LTC-IC and NOD/SCID repopulating cells (assessed using a ≥ 6-week CFC output endpoint) and indicate the predictive value of gene transfer measurements to such LTC-IC for the design of clinical gene therapy protocols.
TRANSDUCTION OF PLURIPOTENT hematopoietic stem cells using recombinant retroviruses forms the basis of most current strategies for the correction of single gene defects. Efficient transfer of genes into murine hematopoietic stem cells with long-term in vivo repopulating ability can now be routinely achieved using this approach.1-4 Encouraging results have also been obtained with human progenitors detectable in vitro as colony-forming cells (CFC) and their more primitive precursors identified as long-term culture-initiating cells (LTC-IC).5-9 More recent findings indicate the possibility of gene transfer to human hematopoietic cells capable of engrafting immune-deficient mice.10-12 However, the application of this technology to clinical transplants has, overall, yielded disappointing results with a few notable exceptions. The latter include results obtained using bone marrow cells from children undergoing hematopoietic recovery13 and a preliminary report of improved gene transfer under conditions that may favor maintenance of proliferating hematopoietic stem cells in vitro.14 15
Our approach has focused on the identification of factors that rapidly stimulate the proliferation of human cell populations that include transplantable progenitors without loss of their original functional potential. Recently, we showed that LTC-IC (defined using a 6-week CFC output endpoint16) and cells able to regenerate human lymphomyelopoiesis in sublethally irradiated nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) mice (referred to as competitive repopulating units [CRU]) are similarly amplified in short-term cultures of CD34+CD38lo human cord blood (CB) cells stimulated by high concentrations of Flt3-ligand (FL), Steel factor (SF), interleukin-3 (IL-3), IL-6, and granulocyte colony-stimulating factor (G-CSF).17 In addition, we found that LTC-IC and CRU in freshly isolated CB cells are similarly distributed between the CD38+ and CD38- subsets of the CD34+ CB population. These findings suggested a close relationship between the cells identified by these two assays and encouraged us to continue to use the LTC-IC assay to identify conditions for optimizing retroviral-mediated gene transfer to CRU. This allowed the development of a supernatant infection protocol that gives reproducibly high levels of retroviral-mediated gene transfer to human CB CRU (≈ 30%), which is significantly correlated with the levels of gene transfer obtained for coinfected 6-week LTC-IC, but not for coinfected CFC.
MATERIALS AND METHODS
Human cells.
Samples of CB from normal, full-term infants delivered by cesarean section were collected in heparin according to protocols approved by the University of British Columbia Clinical Screening Committee for Research Involving Human Subjects. This included obtaining informed consent from the mother before delivery. A light density (<1.077 g/mL) cell fraction was first isolated by centrifugation of the CB cells on ficoll-hypaque (Pharmacia, Uppsala, Sweden). These cells were then either used directly or were further fractionated on a StemSepÔ column (StemCell Technologies Inc, Vancouver, BC) to isolate a CD34+ cell-enriched population (by removal of cells expressing surface antigens characteristic of various mature hematopoietic cells18), according to the manufacturer's instructions. In some experiments, highly purified (>99.9%) CD34+ or CD34+CD38lo cells were isolated from these lin- cells by fluorescence-activated cell sorting (FACS), as described in detail elsewhere.17 In the remainder, the enriched CD34+ cells were used without further purification. Surplus human bone marrow (BM) cells were obtained with informed consent from normal adult donors of allogeneic BM transplants or were cadaveric samples obtained from the Northwest Tissue Centre (Seattle, WA). To generate marrow fibroblasts for the infection experiments, fresh BM cells were first cultured for at least 5 weeks in Iscove's medium with 20% fetal calf serum (FCS; StemCell) and then subcultured repeatedly until a pure fibroblast monolayer was obtained.
Human cytokines.
Highly purified recombinant IL-3 and granulocyte-macrophage CSF (GM-CSF) were gifts from Novartis (formerly Sandoz, Basel, Switzerland). IL-6 and SF were purified from media conditioned by COS cells that had been transiently transfected in the Terry Fox Laboratory with the corresponding human cDNAs. FL was a gift from Immunex Corp (Seattle, WA) and purified human erythropoietin (Ep) and G-CSF were kindly provided by StemCell.
Retroviral vector.
An MSCV-NEO virus19 constructed using the MSCV 2.1 vector (kindly provided by Dr R. Hawley, University of Toronto, Toronto, Canada) was used to establish a GP-env AM12 MSCV-NEO producer cell line as described.18 The titer of these producer cells was 107 colony-forming units/mL as assessed by the transfer of G418 resistance to NIH-3T3 cells.20 The producer cells were shown to be free of helper virus, as indicated by the inability to recover infectious virus from MSCV-NEO–infected NIH-3T3 cells (capable of transferring G418 resistance to a culture of naive NIH-3T3 cells). Supernatants were collected from confluent cultures of MSCV-NEO virus-producing cells after incubation of these overnight with fresh Iscove's medium containing 20% FCS or bovine serum albumin, insulin, and transferrin (BIT, StemCell) as indicated. The medium was then obtained, filtered through 0.4-mm filters, and stored frozen at −196°C, or was used directly.
CFC and LTC-IC assays.
Methylcellulose assays (all reagents from StemCell) were performed essentially as previously described.16 After infection, some cells were plated in methylcellulose both with and without G418 (1.6 mg/mL, dry weight, GIBCO-BRL, Burlington, Canada). At these concentrations of G418, no colony growth from uninfected cells was seen in control groups (mock-infected cells) included in every experiment. LTC-IC assays were performed also as described16 with maintenance of the cultures at 37°C with weekly half-medium changes for 6 weeks, at the end of which the nonadherent and adherent fractions were obtained, pooled, and plated in methylcellulose with and without G418 as indicated.
Assessment of mice transplanted with human cells.
NOD/LtSZ-scid/scid mice21 bred in the animal facility at our institution were housed in microisolator cages and given autoclaved food and water, acidified just before and after total body irradiation (350 cGy). Human CB cells plus 106irradiated (1,500 cGy) normal human BM cells as carrier cells were then injected intravenously. Mice were maintained at least 6 weeks after transplantation, at which time they were killed and the cellular contents of both femurs and both tibias flushed out with HFN (Hanks' buffered salt solution containing 2% fetal calf serum and 0.1% sodium azide) and a single cell suspension obtained from each mouse. Aliquots were stained as previously described17 with anti–CD45-fluorescein isothiocyanate (FITC; HLe 1; Becton Dickinson, Mountain View, CA) and anti–CD71-FITC (OKT9),22 anti–CD19-phycoerythrin (PE; Leu12; Becton Dickinson) and anti–CD34-CY5 (8G12),23 or FITC-conjugated and PE-conjugated mouse Ig as negative controls. Normal mouse BM cells showed less than 0.1% nonspecific staining with these antibodies. In animals containing both human lymphoid (CD19+) and human CD34+ populations, the human CD34+ cells were sorted and plated in CFC assays with and without G418 as described above.
Polymerase chain reaction (PCR) analysis.
Colonies generated in CFC assays were plucked and analyzed individually using the PCR and Southern blotting with a NEOr probe to amplify and identify incorporated NEO-specific sequences as previously described.24
RESULTS
Validation of the supernatant infection protocol.
In an initial series of experiments, the efficiency of infecting human CB CFC and LTC-IC when the target cells were incubated with MSCV-NEO virus-containing supernatants under various culture conditions was compared with the levels of gene transfer obtained by cocultivation with MSCV-NEO viral-producer cells. The conditions chosen were based on previously reported findings that coincubation of the target cells on fibronectin25,26 or fibroblasts5 27 could improve the efficiency of gene transfer to primitive human hematopoietic cells. Either light density (106/mL) or lin- (36% ± 6% CD34+, 105/mL) CB cells were first incubated in the absence of virus for 48 hours in Iscove's medium with 20% FCS and 20 ng/mL IL-3, 10 ng/mL IL-6, and 50 ng/mL SF. Aliquots of these prestimulated cells were then incubated in the same culture volume for an additional 48 hours either in cell-free virus-containing medium supplemented with the same cytokines in petri dishes or in dishes that had been precoated with human full-length fibronectin (Sigma, St Louis, MO) at a concentration of 5 μg/cm2, or on top of a monolayer of irradiated (1,500 cGy) allogeneic human marrow-derived fibroblasts, or in fresh medium containing the same cytokines on top of a monolayer of irradiated (150 cGy) producer cells, as indicated. Polybrene was added to all media to give a final concentration of 4 μg/mL. The cytokine-supplemented viral supernatants (and control media) were replaced halfway through the 48-hour infection period, at the end of which all nonadherent cells were obtained, washed, and assessed for G418-resistant CFC and LTC-IC. The results are summarized in Table 1. Supernatant infection on fibronectin-coated plates gave similarly high levels of gene transfer to LTC-IC, as were obtained by cocultivation (44% v 39%) and both conditions also gave a high level of gene transfer to CFC. Supernatant infection in the absence of either fibronectin or human marrow fibroblasts produced very low levels of gene transfer to any type of progenitor. The presence of human fibroblasts improved gene transfer efficiencies to CFC, but the gene transfer efficiencies and recoveries of LTC-IC were reduced to levels that precluded their assessment.
Retention of CRU activity during infection.
A series of six experiments were then undertaken to determine how the maintenance of CRU activity might be influenced by incubation of the cells with a retroviral supernatant generated in medium containing 20% FCS or medium supplemented with a defined serum substitute (BIT; StemCell). At the time of starting these experiments, we had just determined that FL in addition to SF, IL-3, and IL-6 (or G-CSF) is important for achieving optimal expansion of LTC-IC and CFC in short-term cultures of normal adult human BM,28 and that this combination of cytokines would also support some expansion of CB LTC-IC and CRU (fourfold and twofold, respectively), in 5- to 8-day cultures.17 Therefore, the cytokines selected for use in this next set of gene transfer experiments were changed from the previous combination to FL and SF (100 ng/mL each) plus IL-3, IL-6, and G-CSF (20 ng/mL each). To avoid the toxicity that polybrene had been found to have on primitive cells29 (which we confirmed), the polybrene was replaced with 5 μg/mL of protamine sulphate. In addition, the period of prestimulation was extended from 48 to 72 hours. This latter change was based on our observations of single CD34+CD38- CB cells, which showed that under the conditions used, all viable cells would divide within 5 days, but not before.17 Four of the experiments were set up with lin- CB cells (at 105 cells/mL), one with FACS-purified CD34+ CB cells (at 105 cells/mL), and one with FACS-purified CD34+CD38lo CB cells (at 104 cells/mL). The rest of the protocol was the same as had been found to be optimal in the previous experiments, ie, the cells were prestimulated in cytokine-supplemented, serum-free medium followed by 48 hours of infection on fibronectin-coated petri dishes with replacement of the cytokine-supplemented viral supernatants (prepared either in medium plus 20% FCS or serum-free plus BIT) after the first 24 hours. At the end of the second 24 hours of infection, the cells were obtained and assayed for CFC, LTC-IC, and for their ability to generate lymphoid and myeloid progeny after their transplantation into sublethally irradiated NOD/SCID mice. The input cell type and numbers and the number of resulting positive mice for human CFC and CD19+ cells is shown in Table2. Figure 1 shows a representative dot plot of the relative numbers of total human cells and human CD34+ cells present in the marrow of one of the mice transplanted with human cells from these experiments. As shown in Table 3, the presence or absence of serum in the cultures from which the cells transplanted were obtained made no consistent difference to any of the endpoints of human engraftment assessed in mice up to 15 weeks posttransplant. In addition, there was also no difference in the total numbers of cells, CFC, or LTC-IC recovered from the two types of infection cultures (ie, viral supernatants prepared in serum-free or serum-replete medium, data not shown). The results from both procedures were therefore pooled to derive mean (± standard error of mean [SEM]) yields of each progenitor cell type at the end of the 5-day infection culture period (per 105 input CD34+ cells) as follows: 3.4 ± 1.1 × 106 total cells, 1.4 ± 0.4 × 106 CFC, and 790 ± 290 LTC-IC (the results from the experiment that was performed with CD34+CD38locells was excluded from this analysis).
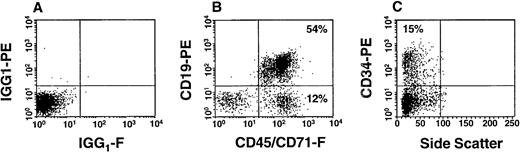
Phenotypic analysis of BM cells derived from a NOD/SCID mouse transplanted 20 weeks previously with the infected progeny of 3.5 × 104 FACS-purified CD34+ human CB cells. In (A), the cells were stained with irrelevant isotype-matched mouse IgG labelled with FITC and PE and the gates shown set to exclude 99.9% of these cells. In (B), the cells were stained with a combination of anti–CD45/71-FITC and anti–CD19-PE. In (C), the cells were stained with anti–CD34-PE.
Phenotypic analysis of BM cells derived from a NOD/SCID mouse transplanted 20 weeks previously with the infected progeny of 3.5 × 104 FACS-purified CD34+ human CB cells. In (A), the cells were stained with irrelevant isotype-matched mouse IgG labelled with FITC and PE and the gates shown set to exclude 99.9% of these cells. In (B), the cells were stained with a combination of anti–CD45/71-FITC and anti–CD19-PE. In (C), the cells were stained with anti–CD34-PE.
Gene transfer to human CB progenitors.
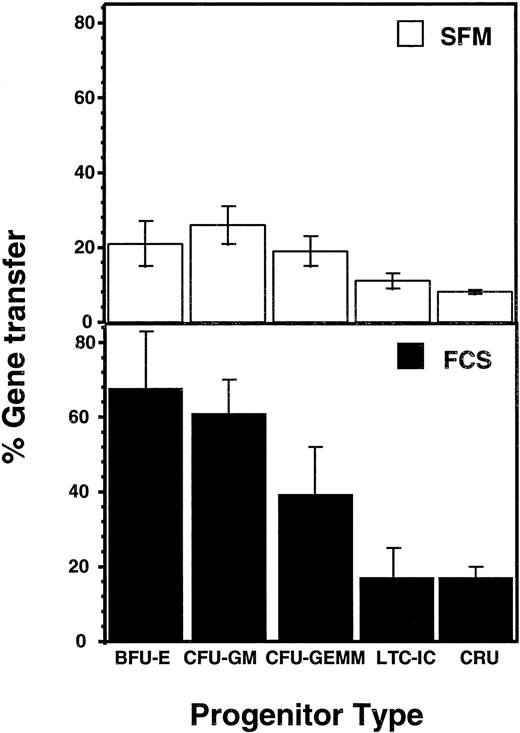
To assess gene transfer efficiencies in these latter experiments, the proportion of G418-resistant CFC, or progeny CFC derived from LTC-IC or (in vivo) from the CRU injected into the NOD/SCID mice was determined. The results, shown in Fig 2, show average gene transfer efficiencies that range from a maximum of 68% (burst-forming unit-erythroid [BFU-E] exposed to FCS-containing supernatants) to a low of 8% (CRU exposed to BIT-containing supernatants). However, for each progenitor type, there was an approximately twofold to threefold higher proportion of G418-resistant cells when these were infected with FCS-containing supernatants, despite the fact that the total number of progenitors present had not been affected. Assessment of the viral titer of the supernatants prepared with FCS and BIT showed a threefold difference (6 × 106 in FCS v 2 × 106 in BIT, n = 2). Thus, the most likely cause of the reduced gene transfer obtained with the BIT-containing supernatants was simply their reduced content of virus.
Comparison of gene transfer efficiencies to different types of human CB progenitors infected under serum-free versus serum-replete conditions and assessed by measurement of G418-resistance. Values represent the mean ± SEM.
Comparison of gene transfer efficiencies to different types of human CB progenitors infected under serum-free versus serum-replete conditions and assessed by measurement of G418-resistance. Values represent the mean ± SEM.
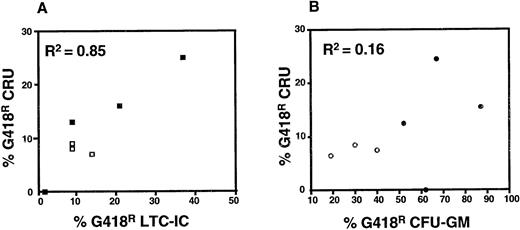
The results shown in Fig 2 also indicate a similar efficiency of gene transfer to human CB CRU and LTC-IC under the best conditions (17% ± 3% and 17% ± 8%, respectively). When gene transfer efficiencies to CRU, LTC-IC, and CFU-GM in individual experiments were compared, the results for LTC-IC and CRU were significantly correlated (r2 = 0.85, P < .01), whereas there was no correlation between the corresponding gene transfer efficiencies to LTC-IC or CRU and CFU-GM (r2 = 0.16 and 0.17, respectively, P > .05) (Fig 3). Results for BFU-E and CFU-GEMM were similar to those shown for CFU-GM, although the numbers of these were lower and hence the data less reliable (data not shown).
Correlation analysis of gene transfer efficiency to CRU and LTC-IC (A) and to CRU and CFU-GM (B). Results shown include data from protocols in which either FCS (solid symbols) or BIT-containing (open symbols) viral supernatants were used. The average number of colonies counted to calculate the efficiency of gene transfer to CFU-GM ranged from 77 to 404 (mean = 154) and from 41 to 350 (mean = 123) in the presence and absence of G418, respectively. For assessment of LTC-IC, the numbers of colonies counted ranged from 12 to 115 (mean = 57) and from 1 to 85 (mean = 20) in the presence and absence of G418, respectively.
Correlation analysis of gene transfer efficiency to CRU and LTC-IC (A) and to CRU and CFU-GM (B). Results shown include data from protocols in which either FCS (solid symbols) or BIT-containing (open symbols) viral supernatants were used. The average number of colonies counted to calculate the efficiency of gene transfer to CFU-GM ranged from 77 to 404 (mean = 154) and from 41 to 350 (mean = 123) in the presence and absence of G418, respectively. For assessment of LTC-IC, the numbers of colonies counted ranged from 12 to 115 (mean = 57) and from 1 to 85 (mean = 20) in the presence and absence of G418, respectively.
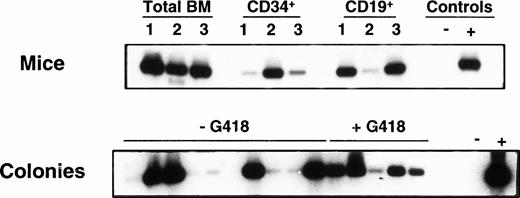
Although assessment of G418-resistant colony formation provides a convenient method of quantitating gene transfer efficiency using NEO-containing retroviruses, such estimates typically underestimate the frequency of infected cells due to a variety of mechanisms that may block or reduce expression of the integrated retroviral cDNA. In addition, some cell types cannot be monitored this way. Therefore, to further characterize the progeny of the infected CB cells that engrafted the NOD/SCID mice, some of the human colonies generated in vitro from the human CD34+ cells isolated from their marrows (6 to 20 weeks posttransplant) were plucked and assessed individually for the presence of the NEO gene by PCR. In addition, in one experiment, highly purified human CD19+ (B lineage) cells sorted from three mice were similarly analyzed. The human lymphoid and human myeloid cells from all mice analyzed showed integration of the NEO gene. Results from a representative experiment are shown in Fig 4. Table 4 shows a detailed comparison of the estimates of gene transfer to the CRU obtained from the infected CB cultures based on the G418 resistance of versus the presence of NEO sequences in human CFC obtained from mice 6 to 20 weeks after they had been injected with the infected human cells. Values determined by PCR analysis were consistently approximately twofold to threefold higher, suggesting that the actual efficiency of gene transfer to the transplanted CRU was correspondingly higher (ie, 32% ± 10%).
PCR detection of NEO sequences in cells obtained from NOD/SCID recipients engrafted with infected human CB cells (Exp. 3 in Table 4). The upper panel shows results for total BM and isolated human CD34+ and CD19+ populations. The lower panel shows results for individual colonies derived from the sorted human CD34+ cells plated with and without G418. Densitometric analysis of the amount of DNA in each lane relative to PCR of the actin gene done on the same colonies were as follows: lanes 1 to 16: 0.8, 0.7, 0.7, 0.4, 0.3, 0.5, 1, 0.4, 0.4, 0.9, 0.7, 0.6, 0.4, 0.4, 0.7, 0.7. Because the NEO signal in lane 1 is weaker than expected relative to the amount of actin present, we have not called this colony positive.
PCR detection of NEO sequences in cells obtained from NOD/SCID recipients engrafted with infected human CB cells (Exp. 3 in Table 4). The upper panel shows results for total BM and isolated human CD34+ and CD19+ populations. The lower panel shows results for individual colonies derived from the sorted human CD34+ cells plated with and without G418. Densitometric analysis of the amount of DNA in each lane relative to PCR of the actin gene done on the same colonies were as follows: lanes 1 to 16: 0.8, 0.7, 0.7, 0.4, 0.3, 0.5, 1, 0.4, 0.4, 0.9, 0.7, 0.6, 0.4, 0.4, 0.7, 0.7. Because the NEO signal in lane 1 is weaker than expected relative to the amount of actin present, we have not called this colony positive.
DISCUSSION
The studies described in this report identify conditions that allow human in vivo repopulating cells to be reproducibly infected by recombinant retroviruses at high efficiency (≈ 30%) using a protocol that should be readily adaptable to clinical applications. This infection procedure builds on a series of previous important observations including the identification of a cytokine cocktail that stimulates the expansion in vitro of human CB in vivo repopulating cells17 and recognition of the ability of fibronectin-coated dishes to enhance gene transfer efficiencies using cell-free viral supernatants.25,26 The toxicity that polybrene has for primitive human hematopoietic cells29 was circumvented by using protamine sulphate as a substitute. We also chose to focus on human CB as a source of the hematopoietic stem cells to be infected. This was based on previous work suggesting that these might be more susceptible to retroviral infection,8,9 and that they also regenerate larger numbers of lymphoid and myeloid progeny in NOD/SCID mice and for more prolonged periods compared with the repopulating cells present in normal adult human BM.30 The adoption of a 5-day infection protocol (3 days of prestimulation plus 2 days of infection) was based on studies indicating that even under conditions of optimized cytokine stimulation, some human CD34+CD38lo cells require this duration of cytokine exposure before they will divide.17,31 32
To document gene transfer to human CB cells with in vivo repopulating activity, sublethally irradiated NOD/SCID mice were injected with the 5-day progeny of relatively large numbers of input CD34+cells, sufficient to obtain greater than 1% engraftment of human cells in greater than 80% of the recipients (20 of 23). Evidence of the expression and/or presence of the NEO gene in the human CFC and CD19+ (B lineage) cells isolated from the bone marrow of the engrafted mice 6 to 20 weeks posttransplant was used to infer the presence of infected human CRU in the cells originally injected (ie, in vivo repopulating human stem cells with lymphomyeloid differentiation potential). We have previously shown that greater than 90% of NOD/SCID mice transplanted with limiting numbers of human CB cells produce both lymphoid and myeloid progeny, indicative of the origin of both of these populations from a common stem cell.17 Thus, although direct evidence of clonal populations containing both retrovirally marked lymphoid and myeloid elements was not obtained in the present experiments, our previous findings would suggest that the genetically marked CFC and pre-B cells detected were generated from infected human CB CRU. It should be noted that we made a conscious effort to transplant nonlimiting numbers of infected CRU into the NOD/SCID mice to minimize variability between recipients in the proportion of regenerated human cells that would be genetically marked. This was then tested by comparing the proportions of G418-resistant and NEO sequence-positive human CFC demonstrable in individual mice injected with aliquots of the same infected CB cell population. The level of gene transfer achieved was sufficient to mark a readily detectable proportion of the human CFC present in the 80% of mice where CFC were regenerated, and the proportion of marked CFC was highly consistent between all mice in a given set, regardless of the method used to identify the marked cells. If the mice had been injected with only one or two CRU, a larger proportion of mice in each experiment would have been expected to not contain any human cells, and all of the human cells in the engrafted mice would have been either marked or not, a situation which, interestingly, fits the findings reported by Larochelle et al.11
Efficient gene transfer to human in vivo repopulating cells present in adult marrow10,33 and fetal liver12 has also been reported recently by other groups. In two of these studies, beige-nude-xid (bnx) mice were used as recipients. These mice allow human myeloid and T-cell progeny to be generated, but not B-lineage cells and overall seem to support much lower levels of human hematopoiesis than NOD/SCID mice. Nevertheless, high level gene transfer to human marrow cells able to engraft bnx mice was reported for cells infected in the presence of stroma, and FL could partially overcome this requirement for stromal cells, which our present studies confirm. In the studies of Yurasov et al,12 who used human fetal liver cell targets, greater infectivity would be expected from their likely increased proliferative activity.34 Our findings thus extend those recently reported by others highlighting the importance of using an infection protocol that optimizes stem cell recovery, as well as infection efficiency. Moreover, the present studies show that these requirements can be met under conditions that are suitable for clinical application. In the future, the possibility of adding other strategies to selectively isolate retrovirally-infected human stem cells,18 as has been achieved with murine stem cells35,36 or other types of human cells,37should, with the gene transfer efficiencies now achievable, allow useful numbers of viable 100% gene-modified human stem cell populations to be obtained.
Many groups have shown that LTC-IC from different sources can be subdivided into biologically distinct subtypes according to the longevity of their CFC-producing ability.38-40 In fact, this principle was first used to discriminate LTC-IC as a population distinct from CFC.38,41 It has also allowed murine cells with short- versus long-term in vivo repopulating abilities to be distinguished.42-45 Thus, some functional heterogeneity among human cells detectable either as LTC-IC or as CRU likely exists, consistent with their heterogeneity in CD38 expression.17However, because human CB CRU are identified at a frequency that is several hundred-fold lower than the frequency of CB LTC-IC, independent of their phenotype,17 it is difficult to establish the precise relationship of CRU and LTC-IC, particularly in the absence of any independent information concerning the relative efficiencies of the procedures used to detect them. Nevertheless, the highly significant correlation shown here between the efficiency of gene transfer to LTC-IC and CRU in human CB (both assessed using a ≥ 6-week endpoint) provides further evidence of a close relationship between these two populations and also serves to emphasize the predictive value of measuring gene transfer to such LTC-IC as a prelude to more ambitious and labor-intensive in vivo experiments.
ACKNOWLEDGMENT
The authors thank Dr R. Hawley (University of Toronto); Dr P. Lansdorp (Terry Fox Laboratory, Vancouver, BC); and StemCell and Novartis for valuable gifts of vectors, antibodies, and other reagents. The expert technical help of Margaret Hale, Gayle Thornbury, Jessyca Maltman, and Maya Sinclaire, and the secretarial assistance of Bernadine Fox are also acknowledged.
Supported by Grant No. HL55435 from the National Institutes of Health (Bethesda, MD), the National Cancer Institute of Canada (NCIC) (Toronto, Ontario, Canada) with funds from the Terry Fox Run, the Medical Research Council of Canada (Ottawa, Ontario, Canada), and Novartis (Basel, Switzerland). E.C. held a Terry Fox Physician Scientist Fellowship and C.J.E. is a Terry Fox Cancer Research Scientist of the NCIC.
Address reprint requests to R.K. Humphries, MD, PhD, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, BC V5Z 1L3, Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.