THE INTERLEUKIN-6 (IL-6) family of cytokines acts via receptor complexes that contain at least one subunit of the signal transducing protein gp130.1 The family comprises IL-6, IL-11, ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1), leukemia inhibitory factor (LIF), and oncostatin M (OSM).1 IL-6, IL-11, and CNTF first bind to specific receptors, and these complexes associate with a homodimer of gp130 in the case of IL-6 and IL-11 or, alternatively, with a heterodimer of gp130 and the related protein LIF receptor (LIF-R) in the case of CNTF. OSM and LIF first bind directly to gp130 and LIF-R, respectively, and form heterodimers with LIF-R and gp130. Recently, a gp130-related protein was described that can heterodimerize with gp130 and that acts as an alternative OSM receptor.2CT-1 binds directly to the LIF-R and induces gp130/LIF-R heterodimer formation.3 Recently, the presence of a specific glycosylphosphatidylinositol (GPI)-anchored CT-1 receptor on neuronal cells was implicated.4
Cytokines of the IL-6 family are involved in various steps of hematopoiesis and have been used for the ex vivo expansion of hematopoietic cells.5-7 Whereas recent reviews have concentrated on soluble cytokine receptors in general,8 on the mechanisms of generation of soluble receptors,9,10 and on various aspects of the IL-6 cytokine family,11 this review will mainly focus on the consequences of direct stimulation of gp130 on hematopoietic cells, through the complex of IL-6 and a soluble form of the IL-6R, in vivo and in vitro.
GENERATION AND OCCURRENCE OF SOLUBLE RECEPTORS
Many if not all transmembrane proteins occur also in a soluble form that consists of the major part of the extracellular domain. This phenomenon has been observed for type I and type II transmembrane proteins.9 10 Two independent mechanisms lead to the generation of such soluble proteins.
Firstly, transmembrane proteins can be cleaved by a transmembrane metalloproteinase that most likely is a protease distinct from matrix-type metalloproteinases to yield the soluble extracellular domain of the proteins. This mechanism has been studied in detail for the human IL-6R.12-16 Cleavage is controlled by protein kinase C and occurs at a distinct site that is not strictly sequence specific.15 The generation of the soluble IL-6R can be prevented by hydroxamic acid compounds16 that previously have been shown to inhibit the processing of the membrane form of tumor necrosis factor (TNF).17,18 A TNF processing metalloproteinase has recently been cloned19,20 and shown to belong to the family of disintegrin domains containing metalloproteinases (ADAMs).21 It is unclear whether different family members of the ADAMs are highly substrate specific or whether one protease is able to cleave more than one protein.
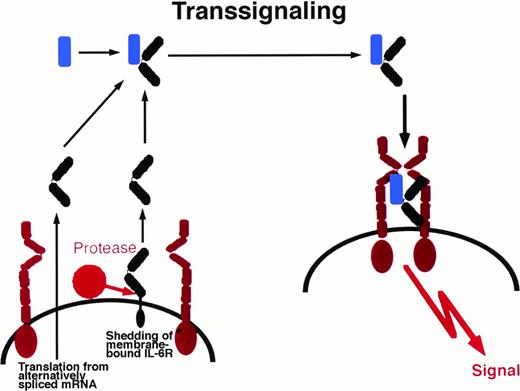
Alternatively, the generation of soluble counterparts of transmembrane proteins has been shown to occur via translation from alternatively spliced mRNAs.9 In particular, a soluble form of the IL-6R can be synthesized by various cells from a spliced mRNA yielding a protein that differs at its COOH-terminus by 14 amino acid residues,22 23 indicating that for one transmembrane protein both mechanisms of generation of a soluble protein may exist.
Soluble IL-6R protein has been detected in the blood of normal individuals at concentrations of 50 to 80 ng/mL.24Increased concentrations have been found during infections and malignant disorders.24-26 Interestingly, bacterial proteins massively induce the shedding of several membrane proteins via the activation of metalloproteinases.27 28
IL-6–TYPE CYTOKINES TRANSSIGNALING VIA SOLUBLE RECEPTORS
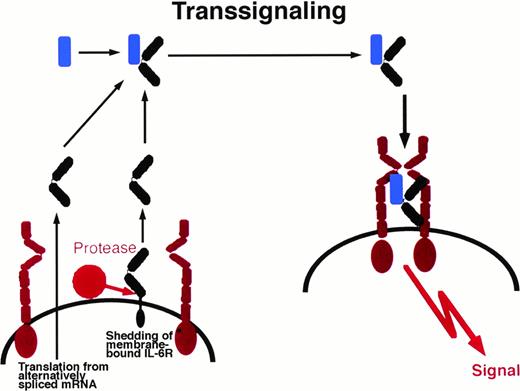
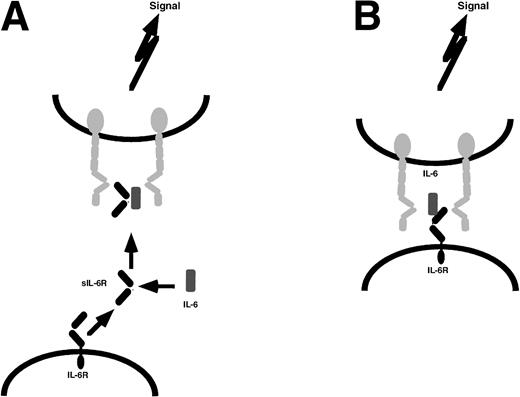
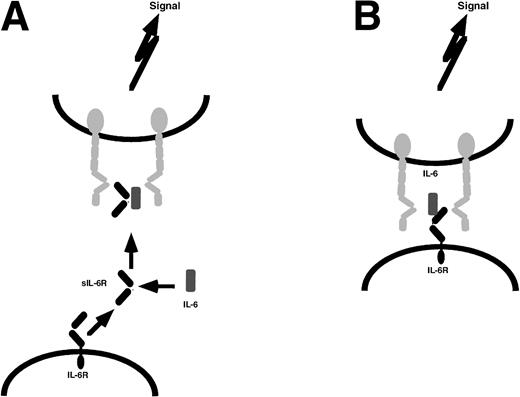
Soluble receptor proteins bind their ligands with similar affinities as the cognate transmembrane receptors.9 Most soluble receptors for cytokines and growth factors compete with their membrane bound counterparts for the binding of the ligand and therefore are antagonists.9 In contrast, the soluble receptors of the IL-6 cytokine family, when complexed with their ligands, exhibit agonistic biological activities. These complexes can directly recruit and activate homodimers of gp130 (in the case of IL-6 and IL-11) and heterodimers of gp130 and LIF-R (in the case of CNTF).29-32Cells that do not express specific receptors for IL-6, IL-11, or CNTF are not able to respond to these cytokines. The presence of soluble receptors leads to responsiveness of these cells (Fig 1). This process has been named transsignaling.9 Of note, soluble forms of gp130 and LIF-R exist in vivo and have been demonstrated to possess antagonistic biological activity.33 34
Transsignaling of soluble receptors of IL-6 family. An IL-6R–expressing cell (left) releases a soluble receptor by shedding or alternative splicing. This soluble receptor binds IL-6 and induces homodimerization of gp130 on a target cell (right) that expresses gp130 but no IL-6R. In this model, the target cell in the absence of soluble IL-6R is not responsive to IL-6. gp130, red; IL-6R black; IL-6, blue. This transsignaling model holds also true for the IL-6 family cytokines IL-11 and CNTF.
Transsignaling of soluble receptors of IL-6 family. An IL-6R–expressing cell (left) releases a soluble receptor by shedding or alternative splicing. This soluble receptor binds IL-6 and induces homodimerization of gp130 on a target cell (right) that expresses gp130 but no IL-6R. In this model, the target cell in the absence of soluble IL-6R is not responsive to IL-6. gp130, red; IL-6R black; IL-6, blue. This transsignaling model holds also true for the IL-6 family cytokines IL-11 and CNTF.
BIOLOGICAL PROPERTIES OF SOLUBLE IL-6R
Taga et al,35 using coimmunoprecipitation techniques, were the first to demonstrate that a soluble form of the IL-6R in the presence of IL-6 associates with gp130. Consequently, release of a soluble IL-6R by human peripheral blood mononuclear cells (PBMC) was demonstrated and it was shown that soluble IL-6R together with IL-6 partly suppressed the Con A-induced proliferative response of PBMC.24 The in vivo biological activity of soluble IL-6R has been demonstrated using a murine tumor rejection model.36 In this assay, highly tumorigenic murine melanoma cells (B78) were used. B78 cells injected into syngeneic mice caused the formation of tumors and metastases, whereas cells transfected with a cDNA coding for IL-6 protected the animals. Surprisingly, transfection of B78 cells with a cDNA coding for the murine soluble IL-6R led to an even more effective protection of the animals, indicating that the soluble IL-6R interacted with the endogenous murine IL-6.36
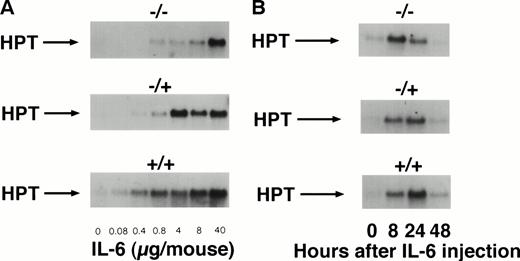
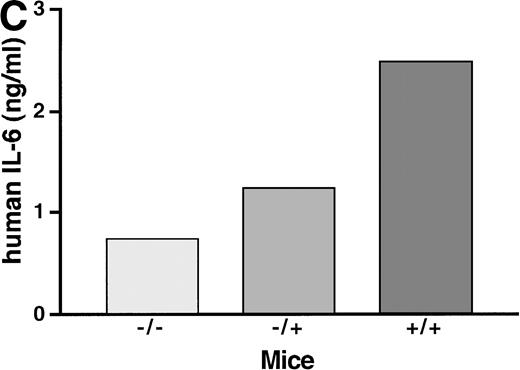
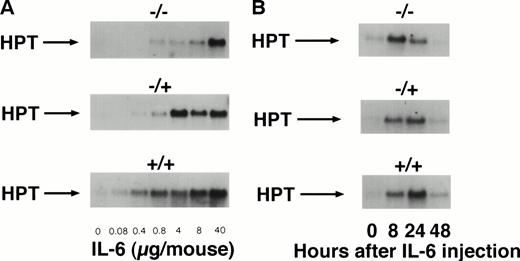
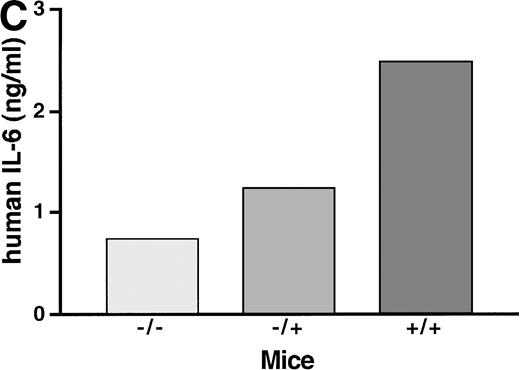
To study the in vivo function of the soluble IL-6R, we have constructed transgenic mice that express a human IL-6R cDNA into which a translational stop codon had been introduced upstream of the transmembrane region. Expression of this soluble receptor was under the transcriptional control of the liver specific phosphoenolpyruvate carboxykinase (PEPCK) promoter.37 Human IL-6 stimulates human and murine cells, whereas murine IL-6 only stimulates murine cells.38 Because of this species specificity of IL-6, the transgenic human soluble IL-6R did not bind the endogenous murine IL-6, and consequently the transgenic animals showed no transgene specific phenotype. Upon injection of human IL-6 into transgenic mice and nontransgenic control mice, the IL-6–specific induction of hepatic genes was analyzed.37 Measuring the IL-6–induced hepatic haptoglobin mRNA expression, it turned out that, in the presence of the soluble IL-6R, hepatocytes were significantly sensitized towards human IL-6 (Fig 2A, left panel). A similar extent of acute phase response was obtained with a 100-fold lower concentration of human IL-6 in mice expressing the soluble IL-6R.37 Time course studies showed that the expression of hepatic haptoglobin mRNA was markedly prolonged in the presence of the soluble IL-6R (Fig 2A, right panel), which was most likely caused by a prolongation of the plasma half-life of IL-6 in mice expressing the soluble IL-6R (Fig 2B).
Sensitization of target cells and increase of the plasma half- life of IL-6 in mice transgenic for the human soluble IL-6R. (A) Dose-response of the IL-6–induced hepatic acute phase protein expression. Haptoglobin expression in the liver of control mice (−/−) and heterozygous (−/+) and homozygous (+/+) mice was analyzed. Mice were injected intraperitoneally with various dosages of human IL-6 as indicated. Mice were killed 4 hours after injection. (B) Time course of the hepatic acute phase protein expression. Mice were injected intraperitoneally with 8 μg of human IL-6 and killed after the time periods indicated. Total RNA was prepared from the liver and subjected to Northern blot analysis. Filters were hybridized with a32P-labeled 0.9-kb HinfI restriction fragment of human haptoglobin cDNA. (C) Serum levels of human IL-6 in control mice or heterozygous (−/+) or homozygous (+/+) transgenic mice expressing the human soluble IL-6R were measured 4 hours after injection with a human IL-6–specific ELISA.
Sensitization of target cells and increase of the plasma half- life of IL-6 in mice transgenic for the human soluble IL-6R. (A) Dose-response of the IL-6–induced hepatic acute phase protein expression. Haptoglobin expression in the liver of control mice (−/−) and heterozygous (−/+) and homozygous (+/+) mice was analyzed. Mice were injected intraperitoneally with various dosages of human IL-6 as indicated. Mice were killed 4 hours after injection. (B) Time course of the hepatic acute phase protein expression. Mice were injected intraperitoneally with 8 μg of human IL-6 and killed after the time periods indicated. Total RNA was prepared from the liver and subjected to Northern blot analysis. Filters were hybridized with a32P-labeled 0.9-kb HinfI restriction fragment of human haptoglobin cDNA. (C) Serum levels of human IL-6 in control mice or heterozygous (−/+) or homozygous (+/+) transgenic mice expressing the human soluble IL-6R were measured 4 hours after injection with a human IL-6–specific ELISA.
Recently, it was shown that human IL-6–dependent myeloma cells were unable to grow in the presence of low IL-6 concentrations when the medium was depleted of soluble IL-6R produced by these cells. This result seems to indicate that the membrane-bound IL-6R was not sufficient to mediate the growth-stimulating signal of IL-6 and might point to a more general importance of the IL-6/soluble IL-6R complex for gp130-mediated signaling.39
DEFINITION OF NEW TARGET CELLS OF THE IL-6/SOLUBLE IL-6R COMPLEX
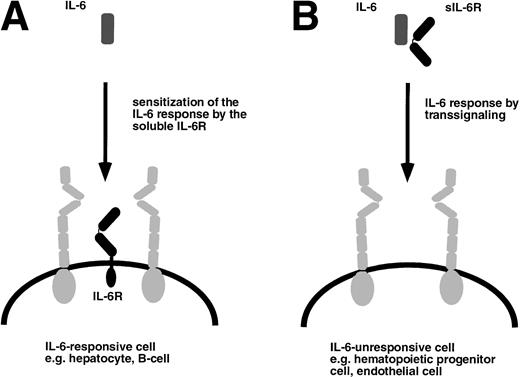
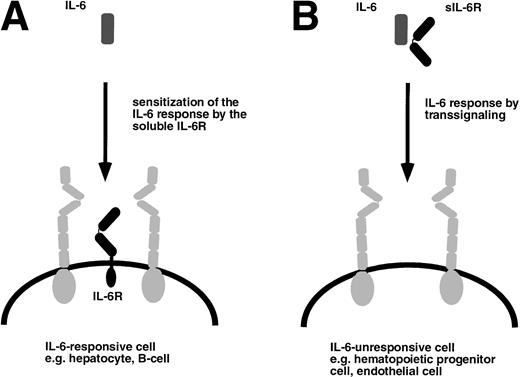
The function of the soluble IL-6R can be deduced from a situation depicted in Fig 3A. Cells that express gp130 and the specific IL-6R can be stimulated with human IL-6 or alternatively by IL-6 complexed to the soluble human IL-6R. Figure 3B schematically shows a hypothetical target cell that would only be responsive to the complex of IL-6 and the soluble IL-6R. To address the question of whether such target cells exist in vivo, we compared the phenotype of mice transgenic for IL-6 alone with the one of mice transgenic for both human IL-6 and human soluble IL-6R.40-42
Target cells of IL-6 and the IL-6/soluble IL-6R complex. (A) Cells that express gp130 (gray) and membrane bound IL-6R (black) are responsive to IL-6 and are sensitized by the presence of the soluble IL-6R. (B) Cells that only express gp130 but no membrane bound IL-6R are unresponsive to IL-6 but can be stimulated by the complex of IL-6 and soluble IL-6R.
Target cells of IL-6 and the IL-6/soluble IL-6R complex. (A) Cells that express gp130 (gray) and membrane bound IL-6R (black) are responsive to IL-6 and are sensitized by the presence of the soluble IL-6R. (B) Cells that only express gp130 but no membrane bound IL-6R are unresponsive to IL-6 but can be stimulated by the complex of IL-6 and soluble IL-6R.
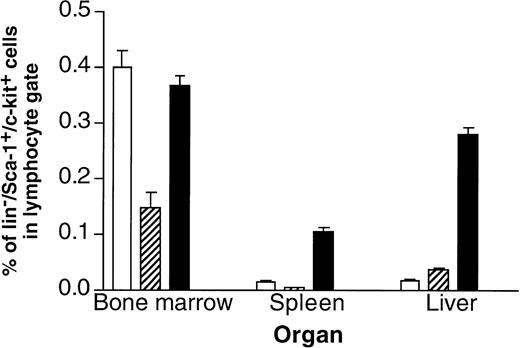
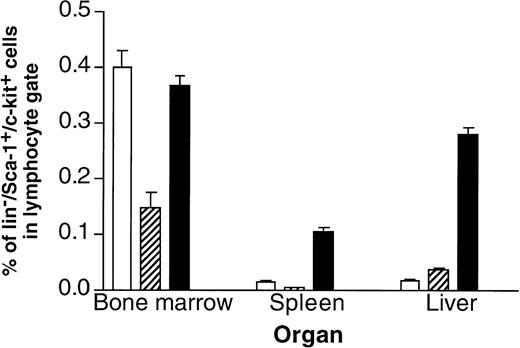
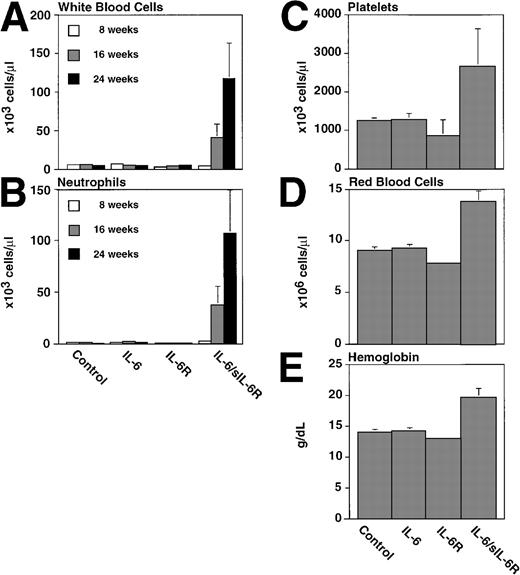
It turned out that one of the main differences between single- and double-transgenic mice was a massive extramedullary hematopoiesis in liver and spleen of the adult animals.40 As shown in Fig 4, the livers and spleens of IL-6/sIL-6R double-transgenic mice contained a highly elevated number of Lin−/Sca1+/c-kit+ cells, which have been demonstrated to contain a very high percentage of hematopoietic stem cells43,44 (Peters et al, manuscript in preparation). Moreover, spleen and liver contained highly elevated numbers of granulocytes, macrophages, Sca1+hematopoietic progenitor cells, and B cells. The presence of hematopoietic progenitor cells in liver and spleen resulted in a time-dependent massive increase of peripheral blood cell numbers in IL-6/sIL-6R double-transgenic mice (Fig 5). These effects were completely absent in single transgenic mice and in nontransgenic control animals.40
Frequency of cells with a hematopoietic stem cell phenotype in control, IL-6, or IL-6/soluble IL-6R transgenic mice. The presence of Lin−/Sca1+/c-kit+cells present in bone marrow, spleen, and liver of (□) control mice, (▨) IL-6 transgenic mice, and (▪) IL-6/soluble IL-6R double-transgenic mice was analyzed by FACS.
Frequency of cells with a hematopoietic stem cell phenotype in control, IL-6, or IL-6/soluble IL-6R transgenic mice. The presence of Lin−/Sca1+/c-kit+cells present in bone marrow, spleen, and liver of (□) control mice, (▨) IL-6 transgenic mice, and (▪) IL-6/soluble IL-6R double-transgenic mice was analyzed by FACS.
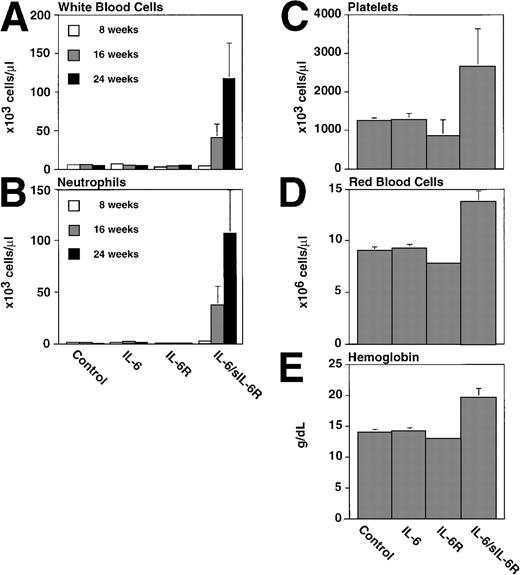
Peripheral blood cells of control, IL-6, or IL-6/soluble IL-6R transgenic mice. White blood cell (A), neutrophil (B), platelets (C), red blood cells (D), and hemoglobin (E) values were analyzed from six transgenic mice and nontransgenic littermates per group at the ages indicated. Mean values with standard deviations are presented.
Peripheral blood cells of control, IL-6, or IL-6/soluble IL-6R transgenic mice. White blood cell (A), neutrophil (B), platelets (C), red blood cells (D), and hemoglobin (E) values were analyzed from six transgenic mice and nontransgenic littermates per group at the ages indicated. Mean values with standard deviations are presented.
In murine embryogenesis, the first hematopoietic cells are generated in the yolk sac at day 7.5 (E 7.5) of gestation. The first intraembryonic tissue with multilineage and long-term repopulating activity is the splanchnopleuric mesoderm/aorta, genital ridge, mesonephros (AGM) region between E 8.5 and 11 of gestation.45 Later, hematopoietic progenitor and stem cells can be found in the fetal liver and around birth in the spleen and bone marrow. The presence of multipotent hematopoietic progenitor cells and cells with a stem cell phenotype in IL-6/sIL-6R double-transgenic adult animals might point to the fact that the adult liver retains a hematopoietic microenvironment and hematopoietic stem cells from the fetal developmental stage independent from other hematopoietic tissues such as spleen and bone marrow. Bone marrow hematopoiesis in double-transgenic mice was unaffected by the presence of IL-6 and IL-6R.40 This suggests that both tissues are affected in a different manner by IL-6 and soluble IL-6R.
Several studies have described functional changes in the hematopoietic system during development (reviewed in Bonifer et al46). Lansdorp et al47 and Vormoor et al48 have recently reported that human fetal liver cells have a higher proliferation and self-renewal rate as compared with cord blood cells and that cord blood cells have a higher proliferative and self-renewal capacity as compared with adult bone marrow cells. There is now growing evidence that the functional difference of stem cells isolated from different developmental stages is reflected by a developmental-specific cytokine/growth factor receptor expression pattern on the surface of hematopoietic cells. Several lines of evidence support this notion: whereas cells isolated from bone marrow are optimally expanded with a combination of Flt-3 ligand, stem cell factor (SCF), and IL-3,49,50 cord blood cells require Flt-3 ligand, IL-6, and the soluble IL-6R for efficient expansion.50 A further example of a developmental-specific growth factor activity of an IL-6 family member is OSM. Mukouyama et al51 report that treatment with OSM leads to the expansion of AGM-derived multipotent hematopoietic progenitors, but no stimulation of colony formation was detected with bone marrow-derived cells.
Taken together, the data from the single- and double-transgenic mice indicate that expansion of early extramedullary hematopoietic progenitor cells could only be stimulated by IL-6 in the presence of the soluble IL-6R. This finding is strongly supported by recent data from Tajima et al,52 who found that CD34+ cells can be subdivided into IL-6R–expressing and nonexpressing cells. Both cell populations express gp130. It was demonstrated that IL-6R–expressing cells can be stimulated to form granulocyte-macrophage colonies, whereas IL-6R negative cells upon stimulation with IL-6 and soluble IL-6R form various types of colonies including erythroid bursts, granulocyte-macrophage colonies, megakaryocytes, and mixed colonies.52 These findings are further supported by data from McKinstry et al,53 who demonstrate that the number of IL-6R on hematopoietic progenitor cells increases significantly with maturation of these cells. The CD34+ subpopulation that does not express IL-6R includes most of the erythroid, megakaryocytic, and primitive human hematopoietic progenitors. Such cells are target cells for IL-6/soluble IL-6R but not for IL-6 alone.
STIMULATION OF HEMATOPOIETIC PROGENITOR CELLS WITH THE IL-6/SOLUBLE IL-6R COMPLEX IN VITRO
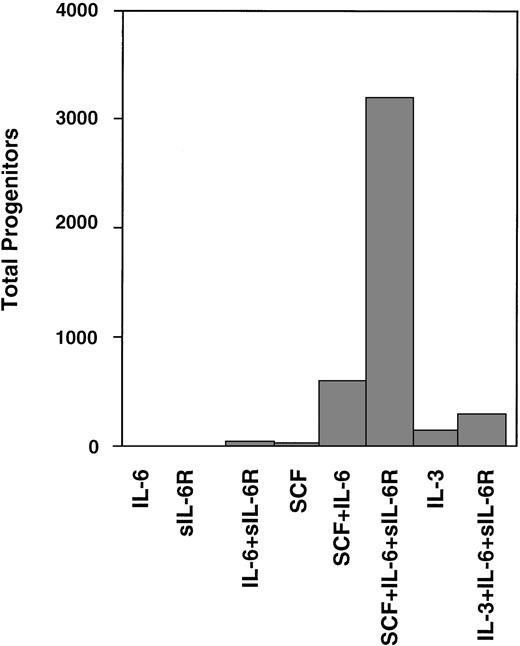
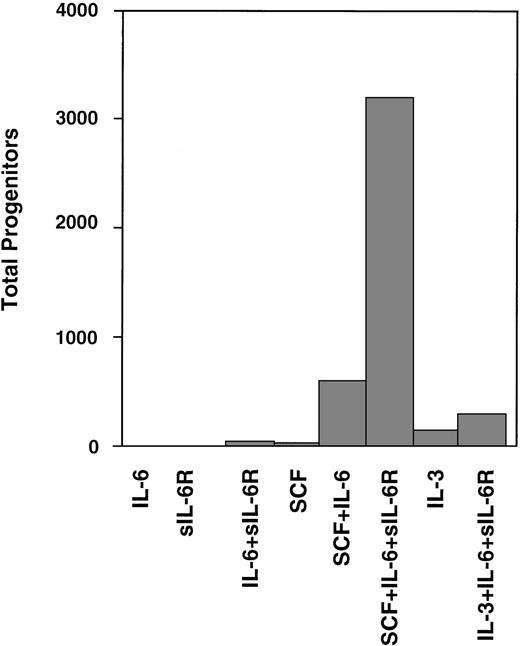
Experimental strategies to expand hematopoietic cells often used cytokines of the IL-6 family.5-7 Sui et al54were the first to demonstrate that stimulation of gp130 by IL-6 and soluble IL-6R resulted in superior ex vivo expansion of primitive human hematopoietic progenitor cells when compared with IL-6 alone. They showed that human cord blood CD34+ cells were stimulated by stem cell factor combined with IL-6 and soluble IL-6R (Fig 6). These studies were extended by the same group52,55 and have meanwhile been confirmed by several laboratories.50,56 The most interesting aspect of these studies is that direct stimulation of gp130 seems not to be a proliferative stimulus by itself. However, IL-6 in combination with stem cell factor and IL-3 has been reported to attenuate differentiation of hematopoietic cells.50,54 56
Expansion of CD34+ human cord blood-derived progenitor cells in the presence of IL-6 or IL-6/soluble IL-6R. Two thousand CD34+ cord blood cells containing 684 progenitors were cultured in serum containing suspension culture medium supplemented with the factors indicated. The expansion of hematopoietic progenitor cells was tested after 2 weeks of liquid culture in methylcellulose assays. Adapted and reprinted with permission from Sui et al.54 Copyright 1995 National Academy of Sciences, U.S.A.
Expansion of CD34+ human cord blood-derived progenitor cells in the presence of IL-6 or IL-6/soluble IL-6R. Two thousand CD34+ cord blood cells containing 684 progenitors were cultured in serum containing suspension culture medium supplemented with the factors indicated. The expansion of hematopoietic progenitor cells was tested after 2 weeks of liquid culture in methylcellulose assays. Adapted and reprinted with permission from Sui et al.54 Copyright 1995 National Academy of Sciences, U.S.A.
A DESIGNER CYTOKINE THAT DIRECTLY STIMULATES gp130
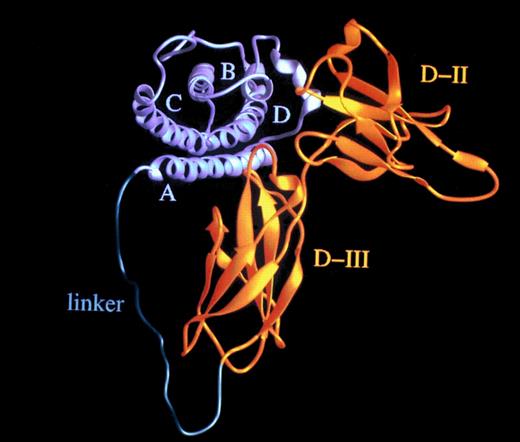
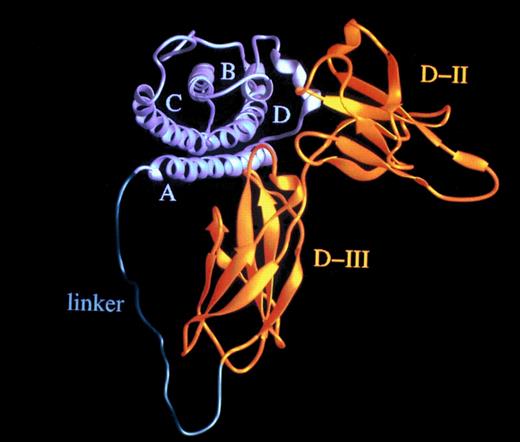
The effective concentration of IL-6 (50 ng/mL) and sIL-6R (>1,000 ng/mL)54 needed for the stimulation of human hematopoietic progenitor cells is high, considering a kd of approximately 1 nmol/L.57,58 Recently, it has been reported that the ligand/receptor interaction is mainly determined by the off-rate,59 suggesting that the average half-life of the IL-6/sIL-6R complex might be shorter than the time needed to assemble the IL-6/sIL-6R/gp130 complex. Accordingly, to lower the effective dose needed for IL-6 bioactivity, IL-6 muteins with a lower off-rate have been generated that render the complexes with IL-6R more stable.60 As a novel approach, we postulated that the formation of the IL-6/IL-6R complex could be enhanced by converting it into an unimolecular protein by using a flexible polypeptide as a linker (Fig 7). The distance between the C-terminus of IL-6R and the N-terminus of IL-6 was calculated from our three-dimensional model of the complex to be in the order of 40 Å.61 Consequently, we used the 16 N-terminal nonhelical and presumably flexible amino acid residues of IL-6 together with a 13 residue sequence rich in glycine and serine to connect IL-6 and the sIL-6R.56
Hyper-IL-6: a highly active designer cytokine consisting of IL-6 and soluble IL-6R. Molecular model of the fusion protein consisting of IL-6 (gray) and sIL-6R (yellow) fused by a flexible peptide linker (green). A, B, C, and D denote the four helices of IL-6; D-II and D-III are the two cytokine-binding receptor domains of the sIL-6R used for the construction of the fusion protein.
Hyper-IL-6: a highly active designer cytokine consisting of IL-6 and soluble IL-6R. Molecular model of the fusion protein consisting of IL-6 (gray) and sIL-6R (yellow) fused by a flexible peptide linker (green). A, B, C, and D denote the four helices of IL-6; D-II and D-III are the two cytokine-binding receptor domains of the sIL-6R used for the construction of the fusion protein.
On gp130-expressing cells, the fusion protein that we call Hyper-IL-6 turned out to be fully active at 100- to 1,000-fold lower concentrations compared with the combination of unlinked IL-6 and IL-6R. The fusion protein was therefore tested for its ability to stimulate expansion of hematopoietic progenitor cells in vitro. It turned out that stimulation with Hyper-IL-6 was at least as effective as IL-6/soluble IL-6R at 100 times lower concentrations than those used for unlinked IL-6 and IL-6R.56 62
gp130 STIMULATION OF HEMATOPOIESIS
Hematopoiesis is arranged in an descending hierachy: clonogenic hematopoietic stem cells pass through several stages of differentiation and finally produce functionally mature blood cells, including erythrocytes, megakaryocytes, granulocytes, monocytes, macrophages, mast cells, and the different classes of lymphocytes.63 The ability of a cell to generate and sustain the production of both mature myeloid and lymphoid cells for the whole lifetime of an hematologically compromised animal after transplantation has now been widely accepted as a useful and functional definition for its assignment to the stem cell compartment.64-66
In the double-transgenic IL-6/soluble IL-6R mice, hematopoietic progenitor cells in adult liver and spleen have been detected.40 As discussed earlier, it cannot be decided whether these hematopoietic progenitor cells originate from the fetal developmental stages or whether they have been washed in via the circulation. Presumably, these cells have expanded during several weeks of hepatic and spleenic hematopoiesis, and therefore renewal of hematopoietic progenitor cells must have occurred. In this respect, it is noteworthy that in double-transgenic IL-6/soluble IL-6R mice, but not in single-transgenic IL-6 mice, we find a massive upregulation of stem cell factor mRNA and cell surface protein expression in the liver that might contribute to stimulation and homing of hematopoietic progenitor cells (M. Peters, unpublished results).
So far, it is not clear whether gp130 stimulation contributes to hematopoiesis in vivo. From our transgenic mice data40together with the data from Zandstra et al,50 it is likely that gp130 stimulation is more important during fetal liver than during adult medullary hematopoiesis. Accordingly, gp130-deficient mice die perinatally between day 12.5 and term and the mutant embryos have reduced numbers of pluripotential and committed hematopoietic progenitors in the liver and reduced differentiated lineages such as T cells in the thymus.67 In contrast, mice that lack the LIF-R have normal hematologic compartments.68 These data argue that either the LIF-R is not important in hematopoiesis or that the biological activity of LIF-R can be substituted.
The case of OSM is more complicated, because human OSM can interact with both gp130/LIF-R and gp130/OSM-R complexes.2 In contrast, murine OSM seems only to interact with the gp130/OSM-R complex.69 In transgenic mice that overexpress OSM, no hematological abnormalities have been reported,70 except for an accumulation of immature and mature T cells in lymph nodes.71 However, it was recently reported that OSM is expressed in the AGM region and that OSM stimulated expansion of AGM-derived multipotential hematopoietic progenitor cells in vitro.51
Functional gp130 is required for normal fetal liver hematopoiesis. Among the cytokines of the IL-6 family, only IL-6 and IL-11, which use gp130 homodimers for signaling, may play a role in hematopoiesis in vivo. IL-11 has been demonstrated to possess thrombopoietic potential and can induce serial repopulating ability of murine hematopoietic stem cells.72 However, mice deficient for the IL-11 receptor do not show hematological abnormalities.73 IL-6 is involved in the regulation of stem cells and committed progenitor cells in vivo, but hematopoiesis still occurs in IL-6–deficient mice.74 A likely explanation for these findings is that IL-6 and IL-11, which both interact with a gp130 homodimer, can complement each other. However, it cannot be excluded that other, as yet unidentified gp130-stimulating cytokines exist that possess hematopoietic activities.
The second question that arises from the reviewed data is whether soluble receptors (eg, soluble IL-6R or soluble IL-11R) are involved in gp130 stimulation during hematopoiesis. Because several reports indicate that hematopoietic progenitor cells do not express IL-6R,40,52,53 gp130 stimulation on these cells might involve soluble receptors (Fig 8A) or intercellular stimulation (Fig 8B). However, it is difficult to discriminate between the two models. The only way to ultimately clarify the biological role of the soluble IL-6R will be to generate mice unable to produce a sIL-6R. However, the generation of such an animal model is a complex undertaking, because generation of the sIL-6R by shedding and splicing would have to be blocked. Therefore, a mouse lacking the exon used for the alternatively spliced sIL-6R23 would have to be constructed. This mutation then would have to be combined with a mutation, resulting in the deletion of the shedding sites of the IL-6R,14,27,28 or, alternatively, with a mutation resulting in the deletion of the shedding protease. Problems in this respect might arise from our finding that at least three different cleavage sites can be used by the shedding protease14,27 28 and that the IL-6R shedding protease has not been molecularly defined.
gp130 stimulation via soluble or membrane-bound IL-6R. (A) A donor cell (bottom) releases the soluble IL-6R that, in the presence of IL-6, stimulates the target cell (top) to dimerize gp130 (gray) and initiate signal transduction. (B) The contact between donor cell (bottom) and target cell (top) is mediated by the membrane-bound IL-6R of the donor cell, IL-6, and the two gp130 molecules of the target cell leading to gp130 dimerization and signaling. For reasons of simplicity, on donor cells, gp130 has been omitted.
gp130 stimulation via soluble or membrane-bound IL-6R. (A) A donor cell (bottom) releases the soluble IL-6R that, in the presence of IL-6, stimulates the target cell (top) to dimerize gp130 (gray) and initiate signal transduction. (B) The contact between donor cell (bottom) and target cell (top) is mediated by the membrane-bound IL-6R of the donor cell, IL-6, and the two gp130 molecules of the target cell leading to gp130 dimerization and signaling. For reasons of simplicity, on donor cells, gp130 has been omitted.
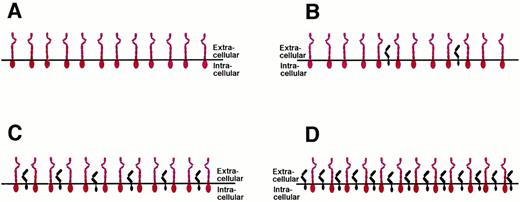
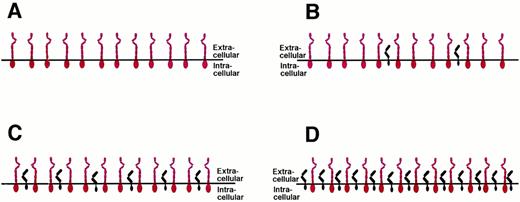
CELLULAR RESPONSIVENESS DEPENDS ON THE RATIO BETWEEN gp130 AND IL-6R
The tissue expression of gp130 is believed to be ubiquitous and the cellular gp130 expression seems not to be the subject to major regulation.1,75 However, the IL-6R is only expressed on certain cell types,1,57 including hepatocytes and B cells, and its expression is regulated by glucocorticoids.58 With the number of gp130 signal transducers being rather constant on all cells of the body, the number of IL-6R molecules expressed on the surface of target cells may vary from one cell type to another (Fig 9). Cells that do not express any IL-6R on their surface can be stimulated only by the IL-6/sIL-6R complex and are insensitive towards IL-6 alone (Fig 9A). Examples for such cells are hematopoietic progenitor cells,40 endothelial cells,76 neuronal cells,77,78 and osteoclasts.79 Osteoclast progenitor cell differentiation can be stimulated by IL-6 and the membrane bound IL-6R on osteoblasts (see Fig 8B) or by the combination of IL-6 and soluble IL-6R.79 It has also been demonstrated recently that osteoblasts can be stimulated via gp130 activation to express osteoclast differentiation factor that might bind to an as yet unidentified osteoclast differentiation factor receptor expressed on osteoclast progenitors and induce their differentiation into osteoclasts.80
Cellular responsiveness to IL-6 or IL-6/soluble IL-6R is determined by the expression levels of IL-6R. The number of the ubiquitously expressed gp130 protein (red) is believed to be rather constant on all cells of the organism. The number of the IL-6R molecules (black) varies on different cell types. See text for explanations.
Cellular responsiveness to IL-6 or IL-6/soluble IL-6R is determined by the expression levels of IL-6R. The number of the ubiquitously expressed gp130 protein (red) is believed to be rather constant on all cells of the organism. The number of the IL-6R molecules (black) varies on different cell types. See text for explanations.
Cells that express fewer IL-6R molecules on their surface than gp130 signal transducers respond towards IL-6 alone, and this response can be enhanced by the presence of the sIL-6R (Fig 9B). Examples for such cells are hepatocytes and plasmacytoma cells. Cells that show a balanced expression of IL-6R and gp130 on their surface respond towards IL-6, and this response is not altered by the sIL-6R (Fig 9C). Theoretically, on cells that express more IL-6R molecules than gp130 proteins, the addition of the soluble IL-6R might inhibit the IL-6 response, because the formation of inactive complexes containing only one gp130 molecule would be favored (Fig 9D). Using transfected cells, such a situation has recently been mimicked using the IL-11 receptor system. Whereas on gp130-expressing cells the complex of IL-11 and soluble IL-11R was agonistic, on cells overexpressing the membrane-bound IL-11R, increasing amounts of soluble IL-11R inhibited the IL-11 response of the cells.81
Interestingly, Wang et al82 recently showed that the stimulation of peripheral T cells with IL-6 results in a downregulation of gp130 molecules that might represent a rescue mechanism of the cytokine receptor system to avoid hyperstimulation.
gp130 STIMULATION AND INHIBITION OF DIFFERENTIATION
As outlined above, the stimulation of gp130 on hematopoietic progenitor cells might result in a differentiation-inhibiting activitiy. This is reminiscent of the activity of LIF83 on embryonic stem cells, which now are widely used to generate knock-out animals. Interestingly, LIF can also be replaced by other cytokines of the IL-6 family that interact with the gp130/LIR heterodimer, such as OSM and CNTF.84 ES cell differentiation is also completely prevented when cells are treated with the combination of IL-6 and sIL-6R.85 It is therefore tempting to speculate that the consequence of gp130 stimulation on early hematopoietic progenitor cells might be an inhibition of differentiation related to the effect of LIF seen in embryonic stem cells. Further experiments are needed to support this hypothesis.
CONCLUSIONS
The reviewed data indicate that gp130 stimulation is of importance for hematopoiesis in vivo. Probably, fetal liver hematopoiesis and medullary hematopoiesis require different stimulating cytokines, reflecting the ontogenic difference between the hematopoietic cells involved. Cytokines that directly stimulate gp130 will be of use for in vitro expansion of hematopoietic progenitor cells and should replace IL-6, which is commonly used in such cytokine cocktails.
ACKNOWLEDGMENT
The authors are thankful to H. Geiger (Freiburg, Germany) for help with the FACS analysis. We thank Dr Connie Eaves (Vancouver, British Columbia, Canada) and Dr Christoph Huber (Mainz, Germany) for reading the manuscript, help, and advice.
Supported by the Deutsche Forschungs-Gemeinschaft (Bonn, Germany), the NMFZ (Mainz, Germany), and the Stiftung Rheinland Pfalz für Innovation (Mainz, Germany).
REFERENCES
Author notes
Address reprint requests to Stefan Rose-John, PhD, I. Medizinische Klinik, Abteilung Pathophysiologie, Johannes Gutenberg Universität Mainz, Obere Zahlbacher Str. 63, D-55101 Mainz, Germany; e-mail: rosejohn@mail.uni-mainz.de.