Abstract
Immunostimulatory oligodeoxynucleotides containing the CpG motif (CpG ODN) can activate various immune cell subsets and induce production of a number of cytokines. Prior studies have demonstrated that both CpG ODN and granulocyte-macrophage colony-stimulating factor (GM-CSF) can serve as potent vaccine adjuvants. We used the 38C13 murine lymphoma system to evaluate the immune response to a combination of these two adjuvants. Immunization using antigen, CpG ODN, and soluble GM-CSF enhanced production of antigen-specific antibody and shifted production towards the IgG2a isotype, suggesting an enhanced TH1 response. This effect was most pronounced after repeat immunizations with CpG ODN and antigen/GM-CSF fusion protein. A single immunization with CpG ODN and antigen/GM-CSF fusion protein 3 days before tumor inoculation prevented tumor growth. CpG ODN enhanced the production of interleukin-12 by bone marrow-derived dendritic cells and increased expression of major histocompatibility complex class I and class II molecules, particularly when cells were pulsed with antigen/GM-CSF fusion protein. We conclude that the use of CpG ODN in combination with strategies involving GM-CSF enhances the immune response to antigen and shifts the response towards a TH1 response and that this approach deserves further evaluation in tumor immunization approaches and other conditions in which an antigen-specific TH1 response is desirable.
A NUMBER OF bacterial products, such as lipopolysaccaride, are known to stimulate mammalian immune responses. Recently, bacterial DNA itself has been reported to be one such molecule.1-9 One of the major differences between bacterial DNA, which has potent immunostimulatory effects, and vertebrate DNA, which does not, is that bacterial DNA contains a higher frequency of unmethylated CpG dinucleotides than does vertebrate DNA.1Select synthetic oligodeoxynucleotides containing unmethylated CpG motifs (CpG ODN) have immunologic effects similar to those seen with bacterial DNA. CpG ODN can induce activation of B cells, NK cells, and antigen-presenting cells (APCs), such as monocytes and macrophages.1-6 It can also enhance production of cytokines known to participate in the development of an active immune response, including tumor necrosis factor-α, interleukin-12 (IL-12), and IL-6.7-9
The molecular mechanisms responsible for CpG ODN-induced immunologic effects are still under investigation. Nevertheless, the immunostimulatory effects of CpG ODN may be useful therapeutically. We have demonstrated that CpG ODN can enhance antibody-dependent cellular cytotoxicity and improve the in vivo efficacy of monoclonal antibody therapy in a syngeneic murine lymphoma model.10 In addition, mice immunized with tumor antigen using CpG ODN as an adjuvant were protected from tumor challenge to a degree similar to that seen in mice immunized using complete Freund’s adjuvant (CFA).11 CpG ODN was as effective as CFA at enhancing production of anti-idiotype (Id) antibody after immunization and was more effective at inducing production of IgG2a anti-Id, suggesting a TH1 response. Evaluation of CpG ODN in other systems has yielded similar findings, including an enhanced cellular response.12-16
Soluble granulocyte-macrophage colony-stimulating factor (GM-CSF) has also been shown to function as an immune adjuvant in tumor antigen immunization.17-20 Other promising approaches based on this cytokine include immunization with antigen/GM-CSF fusion proteins,21 immunization using tumor cells transfected with GM-CSF,22 immunization using dendritic cells cultured in GM-CSF,23,24 and immunization using dendritic cells pulsed with antigen/GM-CSF fusion proteins.25 Although some of these studies have reported enhanced cellular immunity, most describe development of a humoral response. A reagent capable of enhancing the adjuvant effect of GM-CSF and directing it toward a TH1 response would be very useful in tumor antigen immunization and other strategies for which an antigen-specific TH1 response is desirable. We therefore assessed whether CpG ODN and immunization approaches using GM-CSF are synergistic and capable of inducing a pronounced antigen-specific TH1 response.
MATERIALS AND METHODS
Tumor model and tumor antigens.
The 38C13 murine B-cell lymphoma model has been used extensively in studies of antibody-based therapy and active immunization of lymphoma.20,26-29 The Id of the 38C13 surface IgM serves as a highly specific tumor-associated antigen.30 Id was obtained from the supernatant of a cell line that secretes 38C13 IgM as described31 and purified by protein A affinity chromatography. Purified Id was conjugated to keyhole limpet hemocyanin (KLH) using gluteraldehyde and used as the immunogen. Id-negative 38C13 (38C13 T1A) is a variant of 38C13 that contains cytoplasmic 38C13 heavy chain but produces no light chain and fails to express surface Id.27 The cell line that produces 38C13 Id/murine GM-CSF fusion protein (Id/GM-CSF) was kindly provided by Dr Ronald Levy (Stanford University, Palo Alto, CA). This cell line was cultured in a hollow fiber reactor (Unisyn Technologies, Hopkinton, MA), and fusion protein was obtained by protein A affinity chromatography. The fusion protein consists of the 38C13 Id heavy and light chain variable regions, the human IgG1 heavy and light chain constant regions, and murine GM-CSF sequences.21Bifunctional reactivity was confirmed by enzyme-linked immunosorbent assay (ELISA) before use. Plates were coated with anti-Id, serial dilutions of fusion protein were added, and the presence of bound GM-CSF moieties was assessed by probing with anti–GM-CSF antibodies. 38C13 Id/human GM-CSF fusion protein was obtained in a similar manner and used as a control.
Immunization.
Three phosphorothioate CpG ODNs were purchased commercially and produced under GMP conditions (Oligos Etc, Wilsonville, OR). Two of these ODN sequences (1758 and 1826) were immunostimulatory and had similar effects in all assays. CpG ODN 1758 was used unless stated otherwise. ODN 1758 had the sequence TCTCCCAGCGTGCGCCAT and ODN 1826 had the sequence TCCATGACGTTCCTGACGTT. ODN 1758 and ODN 1826 were unmethylated. Prior studies demonstrated that nonimmunostimulatory ODN has little adjuvant effect.11 Nevertheless, ODN 1812 was used as a control in select studies. ODN 1812 is identical to ODN 1758 but contains CpG motifs with methylated cytosines. No detectable endotoxin was present in the CpG ODN preparations by LAL assay. Murine GM-CSF for in vitro production of dendritic cells was purchased commercially (PeproTech, Rocky Hill, NJ). GM-CSF for in vivo studies was kindly supplied by Immunex (Seattle, WA).
Female C3H/HeN mice, obtained from Harlan-Sprague-Dawley, were housed in the University of Iowa Animal Care Unit and used at 6 to 9 weeks of age. Each mouse was immunized subcutaneously with indicated antigen and adjuvant in a total volume of 200 μL, using phosphate-buffered saline (PBS) as a vehicle.
ELISA determination of anti-Id levels.
Serum was obtained by retroorbital puncture from mice after inhalation anesthesia with metophane. Microtiter plates were coated with 5 μg/mL 38C13 IgM or irrelevant IgM overnight. IgM-coated plates were blocked with 5% milk, and serial dilutions of serum were added. Serum from naive mice to which a known concentration of monoclonal anti-Id was added served as a standard. Plates were washed, and alkaline phosphatase-labeled heavy chain-specific goat antimouse IgG, IgG1, or IgG2a (Southern Biotechnology Associates, Birmingham, AL) was added, followed by the colorimetric substrate p-nitrophenylphosphate. Plates were evaluated using a microplate reader. Test curves were compared with standard curves to determine the concentration of anti-Id. Values were considered valid only if the standard curves and the sample curves had the same shape. Reactivity of serum with a control, irrelevant murine IgM was evaluated in parallel and was negative in all assays, confirming that the immune response was not due to development of an isotypic response.
In vivo survival studies.
Three days after a single subcutaneous immunization using the indicated antigen and adjuvant, mice were inoculated intraperitoneally with 1,000 viable 38C13 cells. Cells were growing in log phase for at least 4 days before inoculation. Mice that developed tumor displayed inguinal and abdominal masses, ascites, and cachexia. All mice that developed tumor died. Survival was determined, and significance with respect to time to death was assessed using Cox regression analysis. For statistical purposes, survival of 60 days was assigned for mice that remained tumor free. All such mice remained tumor free indefinately and were monitored for a minimum of 100 days.
Dendritic cell production and stimulation.
Dendritic cells were obtained using a modification of the approach previously described.23,32 Briefly, bone marrow cells were obtained by flushing the femurs and tibias of naive 6- to 8-week-old C3H/HeN mice. Red blood cells were lysed and T cells were removed by complement-mediated lysis using a mixture of anti-CD3 (145.2C11), anti-CD4 (GK1.5), and anti-CD8 (53.6.7) antibodies. B cells were then removed by panning using a flask coated with anti-B220. The remaining cells were allowed to adhere overnight. Nonadherent cells were cultured in media supplemented with 1,000 U/mL GM-CSF and 1,000 U/mL murine IL-4 (muIL-4; PeproTech) at a concentration of 1.25 × 105cells/mL. Media was changed after 4 days, and dendritic cells were harvested 7 days after bone marrow harvest. Dendritic cell phenotype and morphology were confirmed by flow cytometric analysis and scanning electron microscopy. Dendritic cells were washed and counted, and 1 × 105 were cultured for 18 hours in a total volume of 200 μL with antigen at a final concentration of 100 μg/mL and CpG ODN at a final concentration of 5 μg/mL. Immunophenotyping was performed by flow cytometric analysis using fluorescein isothiocyanate (FITC)-labeled anti-class I (anti–H-2Kb), anti-class II (anti–I-Ak), anti–B7-1 (1G10), and anti–B7-2 (GL1; PharminGen, San Diego CA). For measurement of cytokine levels, all samples were run in quadruplicate. Supernatant was harvested and assayed by ELISA for the presence of IL-6 and IL-12, as described.9 33
RESULTS
CpG ODN further enhances development of an antibody response to Id-KLH immunization when using GM-CSF as an adjuvant.
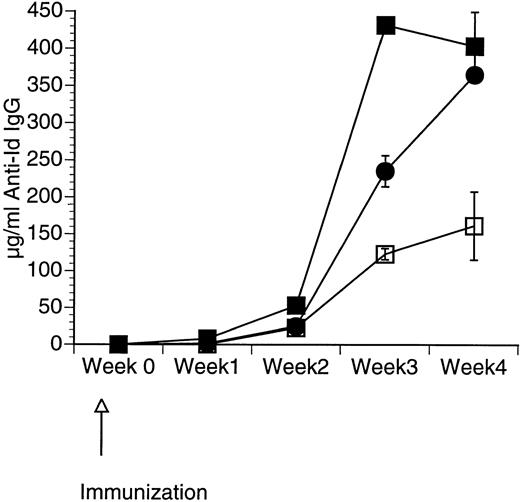
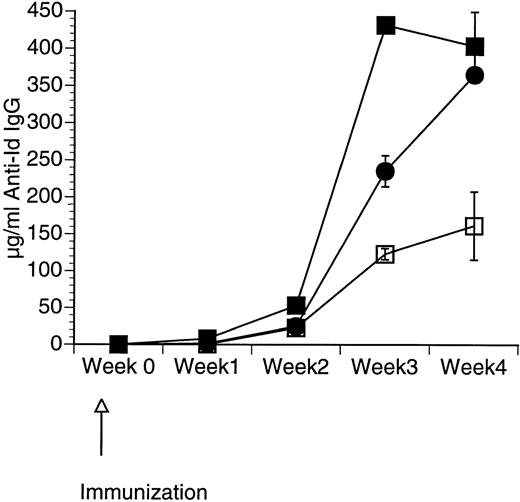
Although CpG ODN is known to induce production by APCs of a number of cytokines including GM-CSF,34 it is unclear whether the addition of CpG ODN to GM-CSF would enhance the immune response further. We therefore assessed the response in mice immunized with a single subcutaneous injection of 50 μg of Id-KLH in PBS mixed in aqueous solution with 50 μg of CpG ODN, 10 μg of GM-CSF, or a combination of CpG ODN and GM-CSF. Serum was obtained weekly and evaluated by ELISA for the presence of antigen-specific IgG (anti-Id IgG). As shown in Fig 1, mice immunized using both CpG ODN and GM-CSF developed the highest levels of anti-Id IgG. The effect of these two adjuvants appeared to be additive.
Production of anti-Id following immunization using a combination of CpG ODN and soluble GM-CSF. Mice were immunized with 50 μg of Id-KLH as a single subcutaneous dose mixed in aqueous solution with GM-CSF, CpG ODN, or both. Blood was obtained weekly, and serum was evaluated for the presence of anti-Id IgG by ELISA. Normal mouse serum supplemented with a known concentration of monoclonal anti-Id was used as a standard. Three mice were included in each group. (□) Id-KLH + GM-CSF; (•) Id-KLH + CpG ODN; (▪) Id-KLH + GM-CSF + CpG ODN.
Production of anti-Id following immunization using a combination of CpG ODN and soluble GM-CSF. Mice were immunized with 50 μg of Id-KLH as a single subcutaneous dose mixed in aqueous solution with GM-CSF, CpG ODN, or both. Blood was obtained weekly, and serum was evaluated for the presence of anti-Id IgG by ELISA. Normal mouse serum supplemented with a known concentration of monoclonal anti-Id was used as a standard. Three mice were included in each group. (□) Id-KLH + GM-CSF; (•) Id-KLH + CpG ODN; (▪) Id-KLH + GM-CSF + CpG ODN.
CpG ODN enhances production of anti-Id antibodies after immunization with Id/GM-CSF fusion protein.
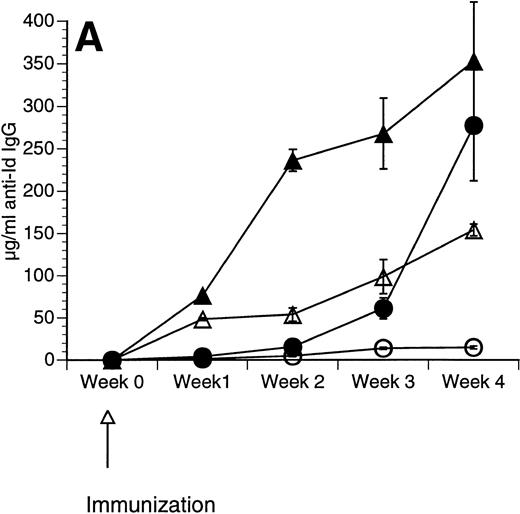
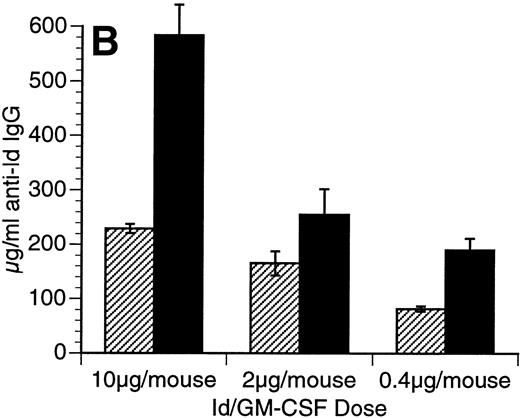
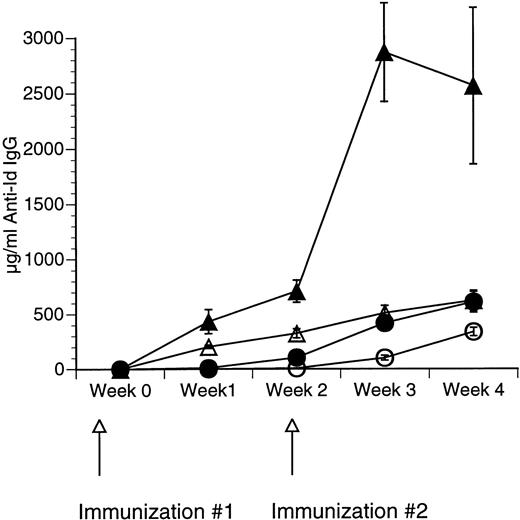
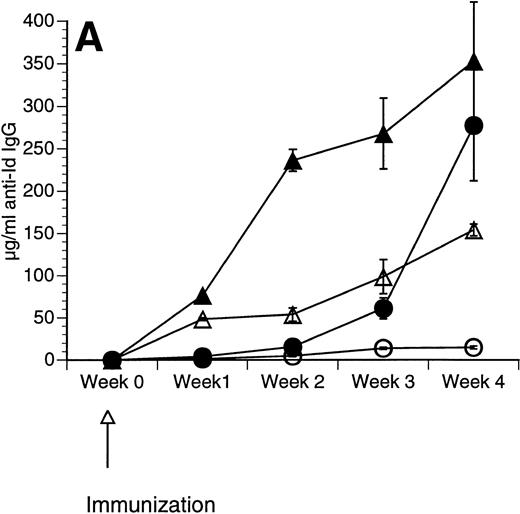
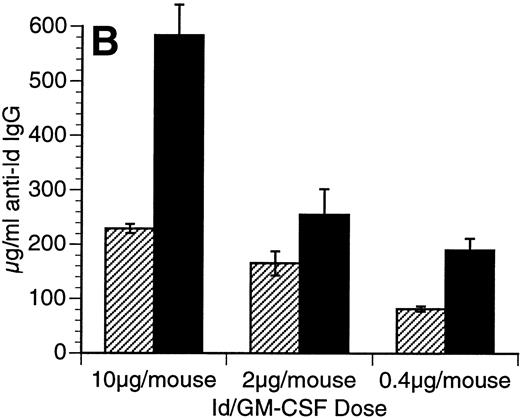
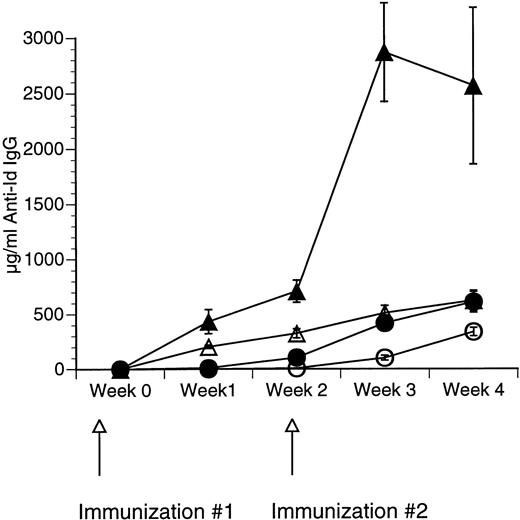
The Id/GM-CSF fusion protein consisting of the 38C13 variable regions, human IgG constant regions, and murine GM-CSF (Id/GM-CSF) has been shown to be an excellent immunogen.21 We evaluated whether CpG ODN can further enhance the specific antibody response induced by Id/GM-CSF. Mice were immunized with Id-KLH or Id/GM-CSF with and without CpG ODN as an adjuvant. Serum was obtained weekly and anti-Id IgG levels were determined. No toxicity was observed in any mice. As shown in Fig 2, CpG ODN enhanced production of anti-Id antibodies in response to Id/GM-CSF. This occurred at a variety of doses of Id/GM-CSF. Anti-Id levels induced by immunization with Id/GM-CSF and control ODN were indistinguishable from those induced by immunization Id/GM-CSF alone (data not shown). In a separate experiment, mice were immunized on day 0 and boosted on day 14 with the same antigen and adjuvant. The combination of Id/GM-CSF and CpG ODN induced remarkably high levels of anti-Id IgG after two immunizations (Fig 3). Serum obtained 1 week after the final immunization contained greater than 2.5 mg/mL anti-Id IgG. A fusion protein consisting of 38C13 Id and human GM-CSF (Id/human GM-CSF) was included as a control, because human GM-CSF is not active in the murine system. Id/human GM-CSF was identical to Id/GM-CSF, except that the murine GM-CSF sequences were replaced with human GM-CSF sequences. Levels of anti-Id produced after immunization using Id/human GM-CSF with or without CpG ODN were significantly lower than those seen after Id/GM-CSF and similar to those seen with Id-KLH (data not shown), demonstrating that biologically active GM-CSF was important for the observed effects.
Immunization using a combination of Id/GM-CSF fusion protein and CpG ODN enhances production of antigen-specific IgG. Mice were immunized with 50 μg of Id/GM-CSF as a single subcutaneous dose with or without CpG ODN. Blood was obtained weekly, and serum was evaluated for the presence of anti-Id IgG by ELISA. Normal mouse serum supplemented with a known concentration of monoclonal anti-Id was used as a standard. Three mice were included in each group. (A) Anti-Id response was measured at various time points after immunization. (○) Id-KLH; (•) Id-KLH; (▵) Id/GM-CSF; (▴) Id/GM-CSF + CpG ODN. (B) Anti-Id response was measured 4 weeks after immunization with various doses of Id/GM-CSF. (▨) Control CpG ODN; (▪) CpG ODN.
Immunization using a combination of Id/GM-CSF fusion protein and CpG ODN enhances production of antigen-specific IgG. Mice were immunized with 50 μg of Id/GM-CSF as a single subcutaneous dose with or without CpG ODN. Blood was obtained weekly, and serum was evaluated for the presence of anti-Id IgG by ELISA. Normal mouse serum supplemented with a known concentration of monoclonal anti-Id was used as a standard. Three mice were included in each group. (A) Anti-Id response was measured at various time points after immunization. (○) Id-KLH; (•) Id-KLH; (▵) Id/GM-CSF; (▴) Id/GM-CSF + CpG ODN. (B) Anti-Id response was measured 4 weeks after immunization with various doses of Id/GM-CSF. (▨) Control CpG ODN; (▪) CpG ODN.
Repeated immunizations with a combination of Id/GM-CSF fusion protein and CpG ODN induce high levels of antigen-specific IgG. Mice were immunized with 50 μg of Id/GM-CSF as a subcutaneous dose with or without CpG ODN on week 0 and again on week 2. Blood was obtained weekly, and serum was evaluated for the presence of anti-Id IgG by ELISA. Normal mouse serum supplemented with a known concentration of monoclonal anti-Id was used as a standard. Three mice were included in each group. (○) Id-KLH; (•) Id-KLH + CpG ODN; (▵) Id/GM-CSF; (▴) Id/GM-CSF + CpG ODN.
Repeated immunizations with a combination of Id/GM-CSF fusion protein and CpG ODN induce high levels of antigen-specific IgG. Mice were immunized with 50 μg of Id/GM-CSF as a subcutaneous dose with or without CpG ODN on week 0 and again on week 2. Blood was obtained weekly, and serum was evaluated for the presence of anti-Id IgG by ELISA. Normal mouse serum supplemented with a known concentration of monoclonal anti-Id was used as a standard. Three mice were included in each group. (○) Id-KLH; (•) Id-KLH + CpG ODN; (▵) Id/GM-CSF; (▴) Id/GM-CSF + CpG ODN.
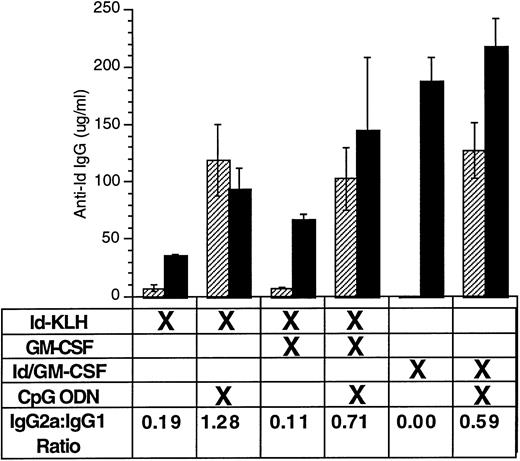
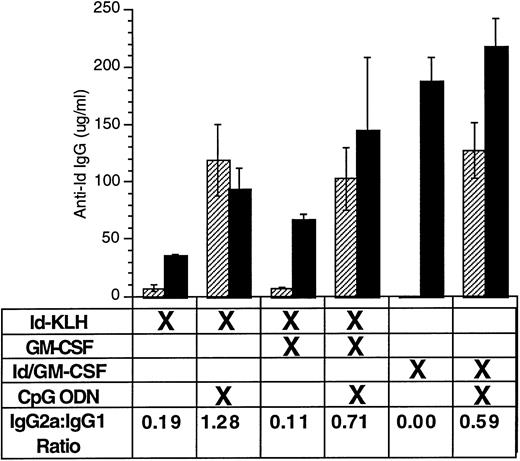
CpG ODN enhances production of antigen-specific antibody of IgG2a isotype.
Enhanced production of IgG1 reflects a TH2 response, whereas predominant IgG2a production indicates a TH1 response.35Moreover, murine IgG2a is more effective than murine IgG1 at mediating antibody-dependent cellular cytotoxicity, and monoclonal IgG2a works better than monoclonal IgG1 with the identical variable region at preventing tumor growth.36 We therefore performed isotype analysis on anti-Id IgG and assessed for the presence of anti-Id IgG1 and IgG2a after immunization (Fig 4). Immunization included various combinations of Id-KLH or Id/GM-CSF with GM-CSF or CpG ODN. Serum was sampled 4 weeks after a single immunization. CpG ODN induced enhanced production of anti-Id IgG2a compared with that seen under the corresponding conditions without CpG ODN. Similar IgG1/IgG2a ratios were seen at other time points (data not shown).
CpG ODN enhances production of antigen-specific antibody of IgG2a isotype. Mice were immunized with a single dose using various combinations of Id-KLH, GM-CSF, Id/GM-CSF fusion protein, and CpG ODN. Serum was obtained 4 weeks after a single immunization. Anti-Id IgG1 and IgG2a was determined by ELISA. Three mice were included in each group. (▨) Anti-Id IgG2a; (▪) anti-Id IgG1.
CpG ODN enhances production of antigen-specific antibody of IgG2a isotype. Mice were immunized with a single dose using various combinations of Id-KLH, GM-CSF, Id/GM-CSF fusion protein, and CpG ODN. Serum was obtained 4 weeks after a single immunization. Anti-Id IgG1 and IgG2a was determined by ELISA. Three mice were included in each group. (▨) Anti-Id IgG2a; (▪) anti-Id IgG1.
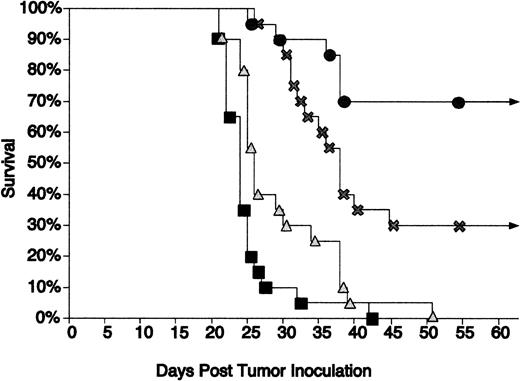
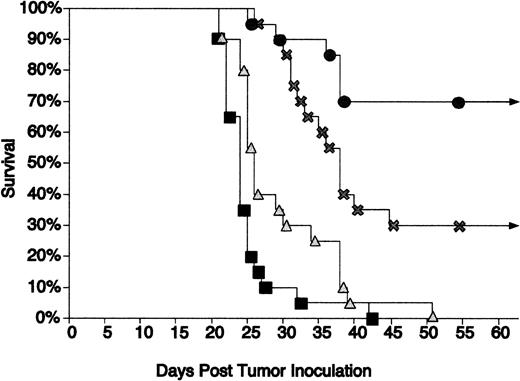
Immunization using CpG ODN as an adjuvant along with Id/GM-CSF fusion protein led to further protection of mice from tumor growth.
Tao and Levy21 found that the Id/GM-CSF fusion protein was superior to Id-KLH at inhibiting growth of the 38C13 tumor. Our previous investigations demonstrated that the addition of CpG ODN to Id-KLH enhanced protection in this same model.11 We therefore evaluated whether CpG ODN can also serve as an effective adjuvant with Id/GM-CSF immunization. Mice were challenged with tumor 3 days after a single immunization with Id/GM-CSF with or without CpG ODN. Immunization using this schedule was only minimally effective in our prior studies with Id-KLH (unpublished data). CpG ODN 1758 and CpG ODN 1826 were equally effective at prolonging survival when used alone or in combination with Id/GM-CSF. The data shown in Fig 5 represent the combined results of mice treated with CpG ODN 1758 and CpG ODN 1826. All unimmunized mice and mice treated with CpG ODN without antigen developed tumor and died within 50 days. Thirty percent of mice immunized with Id/GM-CSF alone remained disease free, whereas 70% of the group immunized with Id/GM-CSF and CpG ODN remained disease free. Mice immunized with Id/GM-CSF and CpG ODN had survival that was statistically superior to that seen with no immunization or treatment with CpG ODN alone (P < .001). The difference between those immunized with Id/GM-CSF alone versus those immunized with CpG ODN plus Id/GM-CSF approached statistical significance (P = .072). To assess whether the effect of immunization was specific, mice were immunized as described above and challenged with either wild-type 38C13 cells or Id-negative 38C13. Mice challenged with WT 38C13 were protected from tumor growth, whereas no protection was seen for those challenged with Id-negative 38C13 (Table 1). This confirmed that the protection was Id specific.
CpG ODN enhances the protective effect of Id/GM-CSF. Mice were immunized with a single injection of Id/GM-CSF and/or CpG ODN and challenged with tumor 3 days later. Survival was followed for 100 days. All mice that were alive after 51 days remained tumor-free for the entire observation period. Twenty mice were included in each group. (▪) No immunization; (▴) CpG ODN; (×) Id/GM-CSF; (•) Id/GM-CSF + CpG ODN.
CpG ODN enhances the protective effect of Id/GM-CSF. Mice were immunized with a single injection of Id/GM-CSF and/or CpG ODN and challenged with tumor 3 days later. Survival was followed for 100 days. All mice that were alive after 51 days remained tumor-free for the entire observation period. Twenty mice were included in each group. (▪) No immunization; (▴) CpG ODN; (×) Id/GM-CSF; (•) Id/GM-CSF + CpG ODN.
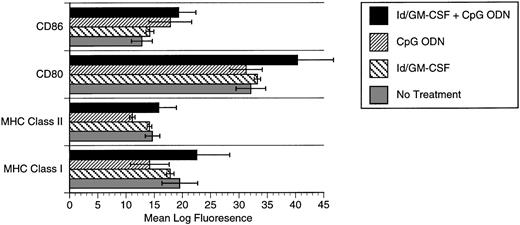
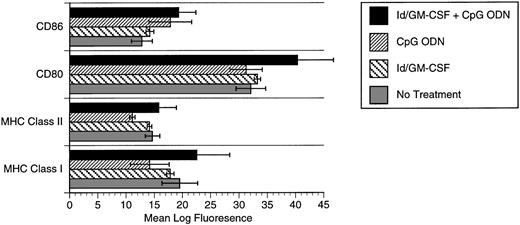
CpG ODN effects on dendritic cell phenotype.
The synergistic effects of CpG ODN and GM-CSF suggested the possibility that these agents together may enhance expression of costimulatory molecules or major histocompatibility complex (MHC) by APCs. We therefore evaluated expression of these molecules by bone marrow-derived dendritic cells. Flow cytometric analysis of dendritic cells pulsed with Id/GM-CSF and/or CpG ODN demonstrated a modest increase in expression of class I MHC in response to the combination of Id/GM-CSF and CpG ODN. Baseline expression of CD80 and CD86 expression by dendritic cells was high and was increased only slightly by CpG ODN (CD86) or CpG ODN plus Id/GM-CSF (CD80; Fig 6).
Expression of MHC class I, MHC class II, CD80, and CD86 after pulsing of bone marrow-derived dendritic cells with Id/GM-CSF and/or CpG ODN. (▪) Id/GM-CSF + CpG ODN; (▨) CpG ODN; () Id/GM-CSF; (▧) no treatment.
Expression of MHC class I, MHC class II, CD80, and CD86 after pulsing of bone marrow-derived dendritic cells with Id/GM-CSF and/or CpG ODN. (▪) Id/GM-CSF + CpG ODN; (▨) CpG ODN; () Id/GM-CSF; (▧) no treatment.
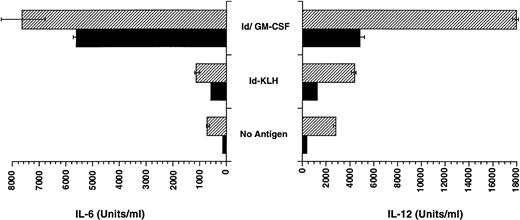
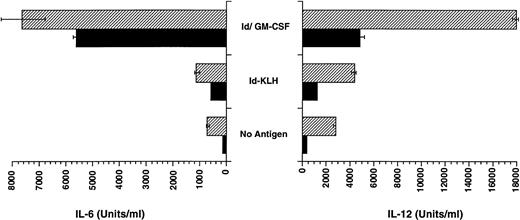
CpG ODN enhances production of IL-12 by dendritic cells pulsed with Id/GM-CSF.
The enhanced TH1 response to antigen could be explained by the ability of CpG ODN to enhance production of IL-12 by APCs such as dendritic cells. We therefore assessed whether CpG ODN is capable of enhancing the production of IL-12 by bone marrow-derived dendritic cells that were pulsed with antigen, including Id/GM-CSF. As shown in Fig 7, pulsing of dendritic cells with CpG ODN increased production of IL-12, particularly when cells were also pulsed with Id/GM-CSF. IL-6 production by dendritic cells was also increased by the addition CpG ODN to Id/GM-CSF, although the effect was less pronounced than for IL-12. The impact of GM-CSF alone on dendritic cell production of cytokines was not studied, because these cells were generated using GM-CSF.
Production of IL-6 and IL-12 by dendritic cells pulsed with Id-KLH or Id/GM-CSF. Bone marrow-derived dendritic cells were pulsed with antigen with and without CpG ODN for 18 hours. Production of IL-12 and IL-6 was determined by ELISA. (▨) CpG ODN; (▪) no CpG ODN.
Production of IL-6 and IL-12 by dendritic cells pulsed with Id-KLH or Id/GM-CSF. Bone marrow-derived dendritic cells were pulsed with antigen with and without CpG ODN for 18 hours. Production of IL-12 and IL-6 was determined by ELISA. (▨) CpG ODN; (▪) no CpG ODN.
DISCUSSION
GM-CSF and CpG ODN both enhance development of an active immune response, yet may play very different roles in that response. Whereas the complex effects of GM-CSF on APCs are still being defined, it is clear that GM-CSF can stimulate granulocyte and monocyte production and activate various aspects of APC function. There is some evidence that much of the adjuvant effect is related to enhanced phagocytosis or pinocytic uptake of antigen as opposed to enhanced presentation of antigen.37 In broad terms, GM-CSF can be thought of as a cytokine that attracts APCs to the site and induces antigen uptake by those APCs.
Even less is known about the mechanisms responsible for the adjuvant effects of CpG ODN. Some hints can be obtained from analysis of the immune activation seen with bacterial DNA, because this is similar to that seen with CpG ODN. Bacterial DNA plays a role in the response to intracellular microbial infections and appears to enhance the response to microbial antigen that is already present within the APC.3,8 Bacterial DNA can induce a cellular, ie, a TH1, response, which is likely beneficial when the invading organisms are intracellular. Thus, bacterial DNA, and CpG ODN, appear to enhance the immune response by upregulating costimulatory molecules and MHC12 and inducing production of TH1-like cytokines that, in turn, enhance the function of effector cells, including T cells, B cells, and NK cells.9 15
The combination of GM-CSF and CpG ODN could therefore stimulate different steps in the induction of the immune response. The primary affect of GM-CSF could be in increasing antigen uptake, whereas the primary affect of CpG ODN could be on enhancing the downstream response to antigen, including production of cytokines involved in effector cell activation. In addition, CpG ODN contributes by synergistically promoting B-cell activation through the antigen receptor and so preferentially activating antigen-specific B cells.1
The data presented above indicate immunization strategies involving the combination of GM-CSF and CpG ODN are particularly effective. CpG ODN and soluble GM-CSF were additive in their ability to induce anti-Id IgG after immunization with Id-KLH. Murine GM-CSF has a short half-life,38 which may explain the lack of a dramatic adjuvant effect. APCs may not have had adequate exposure to GM-CSF. CpG ODN was particularly effective at increasing production of anti-Id IgG after immunization with an Id/GM-CSF fusion protein. Prior studies by Tao and Levy21 demonstrated that this fusion protein has enhanced GM-CSF activity compared with native GM-CSF, most likely due to the bivalent nature of the construct. In addition, this fusion protein may have been more effective at delivering both the GM-CSF and antigen to the APC. In our studies, remarkable levels of anti-Id IgG were achieved after repeated immunization with Id/GM-CSF and CpG ODN. Importantly, CpG ODN shifted the response to a IgG2a under all conditions studied, including immunization with soluble GM-CSF and the Id/GM-CSF fusion protein, suggesting an enhanced TH1 response.
Immunization using this approach translated into protection from tumor growth only 3 days after immunization with Id/GM-CSF and CpG ODN. This is the most effective protection reported to date in this extensively studied model. As with any model system, we need to consider the specifics of the model when interpreting results. Passive administration of anti-Id monoclonal antibody has antitumor activity against 38C13.26,28,36 Although monoclonal antibodies of the IgG2a isotype are the most effective therapeutically, those of the IgG1 isotype also demonstrate antitumor activity. This may explain the significant antitumor activity of Id/GM-CSF alone, despite its relatively limited ability to induce a TH1 response. Other investigations suggest cellular immunity, which is enhanced by CpG ODN,14,15 also plays a role in the antitumor response.20 For technical reasons we have been unable to demonstrate unequivocally that a cellular response was playing a role in the observed antitumor activity. This is currently being explored in other systems. Nevertheless, it is likely that the enhanced antitumor activity of the combination of Id/GM-CSF and CpG ODN documented above was related to enhancement of both the humoral and cellular response.
In summary, GM-CSF can enhance antigen uptake by APC and is known to be effective as an immune adjuvant. CpG ODN is an exciting new immune adjuvant that appears to function by different mechanisms, including activating immune effector cell function. In the studies outlined above, we demonstrate that the combination of immunization strategies involving GM-CSF and CpG ODN are effective at inducing an enhanced TH1 type response and protecting from tumor growth. Further investigation into the precise mechanisms involved in the response to these two agents, both separately and together, is required before the true promise of this approach can be elucidated.
ACKNOWLEDGMENT
The authors thank Ronald Levy, MD (Stanford University, Stanford, CA) for supplying the cell lines that produce the Id/GM-CSF fusion protein; Elaine Thomas, PhD (Immunex) for murine GM-CSF; Justin Fishbaugh (University of Iowa Flow Cytometry Facility); Charles Davis, PhD, for statistical consultation; Anna M. Acosta and Laurie Love-Homan for expert technical assistance; and Timothy Ratliff, PhD, and Donald MacFarlane, MD, for helpful suggestions.
Supported by the Department of Veteran’s Affairs Medical Research Service and the University of Iowa Cancer Center.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to George J. Weiner, MD, Department of Internal Medicine, University of Iowa, C32K GH, 200 Hawkins Dr, Iowa City, IA 52242.