At inflammatory sites, proteoglycans are both secreted by activated mononuclear leukocytes and released as a consequence of extracellular matrix degradation. Chondroitin 4-sulfate proteoglycans constitute the predominant ones produced by activated human monocytes/macrophages. In this study, we show that two chondroitin 4-sulfate forms, CSA and CSB, can activate distinct peripheral blood mononuclear cell types. Whereas CSA activates monocytes (to secrete monokines), CSB activates B-cells (to proliferate). In contrast, the chondroitin 6-sulfate CSC and heparin do not exert these functional effects. We further show that CD44 monoclonal antibodies block CSB-induced B-cell proliferation. These findings point to glycosaminoglycans, and specifically chondroitin 4-sulfates, as a novel class of immunological mediators at inflammatory sites. Furthermore, the data link CD44 to B-cell activation, paralleling the established roles of CD44 in T-cell and monocyte activation.
PROTEOGLYCANS (PGs) are macromolecules composed of a central core protein to which one or more glycosaminoglycan (GAG) chains are covalently attached.1GAGs are large carbohydrates that are composed of repeating disaccharide units and exist in four forms: heparan sulfate (HS) and heparin, chondroitin sulfate (CS, mainly CSA and CSC, and dermatan sulfate, CSB), keratan sulfate, and hyaluronic acid (HA). The first three occur predominantly as protein-bound GAGs and contain sulfate; HA is made as a free GAG and lacks sulfate. In the case of the chondroitin forms, CSA and CSB are structurally related, since both are 4-sulfated, whereas CSC is 6-sulfated.
Stimulated monocytes and macrophages secrete a diverse set of mediators that influence cellular immune functions and inflammation. These mediators include pro- and anti-inflammatory cytokines, prostaglandins, leukotrienes, and reactive oxygen metabolites.2 Soluble PGs constitute another class of molecules produced by human monocytes3,4 and macrophages.5 Chondroitin sulfate proteoglycans (CSPG) have been reported to be the predominant proteoglycan produced by cells of the monocyte/macrophage lineage, and 80% to 90% of this CSPG is chondroitin 4-sulfate (ie, CSA or CSB).5 6
Although the production of CSPGs by monocyte/macrophages during immune activation has been well documented, information pertaining to their functional significance is sparse. CS-modified invariant chain (Ii) associated with major histocompatibility complex (MHC) class II has been shown to enhance stimulation of T-cell responses through interactions with CD44.7 Serglycin, a CSPG secreted by hematopoietic cells,5 activates cytotoxic T-cell clones.8 Chondroitin 4-sulfate released by stimulated polymorphonuclear leukocytes during phagocytosis stimulates their chemotaxis.3 9-12
These disparate studies focused narrowly upon the effects of CSPGs/CSs on isolated immune cell populations. In the present study, we have instead monitored the effects of GAGs, and in particular CSs, on the mixed cell populations present within human peripheral blood mononuclear cell (PBMC) preparations. In this way, we have sought to search in a more open-ended way for effects on different immune cell subpopulations, using a more complex cellular experimental milieu where critical intercellular contacts can take place. What have emerged from these analyses are new insights into the immunomodulatory potential of the chondroitin 4-sulfate forms, with differing properties associated with CSA versus CSB. Whereas CSA is directed toward monocytic cells, CSB exerts its activating effects on B cells. These findings provide the first immunoregulatory links between CSs and both the monocytic and B-cell compartments. Moreover, the apparent role of CD44 in mediating the CSB effect on human B cells provides a new functional niche for CD44 in immunoregulation that goes beyond its documented effects on monocytes and T cells.
MATERIALS AND METHODS
Chemicals and antibodies.
Chondroitin sulfates A (bovine trachea), B (pocrine intestinal mucosa), and C (shark cartilage), heparin (pocrine intestinal mucosa), hyaluronic acid (human umbilical cord), and chondroitinase AC were obtained commercially (Sigma Chemical Co, St Louis, MO). Phycoerythrin-conjugated CD19 (clone 89B, IgG1) and control monoclonal antibody (MoAb) MsIgG1 were purchased from Coulter Clone (Miami, FL). Monoclonal mouse anti-human CD44 (clone A3D8, IgG1) was obtained from Harlan (Sussex, UK). The monoclonal and polyclonal anti–interleukin-1 (anti–IL-1) antibodies13 were kindly provided by Dr M.A. Friedlander (Case Western Reserve University). Recombinant human transforming growth factor-β1 (rTGFβ1) was purchased from R&D Systems (Minneapolis, MN).
Cell culture.
PBMC were separated from heparinized venous blood of healthy donors by density gradient centrifugation over Ficoll-paque gradients and maintained as described.14 The U937 cell line was purchased from the American Type Cell Culture Collection (Rockville, MD) and was maintained in the above medium.
Cytokine production.
GAGs were added to PBMC cultures at the indicated concentrations. Cultures were incubated for either 24 or 48 hours, and conditioned media were then obtained and stored at −20°C. IL-1β was measured in 24-hour conditioned media by a sandwich enzyme-linked immunosorbent assay (ELISA) as described previously.13
Proliferation assay.
PBMC (1 × 105 cells in 0.2 mL per well) were cultured in 96-well plates (Falcon, Lincoln Park, NJ) in triplicate. After stimulation, cultures were pulsed for the last 16 hours with 0.5 μCi per well [3H]-methyl-thymidine (New England Nuclear, Boston, MA). Cells were collected with a Harvester 96 cell harvester (Tomtec, Orange, CT), and the filters were dried and counted with a 1205 Betaplate counter (Wallac, Turku, Finland).
Immunofluorescence and flow cytometry.
PBMC were stained with the phycoerythrin-conjugated CD19 MoAb, or the appropriate phycoerythrin-conjugated isotype-matched control MoAb, as described.15 Immunostained cells were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA) using Lysis II software.
Northern blot analysis.
Total cellular RNA isolation and Northern blot hybridization were performed as previously described16 using an IL-1β probe comprising a 530-bp Nde I-BamHI cDNA fragment corresponding to amino acids 1-139 of the IL-1β precursor. Hybridization signals were quantitated by phosphoimaging using InstantImager (Packard, Meriden, CT).
RESULTS
Induction of IL-1β secretion by CSA.
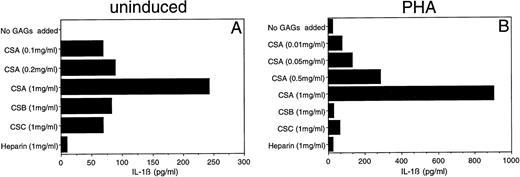
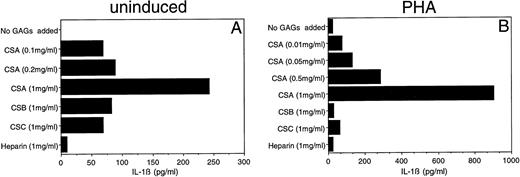
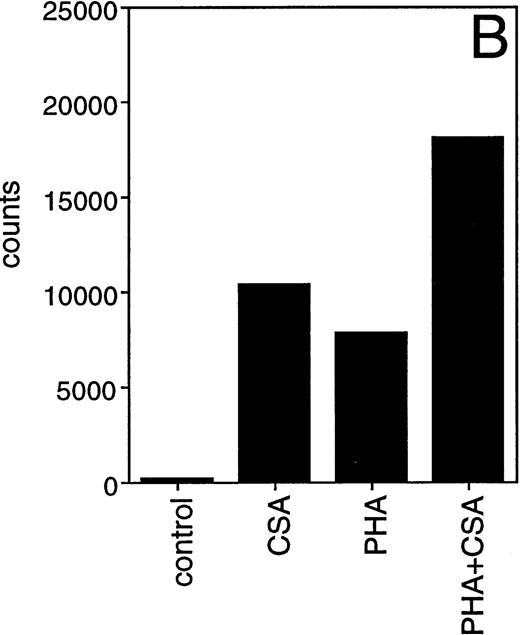
Given the importance of IL-1β in the initiation and modulation of immune responses, we focused initially upon this monokine. Previous studies have documented the capacity of HA to induce IL-1β and other monokine secretions.17,18 We surveyed the capacity of five different GAGs (CSA, CSB, CSC, heparin, and HA) to induce IL-1β secretion. Both HA and CSA reproducibly induced IL-1β secretion by PBMC. The HA-induced IL-1β secretion was evident at concentrations as low as 1 μg/mL (data not shown), consistent with the findings of Hiro et al.17 CSA, on the other hand, induced detectable levels of IL-1β in PBMC only at substantially higher concentrations (100 μg/mL to 1 mg/mL) (Fig 1A). After phytohemagglutinin (PHA) induction, PBMC continued to show a dose-dependent increase in IL-1β secretion over a CSA concentration range of 10 μg/mL to 1 mg/mL (Fig 1B).
Effect of CSA on secretion of IL-1β by human PBMC. PBMC were cultured with (B) or without (A) PHA (1 μg/mL) in the presence of the indicated concentrations of CSA or 1 mg/mL of the other GAGs. After 24 hours supernatants were obtained and IL-1β levels were determined by ELISA.
Effect of CSA on secretion of IL-1β by human PBMC. PBMC were cultured with (B) or without (A) PHA (1 μg/mL) in the presence of the indicated concentrations of CSA or 1 mg/mL of the other GAGs. After 24 hours supernatants were obtained and IL-1β levels were determined by ELISA.
In contrast, the other GAGs (CSB, CSC, and heparin) did not show comparable effects upon IL-1β secretion. Low-level IL-1β secretion (over control) was detected when non–PHA-treated PBMC were exposed to CSB or CSC (1 mg/mL); this cytokine level corresponded to that seen when cells were treated with a 10-fold lower concentration (0.1 mg/mL) of CSA (Fig 1A). This low stimulation may be due to the presence of contaminant CSA in the CSB and CSC preparations (about 10% to 15% in the form provided by the supplier). An even lower level of IL-1β stimulation was observed for heparin.
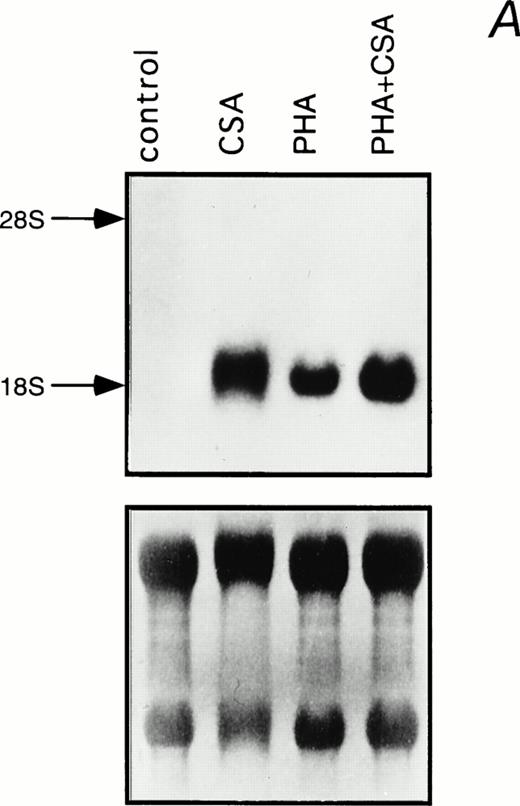
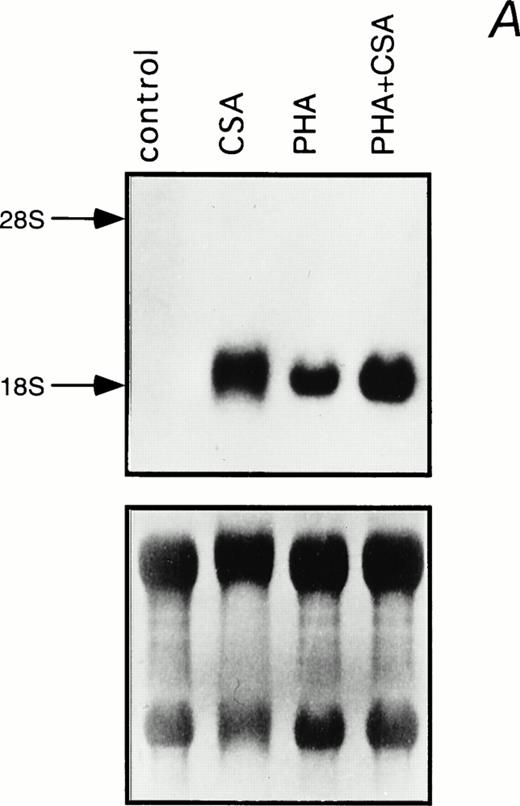
Northern blot analysis was performed to determine the effect of CSA on steady-state IL-1β mRNA levels in PHA-stimulated and unstimulated PBMC (Fig 2). A substantial induction of IL-1β mRNA was observed when cells were treated with 1 mg/mL CSA. This parallels the increase in IL-1β mRNA levels previously associated with HA treatment in murine macrophages.18Moreover, an additive effect was observed when CSA and PHA were combined (Fig 2).
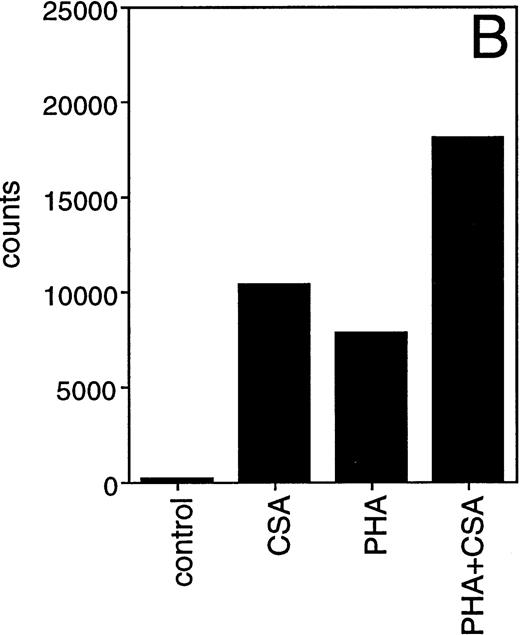
Induction of IL-1β mRNA in human PBMC exposed to CSA. (A) Total RNA isolated from PBMC cultured in the presence of CSA (1 mg/mL), PHA (1 μg/mL), or both, was subjected to Northern blot hybridization using IL-1β cDNA as a probe. The positions of the 28S and 18S ribosomal RNAs are indicated. Relative amounts of RNA loaded in each lane are visualized in the lower panel by methylene blue staining. (B) The intensities of the IL-1β hybridization signals shown in (A) were measured using an InstantImager analyzer, and the results were plotted as a histogram to highlight the relative IL-1β mRNA levels.
Induction of IL-1β mRNA in human PBMC exposed to CSA. (A) Total RNA isolated from PBMC cultured in the presence of CSA (1 mg/mL), PHA (1 μg/mL), or both, was subjected to Northern blot hybridization using IL-1β cDNA as a probe. The positions of the 28S and 18S ribosomal RNAs are indicated. Relative amounts of RNA loaded in each lane are visualized in the lower panel by methylene blue staining. (B) The intensities of the IL-1β hybridization signals shown in (A) were measured using an InstantImager analyzer, and the results were plotted as a histogram to highlight the relative IL-1β mRNA levels.
Because IL-1β is secreted predominantly by monocytes,19we asked whether the CSA effect could be reproduced in an established monocytic cell line. U937 is a histiocytic lymphoma cell line20 that can be induced by PMA to differentiate along the monocytic lineage. In their differentiated state, these cells secrete IL-1β.21 22 CSA induced an approximately 50% increase in IL-1β release by PMA-treated U937 cells (from 115 pg/mL to 191 pg/mL in one representative experiment), but had no observable effect on undifferentiated cells which showed no IL-1β production (data not shown). This is consistent with the notion that the CSA effect is dependent on commitment to the monocytic lineage. Monocytes purified from PBMC were also induced to secrete IL-1β by CSA (from 0 to 115 pg/mL in one representative experiment) (data not shown).
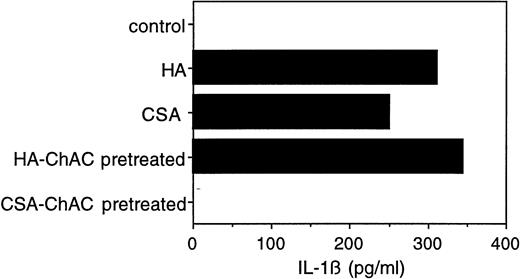
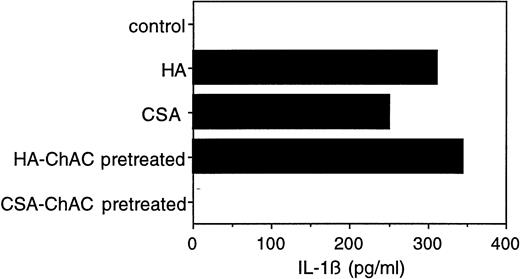
To verify that the observed cytokine effects were indeed attributable to CSA (and not to impurities, eg, HA, in the CSA preparation), we digested the commercial CSA preparation before its use with chondroitinase AC, a polysaccharide lyase that acts endolytically on CSA and CSC. After chondroitinase AC pretreatment, there was only negligible IL-1β release (Fig 3). When HA (at 5 μg, a concentration yielding IL-1β secretion equivalent to 1 mg of CSA) was similarly pretreated with chondroitinase AC, its capacity to induce IL-1β secretion was not affected (Fig 3). Neither chondroitin sulfate nor hyaluronic acid disaccharide had any effect on IL-1β secretion (data not shown), suggesting that the observed activity of the two GAGs is specific for their polymeric forms. These results taken together indicate that CSA induces monocytes to secrete IL-1β, and that this effect is not attributable to impurities.
Effect of chondroitinase AC digestion on the IL-1β–inducing activity of CSA. CSA (10 mg/mL) or HA (50 μg/mL) was incubated with chondroitinase AC (100 mU/mL) in RPMI (without fetal calf serum) at 37°C for 2 hours. The reaction mixtures were then postincubated at 65°C for 10 minutes to inactivate the enzyme. PBMC were cultured in the presence of untreated and chondroitinase AC-treated CSA (1 mg/mL) or HA (5 μg/mL) for 24 hours and the level of IL-1β in the medium was determined as in Fig 1.
Effect of chondroitinase AC digestion on the IL-1β–inducing activity of CSA. CSA (10 mg/mL) or HA (50 μg/mL) was incubated with chondroitinase AC (100 mU/mL) in RPMI (without fetal calf serum) at 37°C for 2 hours. The reaction mixtures were then postincubated at 65°C for 10 minutes to inactivate the enzyme. PBMC were cultured in the presence of untreated and chondroitinase AC-treated CSA (1 mg/mL) or HA (5 μg/mL) for 24 hours and the level of IL-1β in the medium was determined as in Fig 1.
CSB stimulates B-cell proliferation.
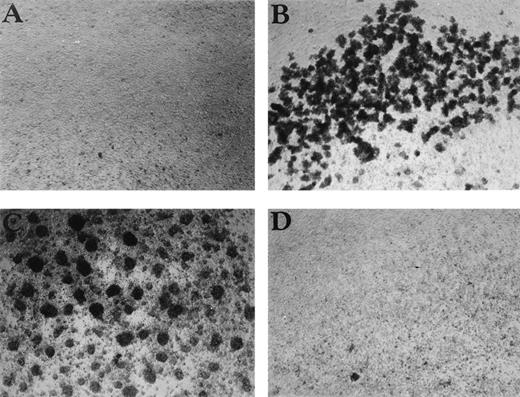
Having shown that CSA and HA induce monokine expression, we next looked for other effects that GAGs might have on PBMC. Cellular aggregation and proliferation are two commonly studied parameters. Although CSA had induced IL-1β secretion in our earlier experiments, this GAG had no observable effects on PBMC proliferation or aggregation (data not shown). The same held for CSC, heparin, and HA (data not shown). However, CSB, which had exhibited minimal cytokine effects, induced both PBMC aggregation (Fig 4) and proliferation in a dose-dependent manner (Fig 5A). Aggregation was visible as early as 2 hours after addition of CSB to cell cultures and increased up to 24 hours. CSB-induced proliferation peaked at day 6 and decreased thereafter (data not shown). TGFβ, an anti-inflammatory cytokine known to have diverse immunoinhibitory activities, completely blocked this CSB-induced proliferation within the PBMC pool (Fig 5B).
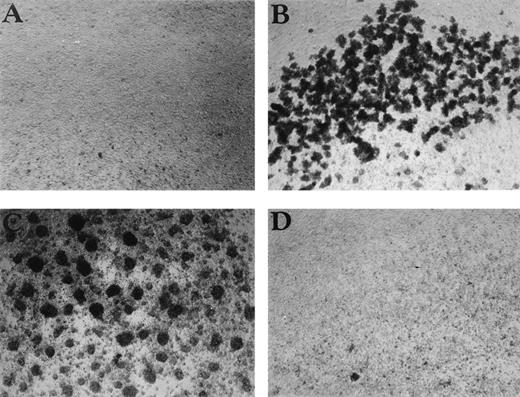
CSB enhances cell aggregation in PBMC cultures. The effect of (A) control, (B) CSB (1 mg/mL), (C) PHA (1 μg/mL), and (D) HA (10 μg/mL) on PBMC aggregation is shown. PBMC were cultured in 24-well plates for 24 hours, and the cultures were then examined by phase microscopy using a Nikon Phase Contrast ELWD 0.3 microscope (original magnification × 100).
CSB enhances cell aggregation in PBMC cultures. The effect of (A) control, (B) CSB (1 mg/mL), (C) PHA (1 μg/mL), and (D) HA (10 μg/mL) on PBMC aggregation is shown. PBMC were cultured in 24-well plates for 24 hours, and the cultures were then examined by phase microscopy using a Nikon Phase Contrast ELWD 0.3 microscope (original magnification × 100).
Characterization of CSB-induced PBMC proliferation. (A) PBMC were cultured as described in Materials and Methods, and CSB was added at the indicated concentrations. (B) PBMC were treated with CSB (1 mg/mL) in the absence or presence of rTGFβ1 (1 ng/mL). (C) Total PBMC or monocyte-depleted PBMC (nonadherent) were incubated with CSB (1 mg/mL) for 6 days. Monocytes were depleted by adherence to tissue culture flasks and nonadherent cells were collected, counted, and plated. Data shown are [3H]-thymidine incorporation during the final 16 hours of a 6-day culture period. In each case, the results shown are representative of at least three similar experiments.
Characterization of CSB-induced PBMC proliferation. (A) PBMC were cultured as described in Materials and Methods, and CSB was added at the indicated concentrations. (B) PBMC were treated with CSB (1 mg/mL) in the absence or presence of rTGFβ1 (1 ng/mL). (C) Total PBMC or monocyte-depleted PBMC (nonadherent) were incubated with CSB (1 mg/mL) for 6 days. Monocytes were depleted by adherence to tissue culture flasks and nonadherent cells were collected, counted, and plated. Data shown are [3H]-thymidine incorporation during the final 16 hours of a 6-day culture period. In each case, the results shown are representative of at least three similar experiments.
To determine the effect of CSB on the proliferation of individual cell types within the PBMC population, monocytes were depleted by adherence to tissue culture flasks, and the residual nonadherent cells were stimulated with CSB. Although this nonadherent cell fraction aggregates when stimulated with CSB, the cells do not proliferate (Fig 5C). Proliferation could be restored when monocytes were reintroduced into the cultures (data not shown). No significant [3H]-thymidine incorporation was observed when monocytes alone were stimulated with CSB (data not shown). In addition, purified T cells did not proliferate when stimulated with CSB alone or in combination with PMA or CD3 antibody (OKT3), or in the presence of monocytes (data not shown). These results suggest that CSB-induced proliferation is monocyte dependent.
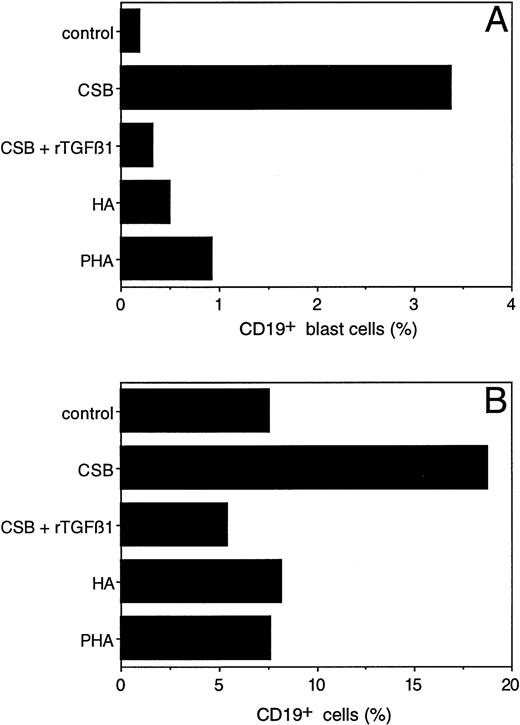
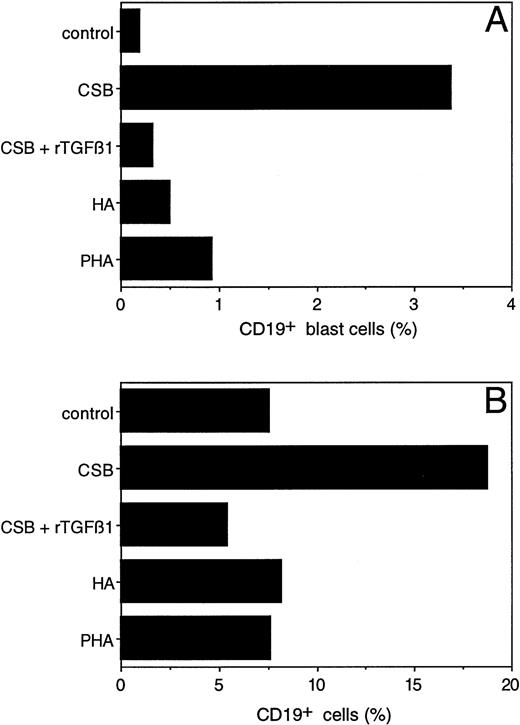
To further dissect the effect of CSB on PBMC, we attempted to determine the particular cell population responding to CSB. PBMC were stimulated with either CSB, HA, or PHA. Six days later, cultures were analyzed for expansion of CD4+, CD8+, and γδ T cells and CD19+ B cells by flow cytometry. Although CSB did not stimulate the different T-cell subsets, it did induce blast transformation (Fig 6A) and expansion (Fig6B) of CD19+ B cells. This effect was specific to CSB, as it was not observed with HA or PHA. The expansion and blast transformation of CD19+ cells as a response to CSB was blocked in the presence of TGFβ1 (Fig 6A and B), consistent with the TGFβ1 inhibitory effect on CSB-induced PBMC proliferation (Fig 5B). Of note, TGFβ1 inhibited in a dose-dependent fashion both CSA- and HA-induced IL-1β secretion (data not shown). These results establish that CSB is a B-cell activator and that its activity is monocyte dependent and TGFβ1 inhibitable.
Blast transformation and outgrowth of human peripheral blood B cells after stimulation with CSB. Control PBMC (1 × 106/mL) or PBMC stimulated with either CSB, HA, PHA, or CSB in combination with rTGFβ1 (1 ng/mL) were cultured for 6 days and then immunostained with phycoerythrin-conjugated CD19 MoAb and analyzed on a FACScan. Two-dimensional plots were generated in which CD19-positivity was plotted on the x-axis and side scatter (as a reflection of cell size) was plotted on the y-axis. In (A), the percentage of CD19+ cells showing blast transformation (as reflected in a size exceeding a threshold value established by control unstimulated cells) has been calculated. In (B), the overall percentage of CD19+ cells detected in these plots is shown. Nonspecific binding was controlled by using appropriate isotype-matched antibodies and was the same for all cultures.
Blast transformation and outgrowth of human peripheral blood B cells after stimulation with CSB. Control PBMC (1 × 106/mL) or PBMC stimulated with either CSB, HA, PHA, or CSB in combination with rTGFβ1 (1 ng/mL) were cultured for 6 days and then immunostained with phycoerythrin-conjugated CD19 MoAb and analyzed on a FACScan. Two-dimensional plots were generated in which CD19-positivity was plotted on the x-axis and side scatter (as a reflection of cell size) was plotted on the y-axis. In (A), the percentage of CD19+ cells showing blast transformation (as reflected in a size exceeding a threshold value established by control unstimulated cells) has been calculated. In (B), the overall percentage of CD19+ cells detected in these plots is shown. Nonspecific binding was controlled by using appropriate isotype-matched antibodies and was the same for all cultures.
CSB proliferative effect is CD44 dependent.
HA's immunomodulatory effects on murine macrophages (IL-1β induction)18 and B cells (proliferation and differentiation)23 have been shown to be CD44 dependent. Although CD44 principally binds to HA, it appears to also interact at a more modest level with CS.24 The hematopoietic CSPG, serglycin, was identified as a ligand for CD4425 and is involved in lymphoid cell activation.8 Furthermore, the CS form of the invariant chain (associating with MHC class II) can enhance T-cell responses through the interaction with CD44.7 To determine whether CD44 is also involved in CSB induction of cell proliferation, PBMC were exposed to CSB in the presence of 10 μg/mL of CD44 MoAb (A3D8). Addition of the CD44 antibody A3D8 significantly blocked CSB-induced [3H]-thymidine incorporation (Fig 7A). However, this antibody did not inhibit or stimulate [3H]-thymidine incorporation when added alone or with any concentration of PHA26 (and data not shown), ruling out toxic effects. Moreover, a CD44-Rg fusion protein24 inhibited CSB-induced PBMC proliferation (Fig7B). Although HA is the principal known CD44 ligand, it did not induce [3H]-thymidine incorporation by itself and did not diminish CSB-induced proliferation when added simultaneously in proliferation assays (data not shown). Therefore, CSB seems to induce B-cell proliferation via CD44, independent of HA ligation.
CD44 MoAbs and CD44-Rg inhibit CSB-induced PBMC proliferation. PBMC were incubated with CSB (1 mg/mL) with or without either the CD44 MoAb A3D8 (10 μg/mL; A), or CD44-Rg fusion protein (12 mg/mL ; B) for 6 days. [3H]-thymidine incorporation was measured in the last 16 hours of the experiment. The data were confirmed in three independent experiments.
CD44 MoAbs and CD44-Rg inhibit CSB-induced PBMC proliferation. PBMC were incubated with CSB (1 mg/mL) with or without either the CD44 MoAb A3D8 (10 μg/mL; A), or CD44-Rg fusion protein (12 mg/mL ; B) for 6 days. [3H]-thymidine incorporation was measured in the last 16 hours of the experiment. The data were confirmed in three independent experiments.
These results suggest novel functions for CSA and CSB as accessory molecules during monocyte and B-cell activation, respectively. Moreover, the data are consistent with CSB functioning through a cell-specific CD44 pathway that is not accessed by HA.
DISCUSSION
Soluble PGs arise at inflammatory sites by secretion from activated monocytes/macrophages and via the degradation of extracellular matrix.5 In turn, PGs, whether soluble or cell-associated, have been functionally linked in a number of isolated reports to immune cell activation and inflammatory processes. The chondroitin 4-sulfate type of serglycin, a CSPG secreted by hematopoietic cells,5is a ligand for CD44 and contributes to the activation of cytotoxic T-cell clones.8 In another study bearing upon T cells, the chondroitin sulfate form of invariant chain (associated with MHC class II) was shown to enhance the stimulation of T cells via interaction with CD44.7 A connection has also been established between PGs/GAGs and macrophage activation. Soluble HA induces the expression of IL-1β, tumor necrosis factor-α, and IGF-1 in macrophages,17,18 and this expression can be blocked by CD44 MoAbs.18
The present study establishes additional links between the CSs and selected immune cell populations. Significant findings include: (1) CSA, like HA, stimulates monokine secretion by PBMC; (2) CSB, another chondroitin 4-sulfate, stimulates aggregation of PBMC and promotes monocyte-dependent B-cell blast transformation and proliferation, which is inhibitable by both the anti-inflammatory cytokine TGFβ and CD44 MoAbs; and (3) CSA and CSB do not appear to overlap in their immunomodulatory activities, and neither CSC, a chondroitin 6-sulfate, nor heparin share in these activities. The data suggest yet another extrinsic pathway leading to the triggering of inflammatory monokine production. Moreover, the data constitute the first direct connection between CSs, and in particular chondroitin 4-sulfates, and both the monocytic and B-cell compartments. Hence, the CSs, the main GAGs secreted by activated monocytes, emerge here as a potentially significant class of inflammatory mediators.
CD44 has previously been implicated in mediating HA-induced activation of human and murine monocytes,17,18 as well as in a recent report, murine B cells.23 Moreover, CD44 has also been implicated in CS-induced activation of T cells.7,25 The findings of the present study significantly expand on these previous CD44-related observations. Our data offer the first evidence that CD44 is involved in human B-cell activation. Furthermore, the present findings show that CD44 is functionally required for CS-induced, and not just HA-induced, activation of monocytes and B cells. While the present data indicate that CD44 is somehow involved in B-cell activation, they do not formally distinguish between the possibilities that the functional CD44 is on B cells versus interacting monocytes, a question prompted by our additional observation that the CSB effect on B-cell proliferation is monocyte dependent. However, it is noteworthy in this regard that previous reports have clearly documented increased CD44 expression after B-cell activation, with associated changes in the CD44 isoforms expressed.27-29 Moreover, HA-induction appears to operate directly on the CD44 of B cells in the murine system.23 Additionally, our data showed no effect of CSB on human monocyte activation (monokine production). Taken together, it seems most likely that the CSB effect on B cells observed here in the human system is mediated by the B cell's CD44.
Although studies have tended to emphasize HA as a principal ligand for CD44, there is in fact evidence that CS also binds to CD44, albeit at a 100-fold lower concentration than does HA.24 In line with this previous binding data, our functional data showing a CD44-dependent CSB-induced effect on B-cell proliferation is likely explained by direct CSB binding to CD44 (on B cells and/or monocytes). However, the data do not rule out other mechanisms. It is also tempting to speculate that the CSA effect on monokine secretion, like the CSB effect on B-cell activation, may be CD44 dependent. This would fit with our observation that CSA stimulates IL-1β secretion by PBMC at a 100-fold excess of CSA as compared with HA, mirroring the differences between CS and HA in their CD44-binding capacities. However, we could not address this interesting question because the CD44 MoAb used here demonstrates agonist activity that induces cytokine secretion by monocytes (data not shown).
The functional differences observed for the various GAGs examined in this study are of special interest. CSA and CSB, both chondroitin 4-sulfates, exhibited markedly different functional profiles, with their primary effects directed toward monocytes and B cells, respectively. Even more strikingly, HA, notwithstanding its high reported binding affinity for CD44, did not share CSB's B-cell modulatory activities nor did it compete with CSB. These differential effects observed here for CSA and HA on monocytes and CSB on B cells might stem from the expression of distinct CD44 isoforms and conformational types with different GAG-binding capacities on these cells.24,27 30-36 Further study of the ability of the variant forms of CD44 expressed during B-cell development and their relative ability to bind CS is clearly warranted.
An important aspect of the present study is the use of whole PBMC pools rather than purified cell subpopulations or cell lines. This experimental approach permits the detection of immunological effects that are dependent on cell:cell interactions, and in our case, was essential for detecting the CSB-dependent B-cell proliferation in the first place because it appears to be dependent on the presence of monocytes. Just how monocytes participate in this phenomenon, as well as how CSB and CD44 play into this, remains to be determined.
B cells can be activated in T-independent fashion using polymeric molecules that bear repeating epitopes. CSB, being composed of repeating disaccharide units, is in principle equipped to provide the first signal for such cross-linkage–dependent B-cell activation. The second signal could come from monocytes, explaining the observed monocyte dependence in our system. Furthermore, the inherently higher valency associated with PGs, as compared with free GAGs, might confer upon them higher functional efficacy, both for CSA-containing ones (in the case of monocyte triggering) and for CSB-containing ones (in the case of B-cell triggering). Hence, although relatively higher concentrations of free CS GAGs (as compared with the HA GAG) are needed to achieve functional immunostimulation in the experimental assays used here, they likely underestimate the potency of these GAG stimulators when PG based.
The present study suggests at least two ways in which GAGs could contribute to inflammatory processes. First, GAGs could tie into pro-inflammatory cytokine secretion. This appears to be the case for both CSA and HA, which can trigger the secretion of monokines. The induction of IL-1β documented here is especially significant in light of the established pivotal role of this cytokine in the inflammatory cascade.19 Second, GAGs could influence lymphoid cells directly, without cytokines as intermediaries. Our studies highlight this point for the CS:B-cell interface. Unravelling of a GAG “pro-inflammatory pathway” could provide novel therapeutic targets within inflammatory sites in pathophysiologic settings. For example, in the case of CSA, there is the potential for therapeutics that intercept this local molecular trigger of IL-1β secretion, as an alternative to other therapies under development (eg, soluble IL-1 receptors and IL-1 receptor anatagonists) which deal with blocking IL-1β's downstream effects. More information pertaining to soluble PG and free GAG accumulation in pathophysiologic settings will first be needed.
ACKNOWLEDGMENT
We thank Dr A. Hochberg, Dr D. Cibrik, and G. Riely for helpful discussions and review of the manuscript. We appreciate Dr M. Lamm's support throughout this project. We also thank Susi Brill for her expert secretarial assistance.
Supported in part by grants from the National Institutes of Health (NIH RO1 AI38960, NIH RO1 CA74958, and NIH RO1 AI31044).
Address reprint requests to Mark L. Tykocinski, MD, Department of Pathology, Biomedical Research Building Room 925, Case Western Reserve University, 10900 Euclid Ave, Cleveland, OH 44106.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 5. Characterization of CSB-induced PBMC proliferation. (A) PBMC were cultured as described in Materials and Methods, and CSB was added at the indicated concentrations. (B) PBMC were treated with CSB (1 mg/mL) in the absence or presence of rTGFβ1 (1 ng/mL). (C) Total PBMC or monocyte-depleted PBMC (nonadherent) were incubated with CSB (1 mg/mL) for 6 days. Monocytes were depleted by adherence to tissue culture flasks and nonadherent cells were collected, counted, and plated. Data shown are [3H]-thymidine incorporation during the final 16 hours of a 6-day culture period. In each case, the results shown are representative of at least three similar experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.223.413k15_223_229/5/m_blod41315005x.jpeg?Expires=1770777939&Signature=M1u7YWhB-Fbfit3ttZJq-wqvMX1zGI4l28VS~mq1fpW3wkz~OKWZBnbfZxyFOX~8tjUG9j9dDIzuBdKN~GjWf9PRaw0LNukxFP2brCe63-mRh0Mcy2DZyRKgqanHLXy8DKCdNkBoJ9dDLVoy0HfJ5-d3eYmesPqNXeJRbi~Cs8ljIZMj5szYkDq-MhkFEMfddJfsc0oVARZEPo~uWQmj6Scu7gODfC61ZzkD1yQ9bzG3D9nah~CD7sVb4k8SNUgpnchqria4foHaIrPpgbUmRdZVVgKMnbAhJTK5l7h-9yNrQGXhW6G9~MGFyk1LvkMLaR3ABRQgbpWu8bgZ6Y7hYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. CD44 MoAbs and CD44-Rg inhibit CSB-induced PBMC proliferation. PBMC were incubated with CSB (1 mg/mL) with or without either the CD44 MoAb A3D8 (10 μg/mL; A), or CD44-Rg fusion protein (12 mg/mL ; B) for 6 days. [3H]-thymidine incorporation was measured in the last 16 hours of the experiment. The data were confirmed in three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.223.413k15_223_229/5/m_blod41315007x.jpeg?Expires=1770777939&Signature=dCgoVkR6r0B2PA5bohPfNTmHtfnzzwJm4TM0j9dvmEIdo1NluvfTpmJe8J3NKc01z7~b5XdClJ084b5-A8ClKsMtzopRwQmNLXHU~eQajUQffMI47u~INOCzmI-d8L3NEh6MkRV~-7QtWBQIASvcBMsNCnGSGaEwlu2623GglQMw2s2JxbIP-iWqhh0QuqATEWwn8FaJcR-2Szcfb3nqDyrO-aFch0bdciHgJfXH~yDhFnW6j-L7IeUhXXZnOWNpM~MGI8WmElWbF26DGMarRC7I0y5udOA1xeepiRauAfo0OnNkoWGLHnI~gy6ARxx4hOLUcMz90kTLlKXNR0JjZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. Characterization of CSB-induced PBMC proliferation. (A) PBMC were cultured as described in Materials and Methods, and CSB was added at the indicated concentrations. (B) PBMC were treated with CSB (1 mg/mL) in the absence or presence of rTGFβ1 (1 ng/mL). (C) Total PBMC or monocyte-depleted PBMC (nonadherent) were incubated with CSB (1 mg/mL) for 6 days. Monocytes were depleted by adherence to tissue culture flasks and nonadherent cells were collected, counted, and plated. Data shown are [3H]-thymidine incorporation during the final 16 hours of a 6-day culture period. In each case, the results shown are representative of at least three similar experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.223.413k15_223_229/5/m_blod41315005x.jpeg?Expires=1770864236&Signature=rbEEQfTWrbiysaU1l2OHfRdlz6V8Uf4eHLIGmLZilqCaoYQUFCsqsAXZf7jgf31NSk-LCxZm3Wvuc7KCN3pd-fjkxvJpKJ06UUhH6bGel~o5iKfRzbStFxH8R9VDnABWCxadqrYCmPCzTJV-vzXM~j7xfWyWTIkVAM1pMBzED-VTppvfUnymfFxFHJKORmR8yXcbBp8o0tBGgYDXE3ZFYZk71SikJ5aU4JySqQvFYWsj~sDouelnZoMGbIe8zUiLpGFeZhFJC7XhFAQ9vdDc9FfSUJ5aQkpt68YG8muR56AjvtV0T3qVX3vJ7tQYQtTmtuyUHPoUAujx8KLDb~3UIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. CD44 MoAbs and CD44-Rg inhibit CSB-induced PBMC proliferation. PBMC were incubated with CSB (1 mg/mL) with or without either the CD44 MoAb A3D8 (10 μg/mL; A), or CD44-Rg fusion protein (12 mg/mL ; B) for 6 days. [3H]-thymidine incorporation was measured in the last 16 hours of the experiment. The data were confirmed in three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.223.413k15_223_229/5/m_blod41315007x.jpeg?Expires=1770864236&Signature=3qzgFk7uKOJte~hsh0epz8xEMXgrH7t-P2LwZsguZSAJGrgzs2AfSM~kD6se12J9uBKUM0DJJoLpHq-ZcQZUq70FHDquxV2P39bSQsfVdy2ftEL2EvaYj6TJXnxmZSYxZgP2CxGyp9CQRHYmUfOxZBRZNwL4rOfHIqRFABtXPkadBI46jC0~AtMR0~as8hcgafYu5WDzbjISXLruzmdKdLf~naNeFnauP27k6HIo95dRZNuuJEx4s7PXYLjVd9-RKgLug3PmHlj2Y7ILFK46bzl1Rcp5N3KHArKHxUSwCYnNHDuNwuxmc8AolA8RHlxRDHyLz~ab-tCVAAgBwYs8vA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)