The human P2Y1 receptor heterologously expressed in Jurkat cells behaves as a specific adenosine 5′-diphosphate (ADP) receptor at which purified adenosine triphosphate (ATP) is an ineffective agonist, but competitively antagonizes the action of ADP. This receptor is thus a good candidate to be the elusive platelet P2T receptor for ADP. In the present work, we examined the effects on ADP-induced platelet responses of two selective and competitive P2Y1 antagonists, adenosine-2′-phosphate-5′-phosphate (A2P5P) and adenosine-3′-phosphate-5′-phosphate (A3P5P). Results were compared with those for the native P2Y1 receptor expressed on the B10 clone of rat brain capillary endothelial cells (BCEC) and for the cloned human P2Y1 receptor expressed on Jurkat cells. A2P5P and A3P5P inhibited ADP-induced platelet shape change and aggregation (pA2 = 5) and competitively antagonized calcium movements in response to ADP in fura-2–loaded platelets, B10 cells, and P2Y1-Jurkat cells. In contrast, these compounds had no effect on ADP-induced inhibition of adenylyl cyclase in platelets or B10 cells, whereas known antagonists of platelet activation by ADP such as Sp-ATPαS were effective. These identical signaling responses and pharmacologic properties suggest that platelets and BCEC share a common P2Y1 receptor involved in ADP-induced aggregation and vasodilation, respectively. This P2Y1 receptor coupled to the mobilization of intracellular calcium stores was found to be necessary to trigger ADP-induced platelet aggregation. The present results, together with data from the literature, also point to the existence of another as yet unidentified ADP receptor, coupled to adenylyl cyclase and responsible for completion of the aggregation response. Thus, the term, P2T, should no longer be used to designate a specific molecular entity.
EXTRACELLULAR ADENINE nucleotides induce various physiologic responses in many tissues. In the cardiovascular system, adenine nucleotides released from damaged cells, especially from red blood cells, endothelial cells, or aggregating platelets contribute to the control of vascular tone and hemostasis.1The central role of adenosine 5′-diphosphate (ADP) as an aggregating agent,2,3 not only in the physiologic processes of hemostasis but also in the development and extension of arterial thrombosis,4 has been established for a long time. Patients who display a rare congenital thrombopathy resulting in a selective defect in ADP-induced platelet activation have been described.5,6 This would suggest a potential clinical importance of the platelet ADP receptor.7-9 This receptor, called P2T, belongs to the nucleotide receptor or P2 superfamily, as distinct from the P1 receptor family specific for adenosine.10 P2 receptors are divided into two main groups: the G protein coupled receptor or “metabotropic” superfamily termed P2Y and the ligand gated ion channel receptor or “ionotropic” superfamily termed P2X. Seven subtypes of P2X and 11 subtypes of P2Y receptor have been identified.11 To date, the P2T receptor has been characterized by second messenger signaling and pharmacologic data, ADP, and related diphosphate analogues being agonists as opposed to adenosine 5′-triphosphate (ATP) and related triphosphate nucleotides such as Sp-ATPαS, 2ClATP, and βγMeATP, which are competitive antagonists.8
Stimulation of platelets by ADP leads to a transient increase in intracellular calcium ([Ca2+]i) due to both rapid calcium influx and mobilization of internal stores12and simultaneously to inhibition of adenylyl cyclase.8,9,13The question of the presence of one or more receptors separately mediating these effects of ADP on calcium movements and adenylyl cyclase has been debated for a long time8,9,13 and still remains open. A good correlation between the effects of agonists and antagonists on aggregation, inhibition of adenylyl cyclase, and [Ca2+]i increases argues for a single ADP receptor mediating these processes.13 However, studies using selective inhibitors of ADP-induced platelet aggregation such as the thienopyridines, ticlopidine and clopidogrel,14 which block ADP-induced inhibition of adenylyl cyclase15 and G protein activation,16 but inhibit only partially the binding of radiolabeled 2MeSADP to intact platelets17-19without inhibiting shape change or the ADP-induced [Ca2+]i increase,15,19 strongly suggest the existence of two receptors separately mediating [Ca2+]i increases and inhibition of adenylyl cyclase. Finally, platelets also exhibit a nonselective cation channel responsible for the rapid calcium influx component of the [Ca2+]i increase unique to ADP stimulation.20 Although this has been shown to be a P2X1 receptor,21,22 its role in the complex process of ADP-induced platelet activation remains to be assessed.23
Recently, we reported cloning24 of the human P2Y1 purinoceptor and its pharmacologic characterization through heterologous expression in Jurkat cells.25 It was demonstrated that this receptor, contrary to common knowledge,26-30 is not an ATP receptor, but an ADP receptor for which adenosine triphosphate nucleotides are competitive antagonists. This pharmacologic profile closely resembles that of the still unidentified platelet ADP receptor. Furthermore, using reverse transcriptase-polymerase chain reaction (RT-PCR) amplification, we found the P2Y1 receptor to be present on blood platelets and megakaryoblastic cell lines. Thus, these results strongly suggested the P2Y1 receptor to be the elusive P2T receptor. The P2Y1 receptor, the first P2 receptor subtype to be cloned,26 has a broad tissue distribution.11Rat brain capillary endothelial cells (BCEC) have been shown to express a specific ADP receptor,31-33 which was more recently identified as a P2Y1 receptor using RT-PCR in a subclone of BCEC termed B10.34 This receptor is linked to the mobilization of internal calcium stores and negatively to adenylyl cyclase, thus bearing a striking resemblance to the ADP receptor of platelets.
The aim of the present study was to further address the question of the molecular identity of the platelet ADP receptor and in particular the possibility of its being of the P2Y1 type. In this objective, we compared the effects of two selective P2Y1antagonists, adenosine-2′-phosphate-5′-phosphate (A2P5P) and adenosine-3′-phosphate-5′-phosphate (A3P5P),35 on ADP-induced platelet activation, on the native P2Y1 receptor expressed on the B10 clone of rat BCEC and on the cloned human P2Y1 receptor heterologously expressed in Jurkat cells. Platelets and BCEC are found to share a common P2Y1 receptor coupled to the mobilization of intracellular calcium stores, which is necessary to allow ADP-induced platelet aggregation. These results, together with data from the literature, support the hypothesis that an ADP receptor coupled to adenylyl cyclase is responsible for completion of the aggregation response.
MATERIALS AND METHODS
Materials.
Adenosine 5′-O-(1-thiotriphosphate) (Sp-isomer) (Sp-ATPαS) was from Boehringer (Mannheim, Germany) and 2-methylthio-adenosine 5′-diphosphate (2MeSADP) from Research Biochemicals Inc (Natick, MA). ADP, ATP, A2P5P, A3P5P, isobutyl methyl xanthine (IBMX), U46619, thrombin, epinephrine, prostaglandin E1 (PGE1), bovine collagen type I, and essentially fatty acid-free human serum albumin were purchased from Sigma (Saint Quentin-Fallavier, France) and human fibrinogen from Kabi (Stockholm, Sweden). Fura-2/acetoxymethyl ester (fura-2/AM) and indo-1/AM were from Calbiochem (Meudon, France). The cyclic adenosine 3′-5′-monophosphate (cAMP) dosage kit was purchased from Amersham (Les Ulis, France) and apyrase was purified from potatoes as previously described.36 A2P5P and A3P5P were checked for purity by high performance liquid chromatography (HPLC) analysis on a Partisil 10 μm SAX column (Interchrom, Interchim, Monluçon, France) eluted with a linear gradient of 0 to 1 mol/L ammonium phosphate buffer, pH 3.8.
Cell cultures.
Jurkat E6.1 cells (ECACC No. 88042803, Cerdic, France) stably expressing the human P2Y1 receptor were grown in RPMI-1640 medium supplemented with 10% (vol/vol) heat inactivated fetal calf serum, 2 mmol/L glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 1 mg/mL geneticin. B10 clone cells from rat BCEC were grown in Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) heat inactivated fetal calf serum, 2 mmol/L glutamine, 100 U/mL penicillin, and 0.1 mg/mL streptomycin. Cultures were kept at 37°C in a humidified atmosphere containing 5% CO2 and cells were subcultured every 3 days so as to maintain a density of approximately 5 × 105 cells/mL.
Preparation of washed human platelets.
Washed human platelets were prepared as previously described.36 Briefly, fresh blood obtained from healthy donors was centrifuged at 175g for 15 minutes at 37°C and platelet-rich plasma was removed and centrifuged at 1,570g for 15 minutes at 37°C. The platelet pellet was washed twice in Tyrode's buffer (137 mmol/L NaCl, 2 mmol/L KCl, 12 mmol/L NaHCO3, 0.3 mmol/L NaH2PO4, 1 mmol/L MgCl2, 5.5 mmol/L glucose, 5 mmol/L HEPES, pH 7.3) containing 0.35% human serum albumin and finally resuspended at a density of 3 × 105 platelets/μL in the same buffer in the presence of 0.02 U/mL of the ADP scavenger apyrase (adenosine 5′-triphosphate diphosphohydrolase, EC 3.6.1.5), a concentration sufficient to prevent desensitization of platelet ADP receptors during storage. Platelets were kept at 37°C throughout all experiments.
Platelet aggregation studies.
Aggregation was measured at 37°C by a turbidimetric method in a dual-channel Payton aggregometer (Payton Associates, Scarborough, Ontario, Canada). A 450-μL aliquot of platelet suspension was stirred at 1,100 rpm and activated by addition of different agonists, in the presence or absence of A2P5P or A3P5P at varying concentrations and in the presence of human fibrinogen (0.8 mg/mL), in a final volume of 500 μL. The extent of aggregation was estimated quantitatively by measuring the maximum curve height above baseline level. ADP-induced shape change was determined turbidimetrically in the presence of 5 mmol/L ethylenediaminetetraacetic acid (EDTA).
[Ca2+]i measurements.
After centrifugation of human platelet-rich plasma at 1,570gfor 15 minutes at 37°C, the platelet pellet was resuspended in Tyrode's buffer containing no albumin or calcium at a density of about 6 × 105 platelets/μL. Platelets were loaded with 2 μmol/L fura-2/AM for 45 minutes at 37°C in the dark, washed in Tyrode's buffer containing 0.35% human serum albumin, and finally resuspended at 37°C at a density of 3 × 105 platelets/μL in Tyrode's buffer containing apyrase and 0.1% essentially fatty acid–free human serum albumin.
Jurkat cells stably expressing the human P2Y1 receptor were washed in basal salt solution (BSS: 25 mmol/L HEPES, pH 7.3, 125 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 5 mmol/L glucose, 0.1% human serum albumin) containing 2 mmol/L CaCl2. After centrifugation at 100g for 5 minutes, the cells were resuspended in BSS without calcium at a concentration of 15 × 106 cells/mL and loaded with 5 μmol/L fura-2/AM at 37°C for 30 minutes in the dark. The cells were then pelleted and resuspended at a density of 2 × 106 cells/mL in BSS containing either 2 mmol/L calcium or no calcium (0.2 mmol/L ethylene glycol-bis[β-aminoethyl ether]N,N,N',N'-tetraacetic acid [EGTA]). Aliquots of fura-2–loaded platelets or cells were transferred to a 10 × 10-mm quartz cuvette maintained at 37°C and fluorescence measurements were performed under continuous stirring, using a PTI deltascan spectrofluorimeter (Photon Technology International Inc, South Brunswick, NJ). The excitation wavelength was alternately fixed at 340 or 380 nm and fluorescence emission was determined at 510 nm.
B10 cells were loaded with 5 μmol/L indo-1/AM for 2 hours in complete culture medium at 37°C. After dissociation from the culture dishes, the cells were centrifuged at low speed and resuspended in Earle's salt solution (25 mmol/L HEPES, pH 7.4, 140 mmol/L NaCl, 5 mmol/L KCl, 1.8 mmol/L CaCl2, 0.8 mmol/L MgSO4, 5 mmol/L glucose). Flow cytometric analysis of indo-1 fluorescence was performed as previously described32using a FacStar Plus apparatus (Becton Dickinson, San Jose, CA). The indo-1 fluorescence ratio was measured in 5,000 single cells and collected in real time at a rate of 500 cells/second.
Measurement of adenylyl cyclase activity.
A 450-μL aliquot of washed platelets was stirred at 1,100 rpm in an aggregometer cuvette, and the following reagents were added at 30-second intervals: 1 μmol/L PGE1, 100 μmol/L A2P5P or A3P5P, and 5 μmol/L ADP or vehicle (Tyrode's buffer containing no Ca2+ or Mg2+). One minute later, the reaction was stopped by addition of 50 μL of ice-cold 6.6 N perchloric acid. B10 cells grown in 6-well tissue culture clusters were first incubated in BSS supplemented with 10-4 mol/L IBMX for 10 minutes at 37°C. Forskolin (1 μmol/L) and/or nucleotides were added (final volume 1 mL per well) and incubation was continued for 5 minutes at 37°C, after which the incubation solution was removed by aspiration and the cells extracted in 10% (vol/vol) ice-cold 6.6 N perchloric acid. The same procedure was applied to Jurkat cells except that the incubation solution was eliminated by centrifuging each tube at 200g for 30 seconds before extracting the cells in perchloric acid. Perchloric acid extracts were centrifuged at 11,000g for 5 minutes to eliminate protein precipitate and cyclic AMP was isolated from the supernatants as described by Khym37 using a mixture of trioctylamine and freon (28/22, vol/vol). The upper aqueous phase was lyophilized and the dry residue dissolved in the buffer provided with the commercial radioimmunoassay kit for cyclic AMP measurement.
Data analysis.
Agonist potencies and apparent dissociation constants of inhibitors (pA2 = −log KD) were calculated using the GraphPad software package (GraphPad, San Diego, CA).
RESULTS
P2Y1 antagonists noncompetitively inhibit ADP-induced platelet aggregation.
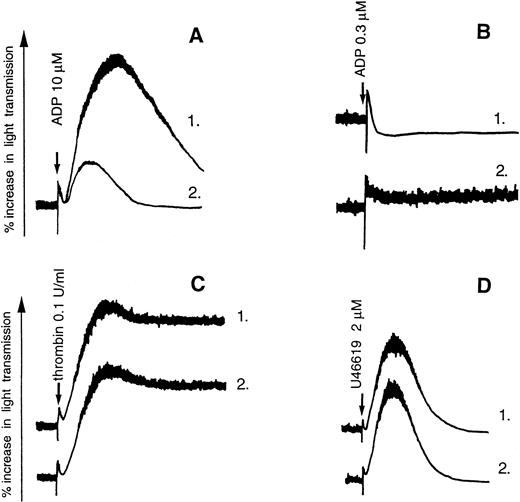
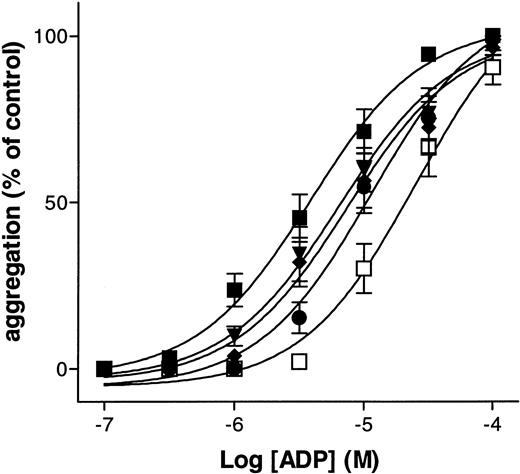
The adenine nucleotide derivatives A2P5P and A3P5P induced no aggregation or shape change of washed human platelets, even at high concentrations (up to 100 μmol/L). On the other hand, ADP-induced platelet aggregation was inhibited by both A2P5P and A3P5P (Fig 1A). The two P2Y1 receptor antagonists were also able to inhibit ADP-induced platelet shape change, as was demonstrated in the presence of 5 mmol/L EDTA, an agent that blocks aggregation by preventing the binding of fibrinogen to platelets (Fig 1B). This effect was selective, as these antagonists did not inhibit the aggregation induced by 0.1 U/mL thrombin or 2 μmol/L U46619 under conditions where the participation of ADP secreted from platelet dense granules was precluded by addition of 0.2 U/mL apyrase, a concentration sufficient to block the aggregation induced by 5 μmol/L ADP (Fig 1C and D). A3P5P produced a parallel concentration-dependent shift to the right of the dose-response curve for ADP (Fig 2). The 50% efficacy concentrations (EC50) of ADP-induced platelet aggregation were 5.2 ± 4.0 μmol/L, 8.5 ± 5.1 μmol/L, 10.2 ± 6.3 μmol/L, 14.8 ± 10.2 μmol/L, and 20.8 ± 9.7 μmol/L in the presence of 0, 3, 10, 30, and 100 μmol/L A3P5P, respectively. Schild analysis of the inhibition by A3P5P resulted in a pA2 value of 5 and a slope of 0.53, which suggests that the antagonism by A3P5P of ADP-induced platelet aggregation is noncompetitive. The isomer A2P5P produced a similar right-hand shift of the dose-response curve for ADP. EC50 of ADP-induced platelet aggregation were 4.4 ± 1.2 μmol/L, 6.2 ± 1.6 μmol/L, 8.1 ± 4.1 μmol/L, 16.7 ± 3.5 μmol/L, and 34.4 ± 14.8 μmol/L in the presence of 0, 1, 3, 30, and 100 μmol/L A2P5P, respectively. Schild analysis of the inhibition by A2P5P gave a pA2 value of 5 and a slope of 0.55, which likewise suggests that the antagonism by A2P5P of ADP-induced platelet aggregation is noncompetitive.
Effects of A3P5P on ADP-induced aggregation of washed human platelets. (A) Platelet aggregation was induced by 10 μmol/L ADP alone (1) or in the presence of 100 μmol/L A3P5P (2). (B) Platelet shape change induced by 0.3 μmol/L ADP in the presence of 5 mmol/L EDTA (1) was inhibited by 100 μmol/L A3P5P (2). (C and D) A3P5P (100 μmol/L) (2) did not inhibit platelet aggregation induced by 0.1 U/mL thrombin (1, C) or 2 μmol/L U46619 (1, D) in the presence of 0.2 U/mL apyrase.
Effects of A3P5P on ADP-induced aggregation of washed human platelets. (A) Platelet aggregation was induced by 10 μmol/L ADP alone (1) or in the presence of 100 μmol/L A3P5P (2). (B) Platelet shape change induced by 0.3 μmol/L ADP in the presence of 5 mmol/L EDTA (1) was inhibited by 100 μmol/L A3P5P (2). (C and D) A3P5P (100 μmol/L) (2) did not inhibit platelet aggregation induced by 0.1 U/mL thrombin (1, C) or 2 μmol/L U46619 (1, D) in the presence of 0.2 U/mL apyrase.
Inhibition by A3P5P of ADP-induced platelet aggregation. Aggregation was induced by increasing concentrations of ADP alone (▪) or in the presence of increasing concentrations of A3P5P: (▾), 3 × 10−6 mol/L; (⧫), 10−5 mol/L; (•), 3 × 10−5 mol/L; (□), 10−4 mol/L. Curves each represent the mean of three independent experiments and bars show the standard error of mean (SEM).
Inhibition by A3P5P of ADP-induced platelet aggregation. Aggregation was induced by increasing concentrations of ADP alone (▪) or in the presence of increasing concentrations of A3P5P: (▾), 3 × 10−6 mol/L; (⧫), 10−5 mol/L; (•), 3 × 10−5 mol/L; (□), 10−4 mol/L. Curves each represent the mean of three independent experiments and bars show the standard error of mean (SEM).
P2Y1 antagonists competitively inhibit ADP-induced [Ca2+]i increases in platelets, B10 cells, and P2Y1-transfected cells.
A3P5P (100 μmol/L) had no effect on intracellular calcium levels in fura-2–loaded washed human platelets, but produced a parallel concentration-dependent shift to the right of the dose-response curve for ADP-induced [Ca2+]i increases in washed platelets resuspended in Tyrode's buffer containing 0.35% human albumin and either 2 mmol/L calcium (Fig3A) or no calcium (0.2 mmol/L EGTA) (data not shown). EC50values for ADP were 0.29 ± 0.1 μmol/L, 0.55 ± 0.14 μmol/L, 1.2 ± 0.4 μmol/L, 4.6 ± 0.5 μmol/L, and 12.1 ± 3 μmol/L in the presence of 0, 3, 10, 30, and 100 μmol/L A3P5P, respectively. Schild analysis of these data gave an apparent pA2 value of 5.3 (KD 5 μmol/L) and a slope of 1.1, which suggests competitive antagonism by A3P5P of ADP-induced [Ca2+]i increases in platelets. Identical inhibition of ADP-induced [Ca2+]i increases was obtained using A2P5P. EC50 values for ADP were 0.54 ± 0.4 μmol/L, 2.4 ± 1.5 μmol/L, 4.5 ± 1.8 μmol/L, and 13.3 ± 5.7 μmol/L in the presence of 0, 10, 30, and 100 μmol/L A2P5P, respectively. Schild analysis of these data led to an apparent pA2 value of 5.5 (KD 3 μmol/L) and a slope of 0.9, which again suggests competitive antagonism by A2P5P of ADP-induced [Ca2+]i increases in platelets. The two nucleotide analogues had, on the contrary, no effect on the [Ca2+]i increases induced by 2 μmol/L U46619 or 0.1 U/mL thrombin in platelets (Fig 3D).
Competitive inhibition by A3P5P of ADP-induced [Ca2+]i increases in washed human platelets (A) and in Jurkat cells stably expressing the human P2Y1receptor (B). [Ca2+]i was stimulated by ADP alone or in the presence of increasing concentrations of A3P5P, in the presence of 2 μmol/L external calcium. (C) Effects of increasing concentrations of A2P5P and A3P5P on [Ca2+]i increases induced by 1 μmol/L ADP in B10 cells. (D) A3P5P (100 μmol/L) (2) did not inhibit [Ca2+]i increases induced by 2 μmol/L U46619 (1, left] or 0.1 U/mL thrombin (1, right) in washed human platelets. Curves each represent the mean of three independent experiments and bars show the SEM.
Competitive inhibition by A3P5P of ADP-induced [Ca2+]i increases in washed human platelets (A) and in Jurkat cells stably expressing the human P2Y1receptor (B). [Ca2+]i was stimulated by ADP alone or in the presence of increasing concentrations of A3P5P, in the presence of 2 μmol/L external calcium. (C) Effects of increasing concentrations of A2P5P and A3P5P on [Ca2+]i increases induced by 1 μmol/L ADP in B10 cells. (D) A3P5P (100 μmol/L) (2) did not inhibit [Ca2+]i increases induced by 2 μmol/L U46619 (1, left] or 0.1 U/mL thrombin (1, right) in washed human platelets. Curves each represent the mean of three independent experiments and bars show the SEM.
A3P5P also produced a parallel right-hand shift of the dose-response curve for ADP-induced [Ca2+]i increases in Jurkat cells stably expressing the human P2Y1 receptor, either in the presence of 2 mmol/L external calcium (Fig 3B) or in its absence (0.2 mmol/L EGTA) (data not shown). EC50 values for ADP were 0.11 ± 0.07 μmol/L, 0.13 ± 0.04 μmol/L, 0.28 ± 0.07 μmol/L, 0.6 ± 0.07 μmol/L, and 2.3 ± 0.6 μmol/L in the presence of 0, 3, 10, 30, and 100 μmol/L A3P5P, respectively. Schild analysis gave an apparent pA2 of 5.1 (KD8 μmol/L) and a slope of 1.1, suggesting competitive antagonism of A3P5P at the P2Y1 receptor. Similar results were obtained using A2P5P. EC50 values for ADP were 0.10 ± 0.05 μmol/L, 0.75 ± 0.03 μmol/L, 1.75 ± 0.08 μmol/L, and 7.6 ± 0.6 μmol/L in the presence of 0, 10, 30, and 100 μmol/L A2P5P, respectively. Schild analysis gave an apparent pA2of 5.5 (KD 3 μmol/L) and a slope of 1, likewise suggesting competitive antagonism of A2P5P at the P2Y1receptor. Once again, the two nucleotide analogues had no antagonistic effect on the [Ca2+]i increase induced by 1 U/mL thrombin (data not shown). In the case of the B10 clone of rat BCEC, A3P5P, and A2P5P both inhibited the [Ca2+]i increase in response to stimulation by 1 μmol/L ADP, with 50% inhibitory concentrations (IC50) of 6.6 ± 0.1 μmol/L and 10.3 ± 0.4 μmol/L, respectively (Fig 3C), corresponding to Ki values of 3.1 μmol/L and 4.8 μmol/L, respectively. At higher concentration (100 μmol/L), the two nucleotide analogues completely abolished the action of 1 μmol/L ADP. However, they once again had no antagonistic effect on the [Ca2+]i increase induced by 0.1 U/mL thrombin (data not shown) in B10 cells.
Lack of effect of P2Y1 antagonists on ADP-induced inhibition of adenylyl cyclase activity.
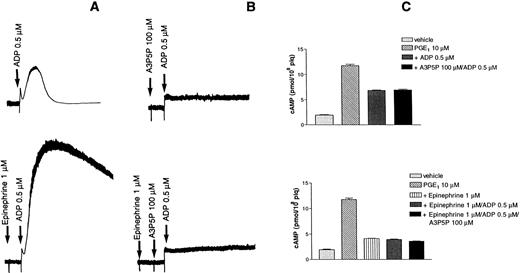
A2P5P and A3P5P (100 μmol/L) had no impact on basal levels of cyclic AMP in human platelets (data not shown). A3P5P likewise had no influence on the increased cyclic AMP levels induced by 1 μmol/L PGE1 (data not shown), whereas 5 μmol/L ADP produced 65% inhibition of the PGE1 response (Fig 4A). A3P5P or A2P5P (100 μmol/L) had no effect on this ADP-induced inhibition of PGE1stimulation, as opposed to Sp-ATPαS (100 μmol/L), which totally reversed the effects of 1 μmol/L ADP on the cyclic AMP levels produced by PGE1 (Fig 4A). Sp-ATPαS is a well-known antagonist of the ADP receptor inhibiting ADP-induced platelet aggregation, intracellular calcium increases, and adenylyl cyclase inhibition.8 13
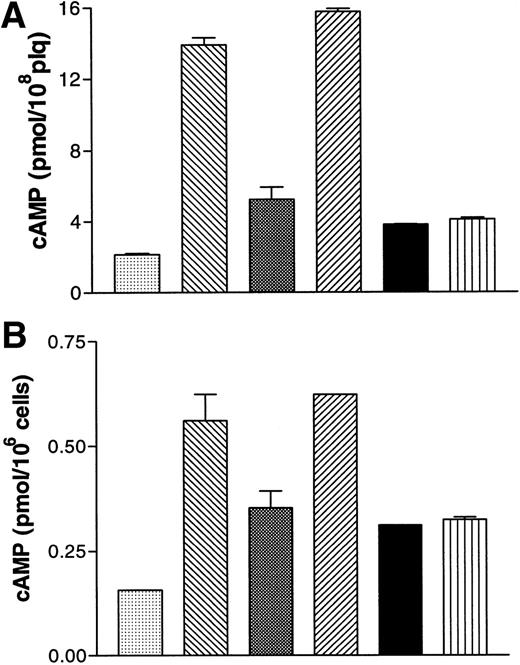
Effects of A3P5P on cAMP levels in washed human platelets (A) and in B10 cells (B). (A) (▧), Vehicle; (▧), PGE1 1 μmol/L; (▧), +ADP 5μmol/L; (▨), +ATPαS 100 μmol/L / ADP 5 μmol/L; (▪), + A3P5P 100 μmol/L / ADP 5 μmol/L; (▥), +A2P5P 100 μmol/L / ADP 5 μmol/L. (B) (▧), Vehicle; (▧), forskolin 1 μmol/L; (▧), +ADP 1 μmol/L; (▨), +ATPαS 100 μmol/L / ADP 1 μmol/L; (▪), +A3P5P 100 μmol/L / ADP 1 μmol/L; (▥), +A2P5P 100 μmol/L / ADP 1 μmol/L. Values are means (±SEM) from one experiment performed in triplicate, representative of three separate experiments giving identical results.
Effects of A3P5P on cAMP levels in washed human platelets (A) and in B10 cells (B). (A) (▧), Vehicle; (▧), PGE1 1 μmol/L; (▧), +ADP 5μmol/L; (▨), +ATPαS 100 μmol/L / ADP 5 μmol/L; (▪), + A3P5P 100 μmol/L / ADP 5 μmol/L; (▥), +A2P5P 100 μmol/L / ADP 5 μmol/L. (B) (▧), Vehicle; (▧), forskolin 1 μmol/L; (▧), +ADP 1 μmol/L; (▨), +ATPαS 100 μmol/L / ADP 1 μmol/L; (▪), +A3P5P 100 μmol/L / ADP 1 μmol/L; (▥), +A2P5P 100 μmol/L / ADP 1 μmol/L. Values are means (±SEM) from one experiment performed in triplicate, representative of three separate experiments giving identical results.
In B10 cells, A3P5P (100 μmol/L) had no influence on basal levels of cyclic AMP. Cyclic AMP increased fourfold in the presence of 1 μmol/L forskolin, and A3P5P had no effect on this stimulation (data not shown). Conversely, addition of 1 μmol/L ADP to forskolin-stimulated cells resulted in a 40% reduction in cyclic AMP levels (Fig 4B). A3P5P or A2P5P (100 μmol/L) did not modify the inhibitory effect of ADP on adenylyl cyclase activity (Fig 4B), whereas under the same conditions, Sp-ATPαS (100 μmol/L) totally reversed the inhibitory action of 1 μmol/L ADP on cyclic AMP levels (Fig 4B). Finally, in Jurkat cells stably expressing the P2Y1 receptor, no positive or negative influence of A2P5P or A3P5P on adenylyl cyclase activity could be detected (data not shown).
Inhibition of ADP-induced aggregation by P2Y1 antagonists is not reversed by epinephrine.
Epinephrine potentiates platelet aggregation induced by low concentrations of ADP (Fig 5A). In the presence of 100 μmol/L A3P5P, which completely inhibited aggregation and shape change, epinephrine could no longer potentiate any platelet response (Fig 5B). Cyclic AMP formation was examined under the same conditions and was found to be inhibited by both ADP and epinephrine (Fig 5C).
Effects of epinephrine on ADP-induced platelet aggregation. (A) Aggregation was induced by 0.5 μmol/L ADP alone (top) or the presence of 1 μmol/L epinephrine (bottom). (B) Aggregation induced by 0.5 μmol/L ADP was inhibited by 100 μmol/L A3P5P in the absence (top) or the presence (bottom) of 1 μmol/L epinephrine. (C) Effects of A3P5P on cAMP levels in the absence (top) or the presence (bottom) of 1 μmol/L epinephrine.
Effects of epinephrine on ADP-induced platelet aggregation. (A) Aggregation was induced by 0.5 μmol/L ADP alone (top) or the presence of 1 μmol/L epinephrine (bottom). (B) Aggregation induced by 0.5 μmol/L ADP was inhibited by 100 μmol/L A3P5P in the absence (top) or the presence (bottom) of 1 μmol/L epinephrine. (C) Effects of A3P5P on cAMP levels in the absence (top) or the presence (bottom) of 1 μmol/L epinephrine.
DISCUSSION
In a previous report, we presented the pharmacologic characteristics of the human P2Y1 receptor heterologously expressed in Jurkat cells.25 ADP was shown to be a selective agonist of this receptor, while freshly purified ATP was an ineffective agonist, but competitively antagonized the action of ADP. Because P2Y1receptor transcripts were found to be present in platelets and megakaryoblastic cell lines, we suggested that the P2Y1receptor could be similar to the platelet P2T receptor for ADP. However, 2MeSATP and 2ClATP were still found to be agonistic to the P2Y1 receptor-transfected cells, contrary to their known antagonistic action on platelets.13 It was suggested that the triphosphate analogues could have been metabolized into the corresponding diphosphates by ectoenzymes, thus explaining their apparent agonistic effect. This was later confirmed by the finding that when the purity of adenine triphosphate nucleotides was controlled with a creatine phosphokinase/creatine phosphate ATP regenerating system, all triphosphate nucleotide derivatives were antagonists to the P2Y1 receptors on Jurkat cells and on the B10 clonal cell line of rat BCEC.38 These data thus support the hypothesis that the P2Y1 receptor common to platelets and endothelial cells could be the P2T receptor.
In the present work, we further addressed the question as to whether the P2Y1 receptor could be the elusive P2T receptor. As probes, we chose A3P5P and A2P5P, two adenine nucleotide isomers recently demonstrated to be competitive and selective antagonists of the P2Y1 receptor.35 The effects of these compounds on human platelets were compared with their effects on the heterologously expressed human P2Y1 receptor and on the P2Y1 receptor endogenously expressed on the B10 clonal cell line of rat BCEC. This clone is useful for studies of the P2Y1 receptor, as in contrast to other endothelial cell lines, which express both P2Y1 and P2Y2 and thus display confusing ligand structure-activity relationships, the B10 clone expresses only the P2Y1 receptor.33 34
A3P5P and its isomer A2P5P both specifically inhibited ADP-induced platelet shape change and aggregation, demonstrating the critical role of the P2Y1 receptor in these events. Inhibition of ADP-induced aggregation was observed at relatively low concentrations of A2P5P and A3P5P and these compounds exhibited potencies (pA2 = 5) similar to that of the natural competitive antagonist ATP (pA2 = 4.6).13 However, this effect on aggregation was found to be noncompetitive, suggesting that more than one receptor could be involved in a highly complex process. A2P5P and A3P5P were nevertheless specific and competitive antagonists of the [Ca2+]i increases induced by ADP in human platelets, in B10 cells, and in Jurkat cells stably expressing the human P2Y1 receptor, which clearly demonstrates the essential role of the calcium pathway triggered by the P2Y1receptor in ADP-induced platelet aggregation. In further support of this view, it was recently shown that platelets from mice lacking the Gαq subunit of the phospholipase C activating G-protein Gq are unable to aggregate in response to ADP, while Inositol-1,4,5-triphosphate formation and calcium signaling are totally abolished.39
In contrast to their inhibitory effect on calcium mobilization, A2P5P and A3P5P had no influence on the inhibition by ADP of stimulated adenylyl cyclase activity in platelets or B10 cells, even at high concentration (100 μmol/L). This indicates that under conditions where platelet aggregation was blocked, ADP could still promote the inhibition of adenylyl cyclase. It is well established that inhibition of adenylyl cyclase is not alone sufficient to support platelet aggregation.40 This is the case, for instance, when platelets are stimulated by epinephrine, which by inhibiting adenylyl cyclase in the absence of an increase in intracellular calcium, does not induce platelet aggregation, but potentiates the response to all other aggregating agents.41 Figure 5 shows that under conditions where the P2Y1 receptor was completely antagonized, epinephrine could no longer potentiate any response, even though adenylyl cyclase was still inhibited by ADP. Thus P2Y1 is absolutely necessary for ADP to induce aggregation, and inhibition of adenylyl cyclase by ADP or epinephrine is not sufficient to promote an aggregation response.
In Jurkat cells stably expressing the human P2Y1 receptor, it was not possible to detect any positive or negative coupling of the receptor to adenylyl cyclase. Although this might be due to weak expression of the P2Y1 receptor in these cells, it more probably reflects the fact that the P2Y1 receptor is selectively coupled to calcium mobilization rather than to adenylyl cyclase inhibition.42 The specific inhibition by A2P5P and A3P5P of the intracellular calcium increases induced by ADP in platelets and endothelial cells, in the absence of any inhibition of the effects of ADP on adenylyl cyclase in these cells, points to the existence of two ADP receptors, the P2Y1 receptor coupled to calcium movements and an as yet unidentified receptor coupled to adenylyl cyclase inhibition. Other lines of evidence reinforce this hypothesis. Thus, thienopyridines inhibit ADP-induced platelet aggregation and inhibition of adenylyl cyclase without affecting the concomitant ADP-induced [Ca2+]iincrease.14,15 Clopidogrel, in particular, inhibits only 70% of the binding of radiolabeled 2MeSADP, leaving residual binding sites even at the highest doses giving maximal blockade of platelet aggregation.18,19 The compound, ARL66096, an ATP analogue that has been proposed as a selective P2T antagonist on the basis of its inhibitory effect on ADP-induced platelet aggregation,43 has been recently reported to block ADP-induced inhibition of adenylyl cyclase without affecting ADP-mediated [Ca2+]i increases or shape change.44
Overall, these data strongly support the view that a full aggregation response when platelets are stimulated with ADP involves the P2Y1 receptor, which triggers calcium signaling, shape change, and initial aggregation, while another ADP receptor coupled to the inhibition of adenylyl cyclase potentiates and completes the initial response. This amplification pathway would also be involved in aggregation induced by other agonists when ADP is released from platelet dense granules. One can then speculate that the antithrombotic properties of clopidogrel and ARL66096 are due to blockade of this ADP pathway whatever the original stimulus. In earlier work, we reported that a full aggregation response could be restored in the platelets of ticlopidine-treated humans by inhibiting adenylyl cyclase with epinephrine.15 Similarly, in the case of the specific defect of ADP-induced platelet aggregation described in two patients5,6 who display a “ticlopidine-like syndrome”,19 one may anticipate that the defect should lie on the receptor coupled to adenylyl cyclase. Indeed, the calcium response is almost normal in these patients and shape change is not abolished, whereas 2MeSADP binding sites are reduced and adenylyl cyclase inhibition is blocked. Sequencing of the P2Y1receptor gene and platelet mRNA along with functional studies using selective P2Y1 antagonists will be required to resolve this question.
The molecular identity of the ADP receptor coupled to inhibition of adenylyl cyclase should be of the P2Y type, as we have previously shown that ADP activates the Gi2 heterotrimeric G-protein in human platelet membranes.45 Such a receptor should exhibit a pharmacologic profile identical to that of the P2Y1receptor, ADP being an agonist and triphosphate nucleotides competitive antagonists, but with subtle differences in the selectivity of a number of ligands. A2P5P and A3P5P, in particular, do not appear to interact with this receptor. Conversely, two other adenine nucleotide derivatives, 2-methylthioadenosine 5′-β, γ-methylenetriphosphonate (2MeSAMPPCP) and 2-ethylthioadenosine 5′-monophosphate (2EtSAMP), reported to selectively and competitively inhibit the effects of ADP on adenylyl cyclase in platelets while only partially inhibiting ADP-induced platelet aggregation,46 should interact with this receptor. Other compounds have long been known to display specificity for shape change and aggregation or for inhibition of adenylyl cyclase. Thus, ADPαS induces platelet aggregation without affecting adenylyl cyclase, whereas the thiol reagent p-mercurybenzoylsulfate (pCMBS) blocks inhibition of adenylyl cyclase, but not shape change (extensively reviewed in Mills8). Altogether, the currently available data best fit a model of two P2Y receptors mediating the effects of ADP on platelet aggregation.
Because of the key pharmacologic feature of agonism by ADP and antagonism by ATP, an adenylyl cyclase-coupled P2Y receptor (P2Ycyc) should display high molecular identity with the P2Y1receptor and be detectable by RT-PCR using wide range primer sets covering the known P2Y receptors. However, such experiments only allowed detection of the P2Y1 receptor of B10 cells,34 leaving open the search for P2Ycyc. A P2Y receptor of this type has been reported to be present in a subclone of glioma cells termed C6-2B,47 which has the advantage of expressing no other P2 receptors, but whether this receptor is the same as that of platelets remains to be established. One would wish to know the effects of compounds like ARL66096 on both C6-2B and B10 cells to clarify this point and such compounds are unfortunately not yet commercially available.
In conclusion, our results demonstrate that platelets and endothelial cells share a common P2Y1 receptor involved in ADP-induced vasodilation and platelet shape change and aggregation and that this receptor is necessary to trigger ADP-induced platelet aggregation. Our findings and other data from the literature also strongly suggest the existence of another as yet unidentified P2Y receptor coupled to the inhibition of adenylyl cyclase. This means that the receptor previously known as “P2T” is probably composed of three distinct receptors, the P2Y1 receptor, the P2Ycyc receptor, and the P2X1 receptor, the role of which appears to be discrete. Thus, the term “P2T” should no longer be used to designate a specific molecular entity. In the near future, it should be possible to establish the respective contributions of each of these receptors not only to platelet aggregation induced by ADP itself, but also to the potentiation of platelet activation by ADP released in different physiologic situations such as adhesion or aggregation in response to thrombin or other strong platelet agonists.
ACKNOWLEDGMENT
The authors thank D. Cassel for expert technical assistance and J.N. Mulvihill for reviewing the English of the manuscript.
Address reprint requests to Christian Gachet, MD, PhD, INSERM U. 311, Etablissement de Transfusion Sanguine de Strasbourg, 10, rue Spielmann, BP N° 36, 67065 Strasbourg, Cédex, France; e-mail: christian.gachet@etss.u-strasbg.fr.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 3. Competitive inhibition by A3P5P of ADP-induced [Ca2+]i increases in washed human platelets (A) and in Jurkat cells stably expressing the human P2Y1receptor (B). [Ca2+]i was stimulated by ADP alone or in the presence of increasing concentrations of A3P5P, in the presence of 2 μmol/L external calcium. (C) Effects of increasing concentrations of A2P5P and A3P5P on [Ca2+]i increases induced by 1 μmol/L ADP in B10 cells. (D) A3P5P (100 μmol/L) (2) did not inhibit [Ca2+]i increases induced by 2 μmol/L U46619 (1, left] or 0.1 U/mL thrombin (1, right) in washed human platelets. Curves each represent the mean of three independent experiments and bars show the SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.152.413k27_152_159/5/m_blod41327003x.jpeg?Expires=1769300628&Signature=ZU-0jgCqASPo2jUUU7c8M9QLNgp8QIPQfUJk-6EHuw5ZGeOdYGrXOYuBCwE2s~i~ftzalf67zl16deCkwx0ZSCwh4TcaGXPCz-aUxNxX8pev6HAaRH4fW9bs0ONchuaHiUWKezqyORn-LPLWgDL1Ql-9gulBaY9PmmaEJDHoU7KviLJnMPnlrNFnk6bM8Eupfb0lgTxGTAZD0H8N~eA~oe9hYq4--dK28kORwceEDghsJsIJn0Ik2R31d4dC4VKcke-IFHZQBJC7h~cKLKYuIyVQSM7kpnUsfYKQLjI2NcMl7ATq-ECovrXlTjtld~1z3A0wXNqDeQ8Pr235fBzNkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)