Abstract
The abnormal adherence of sickle red blood cells (SS RBC) to endothelial cells has been thought to contribute to vascular occlusion, a major cause of morbidity in sickle cell disease (SCD). We determined whether the interaction of SS RBC with cultured endothelial cells induced cellular oxidant stress that would culminate in expression of cell adhesion molecules (CAMs) involved in the adhesion and diapedesis of monocytes and the adherence of SS reticulocytes. We showed that the interaction of SS RBC at 2% concentration in the presence of multimers of von Willebrand factor (vWf), derived from endothelial cell-derived conditioned medium (E-CM) with cultured human umbilical vein endothelial cells (HUVEC), resulted in a fivefold increased formation of thiobarbituric acid-reactive substances (TBARS) and activation of the transcription factor NF-kB, both indicators of cellular oxidant stress. Normal RBC show none of these phenomena. The oxidant stress-induced signaling resulted in an increased surface expression of a subset of CAMs, ICAM-1, E-selectin, and VCAM-1 in HUVEC. The addition of oxygen radical scavenger enzymes (catalase, superoxide dismutase) and antioxidant (probucol) inhibited these events. Additionally, preincubation of HUVEC with a synthetic peptide Arg-Gly-Asp (RGD) that prevents vWf-mediated adhesion of SS RBC reduced the surface expression of VCAM-1 and NF-kB activation. Furthermore, SS RBC-induced oxidant stress resulted in a twofold increase in the transendothelial migration of both monocyte-like HL-60 cells and human peripheral blood monocytes, and approximately a sixfold increase in platelet-endothelial cell adhesion molecule-1 (PECAM-1) phosphorylation, each of which was blocked by protein kinase C inhibitor and antioxidants. These results suggest that the adherence/contact of SS RBC to endothelial cells in large vessel can generate enhanced oxidant stress leading to increased adhesion and diapedesis of monocytes, as well as heightened adherence of SS reticulocytes, indicating that injury/activation of endothelium can contribute to vaso-occlusion in SCD.
ENDOTHELIAL CELLS forming the lining of the blood vessels throughout the vasculature come in continuous contact with circulating blood cells and plasma components. Under normal physiological conditions, blood cells are, at most, loosely attached to endothelial cells as in the marginal pools of polymorphonuclear leukocytes (PMN). However, under other conditions, blood cells do adhere to localized vascular sites as part of hemostasis. For example, PMN adhere to endothelium before they migrate to sites of extravascular infection or acute inflammation, and platelets accumulate at injured sites in the vessels. These are normal processes required for host defense and hemostasis, respectively.1 However, sickle red blood cells (SS RBC) from patients with sickle cell anemia exhibit increased adherence to cultured endothelial cells under both static and flow conditions2-5 and the extent of adhesion in vitro appears to parallel the clinical severity of vaso-occlusive events in sickle cell disease (SCD).6 Such abnormal adhesion of SS RBC, when compared with the behavior of normal RBC, is thought to be important in the mechanism of vaso-occlusion, a hallmark of sickle cell crises.6-8 In addition to adhesion events, the interaction of SS RBC with endothelium may cause endothelial injury, culminating in the dislodgment of cells from vessel walls9 or the altering of endothelium characteristics, such as vascular tone.10 It has been shown that the genes coding for vasoconstrictor endothelin-1 (ET-1)10 are induced as a result of interaction of SS RBC with endothelium, which may affect vascular tone and thus affect blood flow.11
Because SS RBC generate excessive amounts of reactive oxygen metabolites due to the presence of unstable hemoglobin S and the spontaneous autoxidation of iron in heme,12-15 we hypothesized that the adherence of SS RBC, mediated by multimers of von Willebrand factor (vWf), to cultured endothelial cells might induce cellular oxidant stress, ie, generation of reactive oxygen intermediates capable of activating transcription factor, NF-kB.16 Numerous studies16,17 have shown that diverse agents, eg, inflammatory cytokines, oxidative stress (hydrogen peroxide), and endotoxins activate NF-kB by distinct intracellular pathways that involve reactive oxygen species (ROS) as a common messenger. The activation of NF-kB is capable of altering gene expression of several genes including those for the cell-adhesion molecules (ICAM-1, E-selectin,and VCAM-1).16,18 As a consequence of the increased expression of cell-adhesion molecules, the adherence of PMN and monocytes to vascular endothelium might be heightened. Moreover, VCAM-1 acts as a receptor for the α4β1 integrin present on SS reticulocytes19 20 thus promoting the additional adherence of SS RBC. As a consequence of the additional adherence of SS RBC, PMN, and monocytes, blood flow could be further obstructed, which may contribute to the recurrent vaso-occlusive events in SCD.
To test our hypothesis, we examined whether the adhesion of SS RBC to cultured endothelial cells generated ROS, and if so, whether it caused activation of transcription factor NF-kB. As a consequence of activation of NF-kB, we expect to find increased expression of VCAM-1, which would allow adherence of sickle reticulocytes via the α4β1 integrin. The activation of NF-kB would also lead to increased expression of CAMs, which participate in the adhesion of monocytes to endothelial cells. Thus, adhesion of both reticulocytes and monocytes to the vascular endothelium could cause further obstruction and participate in vaso-occlusive phenomenon.
Our results show that the interaction of SS RBC in the presence of multimers of vWf, derived from endothelial cell-derived conditioned medium (E-CM) with cultured human umbilical vein endothelial cells (HUVEC), indeed results in an increased formation of thiobarbituric acid-reactive substances (TBARS) and the activation of the transcription factor NF-kB, both indices of cellular oxidant stress.16,17,21 Furthermore, we show for the first time that the oxidant stress induced by the adhesion of SS RBC causes a several-fold increase in the phosphorylation of PECAM-1, a multifunctional cell-junction molecule,22-27 and importantly, a concomitant increase in the migration of both monocyte-like HL-60 cells and human peripheral blood monocytes (PBM) across the endothelial cell monolayer. In SCD, the accumulation of monocytes in large vessels, eg, cerebral vasculature in response to the oxidant stress generated by the adhesion of SS RBC, may contribute to the stroke syndrome.
MATERIALS AND METHODS
Materials.
32P[carrier free] was obtained from ICN Biomedical Inc (Irvine, CA); [γ-32P]ATP (adenosine triphosphate) was obtained from Amersham Corp (Arlington Heights, IL). 1α, 25-dihydroxyvitamin D3, Calyculin A, and SQ-22536 were obtained from Biomol Research Laboratories (Plymouth Meeting, PA); GF-109203X was obtained from Calbiochem-Novabiochem International (San Diego, CA). Acetylated low-density lipoprotein (LDL) labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate was obtained from Biomedical Technologies, Inc (Stoughton, MA). Monoclonal antibodies (MoAb) to ICAM-1, ICAM-2, E-selectin, VCAM-1, and PECAM-1 were obtained from Immunotech Inc (Westbrook, MN). A MoAb to bovine PECAM-1, a purified ascites fluid from clones XVD2, was prepared as described.22 All other reagents were obtained from Sigma (St Louis, MO) unless otherwise specified.
Isolation of RBC from whole blood.
Fresh heparinized or citrated blood was obtained from adult sickle cell anemia patients (homozygous SS) and sickle cell trait subjects (AS) in the adult clinic of the Comprehensive Sickle Cell Center, LAC-USC Hospital (Los Angeles, CA); or from normal (AA) healthy volunteers (ages 25 to 37 years) after obtaining informed consent according to a protocol approved by the Institution Review Committee. Sickle cell patients who had received blood transfusions within 3 months were excluded from these studies. Whole blood (8 to 10 mL) was centrifuged at 450g for 20 minutes. Plasma and buffy coat were removed by aspiration. The pellet was suspended in 3 volumes of phosphate-buffered saline (PBS), pH 7.4, and passed through a column (3.5 cm in a 30 mL syringe) of cellulose-microcrystalline cellulose (wt/wt 1:1) as described.28 The eluted fraction was passed twice through a new column of cellulose-microcrystalline cellulose (1:1 wt/wt) to remove platelets and white blood cells (WBC). The eluate was centrifuged at 450g for 10 minutes. The pellet of RBC was resuspended in 2 mL of PBS for differential count by the MINOS-STX automatic cell analyzer (Roche Diagnostic Systems, Nutley, NJ). By this method, we routinely obtained RBC preparations that were less than 1% contaminated by platelets and WBC.
Isolation of human PBM.
Blood (80 to 100 mL) was collected by venipuncture from adult donors of both sexes. To the whole blood collected into a heparinized 60 mL plastic syringe was added Dextran (13.5 mL of 5% Dextran) followed by gravity sedimentation for 40 minutes as described.29 The supernatant containing leukocytes were fractionated into monocytes by gradient centrifugation on Isolymph. The monocyte fraction was labeled with MO2-FITC antibody (Coulter Diagnostics, Hialeah, FL) followed by FACscan analysis as previously described.29 Cells obtained by this procedure had purity in the range of 70% to 85% and a yield of 50% to 75%.
Cell cultures.
HUVEC were harvested from umbilical cord veins by collagenase digestion as previously described.29 The cells were evaluated for cobblestone morphology, the binding of vWF-antibody, and the uptake of acetylated LDL labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate. Cells were used from passages two through six. The human promyelocytic cell line HL-60 (American Type Culture Collection, Rockville, MD) was cultured in RPMI-1640 containing 20% heat-inactivated fetal calf serum (Gemini Bioproducts, Calabasas, CA). HL-60 cells differentiate to a monocyte-like phenotype by incubation in the presence of 1 × 10-7 mol/L 1α-25-dihydroxyvitamin D3 after 3 to 4 days under culture conditions, as previously described.30 31
Preparation of E-CM.
To the HUVEC (2 × 106 cells) grown to confluence, 3 mL of RPMI-1640 (devoid of fetal bovine serum), 1 mmol/L CaCl2, and 10 μmol/L A23187 calcium ionophore were added followed by incubation for 2 hours at room temperature.32 The supernatant was collected and concentrated in dialysis tubing. The concentrated sample was subjected to an agarose (2.5%) gel electrophoresis in a running buffer containing 25 mmol/L Tris buffer, pH 8.3, 0.192 mol/L glycine, and 0.1% sodium dodecyl sulfate (SDS). The molecular sizes of vWf multimers were estimated by comparison with Jack bean urease as a standard (Mr. 240 and 480 kD, Sigma) after staining the gel with 0.25% Coomassie brilliant blue dye. The analysis of conditioned medium showed triplet bands of high-molecular weight polypeptides to be estimated in the range of 400 to 950 kD that cross-reacted with antibody to vWf as has been previously observed.33 We used E-CM at 100 μg/mL for the studies described here because this concentration showed optimal adherence of SS RBC to HUVEC (Table1).
Measurement of lipid peroxides as thiobarbituric acid-reactive substances (TBARS) in endothelial cells.
Confluent HUVEC (2.5 × 106 cells in 100 mm tissue culture dishes) were washed with RPMI-1640 and incubated at 37°C in the presence and absence of RBC (hematocrit 2%) with E-CM (100 μg/mL) in 3 mL serum-free RPMI-1640 for 4 hours. At the end of incubation, nonadherent RBC were aspirated and plates washed three times with 3 mL of PBS. Endothelial cells were harvested from the culture dish by treatment with a 0.25% trypsin-0.02% EDTA solution. The contents were centrifuged at 500g for 5 minutes at 4°C. Cell pellets were washed three times with TBS (10 mmol/L Tris-buffer, pH 7.4, containing 140 mmol/L NaCl). The content of lipid peroxides formed was determined by the reactivity of malondialdehyde (MDA), an end product of lipid peroxidation, with thiobarbituric acid (TBA) as described.34 Briefly, 0.8 mL of water was added to the pellet, followed by the addition of 0.2 mL of 8.1% sodium dodecyl sulfate (SDS), 1.5 mL of 20% acetic acid, and 1.5 mL of a 0.8% aqueous solution of TBA. The mixture was heated for 60 minutes in a boiling water bath. After cooling, 1 mL of water and 5 mL of a mixture of n-butanol-pyridine (15:1 vol/vol) were added. Contents were vortexed, then centrifuged at 3,000g for 10 minutes. The upper (organic) layer was removed for measurement of absorbency at 532 nm (Hitachi U-3110 spectrophotometer; Hitachi, San Jose, CA). The TBARS values were calculated using the molar extinction coefficient of the MDA-TBA complex of 1.5 × 106 cm-1M-1 as previously described.35
Static assay for the adherence of RBC to cultured HUVEC.
RBC in PBS (2 mL at Hct of 10%) were incubated with51Cr-sodium chromate (0.5 mCi) at room temperature for 60 minutes. Labeled RBC were washed three times with 10 vol of PBS and used at a hematocrit of 2% in PBS. HUVEC grown to confluence in 6-well plates were washed twice with PBS (3 mL) followed by the addition of51Cr-labeled RBC (Hct 2%) and PBS to a final volume of 1 mL. The contents were incubated for 45 minutes at room temperature. Nonadherent RBC were removed by aspiration and monolayers were washed three times with 1 mL of PBS containing 0.5% bovine serum albumin (BSA). The radioactivity in the cell monolayer-containing adherent RBC was determined and the percentage of RBC adherent was calculated.2 36
Electrophoretic mobility shift assay (EMSA) for transcription factor NF-kB activity.
Confluent HUVEC (6 × 106 cells) were incubated at 37°C with RBC (2% hematocrit) from either sickle cell anemia (SS) or normal (AA) subjects in 3 mL serum-free RPMI-1640 containing E-CM (100 μg/mL) for time periods ranging from 30 to 240 minutes. At the end of the incubation period, the medium was aspirated and cells were washed twice with 3 mL ice-cold PBS. Cells were scraped in 1 mL PBS and nuclear extracts prepared as described.37 Briefly, cells were suspended in 400 μL of buffer A (10 mmol/L Hepes, pH 7.9, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 1 mmol/L dithiothreitol (DTT), 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF), 0.3 μg/mL leupeptin, and 0.7 μg/mL pepstatin A) and incubated on ice for 10 minutes. Cells were pelleted and resuspended in 50 μL of buffer B (20 mmol/L Hepes, pH 7.9, 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L Na-EDTA, 1 mmol/L DTT, 0.5 mmol/L PMSF; 0.3 μg/mL leupeptin, and 0.7 μg/mL pepstatin A). After a 20-minute incubation at 4°C, the homogenate was centrifuged at 8,000g for 20 minutes. The supernatant, containing the nuclear proteins, was stored at −80°C, and used for assay of NF-kB activity within 10 days of storage. Five nanograms of double-stranded oligonucleotide containing a tandem repeat of the consensus sequence of the NF-kB DNA binding site, -GGGGACTTTCC-with the following sequence:
was end labeled with 100 μCi [γ-32P] ATP using T4 polynucleotide kinase as suggested in the manufacturer’s kit (GIBCO-BRL, Gaithersburg, MD). DNA–protein-binding reactions were performed by preincubating 3 to 5 μg nuclear extract protein on ice for 15 minutes in a total volume of 25 μL containing 10 mmol/L Tris, pH 7.6, 50 mmol/L NaCl , 1 mmol/L DTT, 0.02 μmol/L ATP, 5 μg BSA, and 10% glycerol in the presence and absence of excess specific-competitor oligonucleotide. This was followed by the addition of the double-stranded 32P-labeled oligonucleotide (1 × 105 cpm) and a second incubation at room temperature for 20 minutes. The samples were subjected to electrophoresis on 6% nondenaturing polyacrylamide gels as previously described37 using 0.25 × TBE running buffer containing Tris (25 mmol/L; pH 8.0), borate (22.5 mmol/L), and EDTA (0.025 mmol/L) at 150 V for 2 to 3 hours. Gels were then dried and exposed to Kodak X-Omat AR film (Eastman Kodak, Rochester, NY) with intensifying screens at −80°C.
Enzyme-linked immunosorbent assay (ELISA) for surface expression of CAMs.
HUVEC were grown to confluence in 24-well tissue culture plates, rinsed with RPMI-1640, and incubated with serum-free RPMI-1640 in the presence or absence of RBC (hematocrit 2%) with E-CM at 37°C. At the end of the indicated incubation periods, ranging from 2 to 24 hours, the medium was aspirated and the wells washed twice with 1 mL of PBS. To each well, 500 μL of 2.5% paraformaldehyde was added to fix the cells. Cell-surface expression of ICAM-1, E-selectin, and VCAM-1 in HUVEC was assayed by separate incubations with MoAb to ICAM-1, E-selectin, and VCAM-1. Incubations were performed at room temperature for 120 minutes at a saturating concentration of antibody (1:500 dilution in 0.5 mL of PBS). Cells were washed, then incubated with alkaline phosphatase-conjugated goat antimouse IgG, diluted 1:1000 in PBS, for 60 minutes. Cells were washed three times with 1 mL of PBS. Binding of the secondary antibody was determined by the addition of 200 μL of p-nitrophenyl phosphate substrate (1 mg/mL in 0.2 mol/L Tris, pH 9.8, containing 5 mmol/L MgCl2). Plates were incubated for 30 minutes in the dark. The reaction was terminated by the addition of 50 μL of 3N NaOH. The absorbency was read at 405 nm in an ELISA plate reader (Model 7520; Cambridge Technology, Watertown, MA) interfaced with an IBM-PC computer. The surface expression of CAMs is shown as mean ± standard deviation (SD) of the optical density (OD) after subtracting the blank value, that is the OD in the absence of primary antibody.
32P Labeling of HUVEC and immunoprecipitation of PECAM-1.
HUVEC grown to confluence in tissue culture dishes (100 × 15 mm) were washed three times with prewarmed phosphate-free RPMI-1640 (GIBCO-BRL) and radiolabeled in 3 mL of phosphate-free medium containing 0.20 mCi of 32P (carrier-free; ICN Biomedicals, Inc, Irvine, CA) for 4 hours at 37°C as previously described.37 The monolayer of radiolabeled HUVEC was washed with phosphate-free medium and incubated in 3 mL of the same medium in the presence or absence of RBC (hematocrit 2%) and E-CM for the indicated time intervals. At the end of incubation, the medium was aspirated and cells washed three times with 3 mL of ice-cold PBS. Endothelial cells were lysed in 1 mL of lysis buffer (50 mmol/L Tris-HCl, pH 8.0; 150 mmol/L NaCl; 0.1% SDS; 50 mmol/L NaF, 10 mmol/L EDTA, 1% Nonidet P-40; 0.1 mmol/L DTT; 0.5% sodium deoxycholate; 2 mmol/L sodium orthovanadate; 100 μg/mL PMSF; 1 μg/mL pepstatin; and 1μg/mL leupeptin). The lysate was centrifuged and immunoprecipitated with an antibody to PECAM-1 (as ascites fluid from clone XVD2) as previously described.37 The immunocomplex was collected by centrifugation, washed with lysis buffer, and the sample was subjected to electrophoresis on a 10% SDS-polyacrylamide gel as previously described.37 Gels were dried and exposed overnight to Kodak XR-5 film at room temperature. The radioactivity in the band corresponding to PECAM-1 (130 kD) was quantitated by using an Ambis radioactivity gel scanner (Model 101; San Diego, CA).
Fluorescent labeling of HL-60 monocyte-like cells.
Vitamin D3-differentiated HL-60 cells were labeled with a fluorescent probe, CellTracker Green BODIPY (8-chloromethyl-4,4-difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a-diazaindacene) according to the manufacturer’s instructions (Molecular Probes Inc, Eugene, OR). Briefly, the medium was aspirated from Vitamin D3-differentiated HL-60 cells cultivated in tissue culture plates, followed by the addition of the medium containing fluorescent probe (5 μmol/L). The cells were incubated for 45 minutes. The loading solution was then replaced with fresh prewarmed medium and the cultures incubated for an additional 30 minutes at 37°C. The fluorescently labeled HL-60 cells were used for a transendothelial migration assay.
Assay for transendothelial migration of monocytes.
HUVEC monolayers were grown to confluence on fibronectin-coated porous membranes (Biocoat Cell culture inserts, 3.0 micron; Collaborative Biomedical Products, Bedford, MA) for 5 to 6 days as described.31 Transendothelial resistance was measured daily on the filters with sterilized electrodes using an EVOM voltohmmeter (World Precision Instruments, Sarasota, FL). At day 5 to 6, HUVEC monolayer exhibited total electrical resistance (endothelial cell monolayer with filter) of 58 ± 8 ohms.cm2 (n = 13) with net transendothelial electrical resistance of 30 ± 4 ohms.cm2 and were used at that time for migration studies. The values for transendothelial electrical resistance for HUVEC cultivated on fibronectin-coated porous membranes are similar to literature values of 28 ± 11 ohms.cm2for HUVEC monolayer cultured on polyethylene terephthalate micropore membranes.38 Confluent monolayers of HUVEC were incubated at 37°C in duplicate with aliquots (1 × 105 cells per well) of fluorescently labeled vitamin D3-differentiated HL-60 cells, in the presence and absence of RBC (hematocrit 2%), containing E-CM for time periods ranging from 30 minutes to 4 hours. The lower compartment of the Transwell chamber contained 1 mL of RPMI-1640, whereas the upper compartment contained 0.5 mL of the same medium, as well as the HL-60 cells. At the indicated time points, aliquots of 50 μL were removed from the bottom compartment of the well. Cells were counted on the coverslip using a fluorescence microscope (Olympus IMT 2 attached with a 10 × 10 mm grid in the eyepiece; Olympus Optical Co, Tokyo, Japan). Only those cells that were viable were counted by this procedure (dead cells release the fluorescent marker). To keep the volume constant in the lower compartment, an equal amount (50 μL) of medium was added at each removal of monocytes. In inhibition experiments, antibodies and inhibitors were added 30 minutes before the addition of RBC to HUVEC.
Data analysis.
All experiments on cell-adhesion molecule expression were performed in triplicate with at least three different batches of endothelial cell preparations. Each treatment (incubation with RBC) was compared with its respective control (untreated). The results for the incorporation of 32P into PECAM-1 are expressed as the percent of control and are mean ± SD. The statistical significance of difference in a treatment series was determined by analysis of variance. Individual treatments in the experimental series were compared with their controls using Dunnet’s test. Statistical analyses were performed using the Instat-2 software program (GraphPad Software).
RESULTS
Effect of SS RBC on the induction of oxidant stress in HUVEC.
We determined whether the adhesion of SS RBC could induce oxidant stress. As shown in Table 1, SS RBC were 2.5 times more adherent to the endothelial cells than were those from normal individuals. Additionally, SS RBC in the presence of multimers of vWf (conditioned medium from endothelial cells, E-CM) exhibited threefold more adherence than in the absence of E-CM, as has been previously observed.23,39 The augmented adherence of SS RBC in the presence of multimers of vWf (E-CM) was observed to be optimal with 100 μg protein/mL of E-CM and inhibited by antibody to vWf. As shown in Table 1, incubation of SS RBC at a concentration approximating a hematocrit of 2% (vol/vol) with HUVEC, in the absence and presence of E-CM led to approximately three- and fivefold increased formation of TBARS, respectively, relative to HUVEC coincubated with normal (AA) RBC with and without E-CM. The adhesion of SS RBC to endothelial cells mediated by multimeric vWF (E-CM) appears to play a role in oxidant stress (lipid peroxide) induction because the addition of the peptide Lys-Tyr-Arg-Gly-Asp-Ser (KYRGDS) containing the arginine-glycine-aspartic acid (RGD) sequence motif, which prevents multimeric vWf-mediated adhesion of SS RBC to HUVEC,23,39completely inhibited the formation of TBARS, over and above that of SS RBC alone, whereas a variant peptide Ala-Gly-Asp-Val (AGDV) did not prevent either the adhesion of SS RBC or the formation of TBARS. Both catalase (200 U/mL) and superoxide dismutase (200 U/mL) together, scavengers of free radicals,40 completely abrogated SS RBC+ E-CM–induced TBARS formation in HUVEC, relative to HUVEC incubated with SS RBC alone. Moreover, in the presence of superoxide dismutase (SOD) and catalase the formation of lipid peroxides (TBARS) in HUVEC was observed to be 50% reduced below the level of HUVEC incubated with SS RBC alone. It is pertinent to note that incubation of SS RBC (2% Hct) alone for 2 to 4 hours did not show the formation of TBARS because its formation was below the detection limit of the assay. However, at a higher concentration (10%) of SS RBC, the amount of MDA formed by SS RBC alone was 8.78 ± 0.62 nmole MDA/g hemoglobin (n = 4) (data not shown) in agreement with previous studies.14 Overall, these results indicate that adhesion of SS RBC to endothelial cells, mediated by multimers of vWf (E-CM), generates greater amounts (approximately twofold) of ROS over and above that observed with SS RBC alone interaction with endothelial cells.
SS RBC-induced activation of NF-kB activity in HUVEC.
We measured, in HUVEC nuclear extracts, the activation of the transcription factor NF-kB, another index of cellular oxidant stress.16 41 As shown in Fig 1, nuclear extracts prepared from HUVEC that had been incubated with SS RBC and E-CM, showed a time-dependent increase in NF-kB activity. The maximal activation of NF-kB activity occurred at 1 hour, followed by a decrease at 2 hours and 4 hours. As shown in Fig 1, gel-shift binding assays in the presence of excess competing oligonucleotide resulted in a decrease in NF-kB activity, indicating specificity of interaction of DNA binding proteins with an NF-kB nucleotide sequence. There was minimal activation of NF-kB activity when HUVEC were incubated with AA RBC in the presence of E-CM at the 1-hour time point (Fig 1), the optimal time period for activation of NF-kB with SS RBC plus E-CM. As shown in Fig 2, incubation of normal red blood cells (AA RBC) with HUVEC for a prolonged time (4 hours) with or without E-CM did not significantly increase NF-kB activity over that of untreated HUVEC. Furthermore, SS RBC incubation with HUVEC alone for 1 hour augmented by twofold activation of NF-kB activity. However, incubation of SS RBC with HUVEC in the presence of E-CM increased by twofold the NF-kB activity over and above that of HUVEC incubated with SS RBC alone. This data is qualitatively similar to the formation of TBARS in HUVEC by SS RBC in the absence and presence of E-CM (Table 1). As shown in Fig 2, incubation of HUVEC with E-CM alone did not affect basal NF-kB activity.
Effect of incubation of RBC and inhibitors on NF-kB activity in HUVEC nuclear extracts by gel-shift assay. HUVEC were incubated with RBC (2% Hct) in the presence of E-CM (100 μg/mL) for 60 minutes, unless otherwise indicated in the presence and absence of inhibitors (genistein, 25 μg/mL; KYRGDS, 100 μmol/L; HFPA, 10 μmol/L; and probucol, 50 μmol/L). Nuclear extracts were prepared and incubated with a double-stranded 32P-labeled NF-kB oligonucleotide probe. Where indicated, excess cold competitor oligonucleotide was added to the nuclear extracts and incubated for 10 minutes before addition of radiolabeled DNA probe. The data are representative of four independent experiments.
Effect of incubation of RBC and inhibitors on NF-kB activity in HUVEC nuclear extracts by gel-shift assay. HUVEC were incubated with RBC (2% Hct) in the presence of E-CM (100 μg/mL) for 60 minutes, unless otherwise indicated in the presence and absence of inhibitors (genistein, 25 μg/mL; KYRGDS, 100 μmol/L; HFPA, 10 μmol/L; and probucol, 50 μmol/L). Nuclear extracts were prepared and incubated with a double-stranded 32P-labeled NF-kB oligonucleotide probe. Where indicated, excess cold competitor oligonucleotide was added to the nuclear extracts and incubated for 10 minutes before addition of radiolabeled DNA probe. The data are representative of four independent experiments.
Relative NF-kB activation in HUVEC in response to interaction of RBC. HUVEC were incubated with either AA RBC (n = 3; two different donors ) for 1 hour and 4 hours or with SS RBC (n = 4; three different donors and one replicate) for 1 hour in the absence and presence of E-CM. Nuclear extracts were probed for NF-kB activity. The NF-kB activity in the gel (not shown) was quantified by densitometric scan of the autoradiograph by Alpha Image 2000 Documentation and Analysis system. The data shown is relative change in NF-kB activity compared to untreated HUVEC. NF-kB activity increased to 365% ± 34% (range, 320% to 425%) on incubation of HUVEC with SS RBC and E-CM. Replicate of the same donor sample showed less than 10% variation.
Relative NF-kB activation in HUVEC in response to interaction of RBC. HUVEC were incubated with either AA RBC (n = 3; two different donors ) for 1 hour and 4 hours or with SS RBC (n = 4; three different donors and one replicate) for 1 hour in the absence and presence of E-CM. Nuclear extracts were probed for NF-kB activity. The NF-kB activity in the gel (not shown) was quantified by densitometric scan of the autoradiograph by Alpha Image 2000 Documentation and Analysis system. The data shown is relative change in NF-kB activity compared to untreated HUVEC. NF-kB activity increased to 365% ± 34% (range, 320% to 425%) on incubation of HUVEC with SS RBC and E-CM. Replicate of the same donor sample showed less than 10% variation.
As shown in Fig 1, SS RBC + E-CM induced activation of NF-kB was inhibited by a tyrosine kinse inhibitor, genistein42; and by antioxidant, probucol.40 Other antioxidants, pyrrolidinedithiocarbamate (PDTC)43 and vitamin E, also inhibited NF-kB activity (data not shown). Additionally, the activation of NF-kB in HUVEC nuclear extracts induced by SS RBC in the presence of E-CM was inhibited when HUVEC were preincubated with the synthetic peptide KYRGDS (Fig 1). We have recently observed that tert-butylhydroperoxide, an inducer of oxidant stress, causes activation of the transcription factor NF-kB as a result of activation of p21ras44; thus we examined whether the NF-kB activation that was induced by SS RBC could be blocked by an inhibitor of p21ras activity. As shown in Fig 1, treatment of HUVEC with a p21ras farnesyltransferase inhibitor41 (α-hydroxyfarnesylphosphonic acid, HFPA), before the addition of SS RBC + E-CM inhibited NF-kB activity. These results suggest that interaction of SS RBC with HUVEC, in the presence of multimers of vWf (E-CM), causes a greater activation of NF-kB over and above that observed with SS RBC alone. Thus, we chose to study the effect of adhesion of SS RBC, mediated by multimers of vWf (E-CM), on the cellular signaling in HUVEC leading to the expression of CAMs and migration of monocytes across the endothelial cell monolayer in the following studies.
Adherence of SS RBC to HUVEC leads to the surface expression of adhesion molecules.
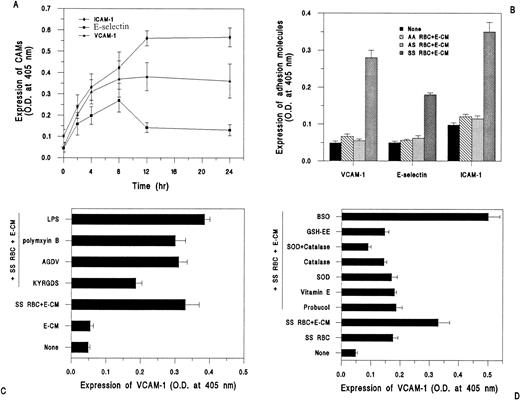
As shown in Fig 3A, incubation of SS RBC (2% hematocrit) in the presence of vWf (E-CM), with HUVEC resulted in a time-dependent increase in the surface expression of ICAM-1, E-selectin, and VCAM-1, as determined by ELISA. The surface expression of ICAM-1 continued to increase above basal level (untreated HUVEC) at 12 hours and remained unchanged up to 24 hours. Similarly, the surface expression of VCAM-1 was also optimal at 12 hours. However, E-selectin surface expression, though it increased up to 8 hours in response to incubation with SS RBC, then declined to almost basal level by 12 to 24 hours. Moreover, there was no affect on the surface expression of constitutively expressed ICAM-2 by SS RBC and E-CM (data not shown). To determine whether the effect observed was specific for SS RBC (Hb SS), HUVEC were incubated with normal RBC (Hb AA) and SC trait RBC (Hb AS) under identical conditions and assayed for CAMs expression. As shown in Fig 3B, there was no significant difference in the surface expression of CAMs in response to the interaction of AA and AS RBC with endothelial cells compared with untreated ECs.
Cell adhesion molecule expression in HUVEC in response to interaction with RBC. HUVEC grown to confluence in 24-well plates were incubated with RBC (2% Hct) and E-CM (100 μg/mL) for 4-hour time periods, unless otherwise indicated. ICAM-1, E-selectin, and VCAM-1 expression was determined by ELISA. (A) Time period of expression of CAMs in response to SS RBC plus E-CM; results are expressed as mean ± SD of OD at 405 nm of triplicate determinations, (n = 5; including three different donors). (B) Effect of AA RBC (n = 3), AS RBC (n = 3), and SS RBC (n = 6; including four different donors) on CAMs expression at 4 hours. (C) HUVEC were preincubated for 30 minutes with peptides (KYRGDS, 100 μmol/L; AGDV, 100 μmol/L) and polymyxin sulfate (5 μg/mL) before incubation with SS RBC plus E-CM or E-CM (100 μg/mL) alone for 4 hours. Incubation with LPS (100 ng/mL) was performed for 4 hours. (D) HUVEC were preincubated for 30 minutes with inhibitors (Probucol, 50 μmol/L; vitamin E, 25 μmol/L; SOD, (200 U/mL); catalase, (200 U/mL); GSH-EE, 0.5 mmol/L; and BSO (100 μmol/L) before incubation with SS RBC for 4 hours, followed by measurement of VCAM-1 expression.
Cell adhesion molecule expression in HUVEC in response to interaction with RBC. HUVEC grown to confluence in 24-well plates were incubated with RBC (2% Hct) and E-CM (100 μg/mL) for 4-hour time periods, unless otherwise indicated. ICAM-1, E-selectin, and VCAM-1 expression was determined by ELISA. (A) Time period of expression of CAMs in response to SS RBC plus E-CM; results are expressed as mean ± SD of OD at 405 nm of triplicate determinations, (n = 5; including three different donors). (B) Effect of AA RBC (n = 3), AS RBC (n = 3), and SS RBC (n = 6; including four different donors) on CAMs expression at 4 hours. (C) HUVEC were preincubated for 30 minutes with peptides (KYRGDS, 100 μmol/L; AGDV, 100 μmol/L) and polymyxin sulfate (5 μg/mL) before incubation with SS RBC plus E-CM or E-CM (100 μg/mL) alone for 4 hours. Incubation with LPS (100 ng/mL) was performed for 4 hours. (D) HUVEC were preincubated for 30 minutes with inhibitors (Probucol, 50 μmol/L; vitamin E, 25 μmol/L; SOD, (200 U/mL); catalase, (200 U/mL); GSH-EE, 0.5 mmol/L; and BSO (100 μmol/L) before incubation with SS RBC for 4 hours, followed by measurement of VCAM-1 expression.
Does adhesion of SS RBC or soluble factor released from SS RBC mediate the surface expression of CAMs?
As shown in Fig 3C, E-CM alone (vWf), in the absence of SS RBC, did not induce VCAM-1 surface expression. However, E-CM induced the surface expression of VCAM-1 in the presence of SS RBC. Moreover, addition of the KYRGDS peptide (100 μmol/L), which blocks vWf-mediated adhesion of SS RBC,39 reduced by approximately 50% the surface expression of VCAM-1, whereas the variant-peptide AGDV failed to inhibit VCAM-1 expression induced by SS RBC and E-CM. The VCAM-1 expression, which is not inhibitable by RGD peptide, may be due to the basal level of oxidative stress generated by SS RBC alone.13 It is pertinent to note that the addition of synthetic peptides KYRGDS and AGDV directly to HUVEC, in the absence of SS RBC, or the SS RBC-conditioned media derived from the incubation of 1 mL of SS RBC (20% Hct) for 2 hours, failed to induce the surface expression of VCAM-1 (data not shown), indicating that binding of small peptide ligands or components present in the medium from SS RBC to the putative receptor on HUVEC was not sufficient to induce VCAM-1 expression. Treatment of HUVEC with LPS (100 ng/mL) as a positive control exhibited an increase in VCAM-1 expression over and above the level observed with SS RBC in the presence of E-CM (Fig 3C). The increase in VCAM-1 surface expression in HUVEC in response to SS RBC E-CM was not due to the contaminating effect of endotoxin because addition of polymyxin B (5 μg/mL), an inhibitor of endotoxin, did not reduce VCAM-1 expression (Fig 3C). These studies indicate that adherence of SS RBC mediated by vWf augments VCAM-1 expression, whereas inhibition of adherence by RGD peptide reduces the VCAM-1 expression.
Effect of antioxidants on the surface expression of VCAM-1 in HUVEC.
Because probucol inhibited the activation of NF-kB activity in HUVEC in response to the interaction of SS RBC +E-CM, we examined the effect of antioxidants on the surface expression of VCAM-1. As shown in Fig 3D, pretreatment of HUVEC with the antioxidants probucol (50 μmol/L) and vitamin E (25 μmol/L) reduced by ∼90% VCAM-1 expression induced by SS RBC plus E-CM. Moreover, the addition of SOD and catalase, completely abrogated the VCAM-1 expression over and above that observed with SS RBC alone. The possibility of the involvement of the glutathione redox cycle in the cellular oxidant stress generated by the interaction of SS RBC with HUVEC was examined through the use of agents, that affect intracellular glutathione levels.45Elevation of intracellular glutathione (GSH) levels by preincubation of HUVEC with the cell-permeable glutathione ester (GSH-ethyl ester) for 24 hours completely inhibited SS RBC, plus E-CM induced VCAM-1 expression (Fig 3D). Conversely, pretreatment of HUVEC with butathionine sulfoximine (BSO), an agent that prevents GSH synthesis,45 augmented VCAM-1 expression by ∼40% over and above that observed with SS RBC plus E-CM (Fig 3D).
Effect of SS RBC-induced oxidant stress on the transendothelial migration of monocytes.
In our recent studies,44 we observed that oxidant stress induced by tert-butylhydroperoxide promoted the transendothelial migration of monocytes; thus we examined whether ROS generated by HUVEC in response to the adhesion of SS RBC also mediated the transendothelial migration of monocytes. First, we examined the effect of adhesion of SS RBC on the transendothelial migration of monocyte-like HL-60 cells. To study the migration of monocyte-like cells in the presence of RBC, we used fluorescent-labeled HL-60 cells, so as to distinguish the migration of monocytes from that of RBC across the endothelial cell monolayer. The migration of fluorescently labeled monocyte-like HL-60 cells was monitored by fluorescence microscopy. As shown in Fig 4A, the addition of SS RBC (hematocrit 2%) with E-CM to confluent monolayers of HUVEC cultured in Transwell chambers resulted in an approximately twofold increase in the number of monocyte-like HL-60 cells migrating across the endothelial cell monolayer at 2 and 4 hours posttreatment with SS RBC. Normal (AA) RBC in the presence of E-CM failed to increase the migration of monocyte-like HL-60 cells above basal level (Fig 4A). As previously observed37 with 15-HPETE-induced transendothelial migration of monocytes, an antibody to PECAM-1 inhibited by 70% ± 11% the SS RBC-induced migration of monocyte-like HL-60 cells across HUVEC monolayers (Fig 4B), whereas a control antibody to ICAM-2 had no effect on transendothelial migration (Fig 4B).
Migration of monocyte-like HL-60 cells and PBM across HUVEC monolayer in response to incubation with RBC. HUVEC were grown to confluence on fibronectin-coated porous membranes (Transwell, Cat. #40492, Biocoat cell culture inserts, Becton-Dickinson). To the upper compartment, RPMI-1640 containing FCS was added, followed by RBC (2% Hct) and E-CM (100 μg/mL) and fluorescently labeled vitamin D3-differentiated HL-60 cells or PBM (0.5 × 106 cells per well). At the indicated time points, aliquots were removed from the lower compartment of the Transwell chamber for counting of monocyte-like HL-60 cells. Data are expressed as mean ± SD of SS RBC, n = 4 (three different donors), and three independent determinations for AA RBC. (A) Time course of HL-60 cells transendothelial migration. (B) HUVEC were incubated with inhibitors (MoAb to human PECAM-1 (10 μg/mL); MoAb to ICAM-2 (10 μg/mL); GF 109203X (20 nmol/L); and Calyculin A, (2 nmol/L) for 45 minutes before the addition of SS RBC (2% Hct) and E-CM. (C) HUVEC were preincubated with either antioxidant (probucol, 50 μmol/L) for 45 minutes or GSH effectors (GSH-EE, 0.5 mmol/L and BSO, 100 μmol/L) for 24 hours, before the addition of SS RBC (2% Hct) and HL-60 cells. (D) HUVEC were incubated with MoAb to human PECAM-1 (10 μg/mL); KYRGDS, (100 μmol/L); and AGDV, (100 μmol/L) for 45 minutes before the addition of SS RBC (2% Hct) and E-CM (100 μg/mL). This was followed by the addition of fluorescently labeled PBM. The transmigrated monocyte cells were counted at 2 hours. Data are expressed as mean ± SD for three different donors RBC.
Migration of monocyte-like HL-60 cells and PBM across HUVEC monolayer in response to incubation with RBC. HUVEC were grown to confluence on fibronectin-coated porous membranes (Transwell, Cat. #40492, Biocoat cell culture inserts, Becton-Dickinson). To the upper compartment, RPMI-1640 containing FCS was added, followed by RBC (2% Hct) and E-CM (100 μg/mL) and fluorescently labeled vitamin D3-differentiated HL-60 cells or PBM (0.5 × 106 cells per well). At the indicated time points, aliquots were removed from the lower compartment of the Transwell chamber for counting of monocyte-like HL-60 cells. Data are expressed as mean ± SD of SS RBC, n = 4 (three different donors), and three independent determinations for AA RBC. (A) Time course of HL-60 cells transendothelial migration. (B) HUVEC were incubated with inhibitors (MoAb to human PECAM-1 (10 μg/mL); MoAb to ICAM-2 (10 μg/mL); GF 109203X (20 nmol/L); and Calyculin A, (2 nmol/L) for 45 minutes before the addition of SS RBC (2% Hct) and E-CM. (C) HUVEC were preincubated with either antioxidant (probucol, 50 μmol/L) for 45 minutes or GSH effectors (GSH-EE, 0.5 mmol/L and BSO, 100 μmol/L) for 24 hours, before the addition of SS RBC (2% Hct) and HL-60 cells. (D) HUVEC were incubated with MoAb to human PECAM-1 (10 μg/mL); KYRGDS, (100 μmol/L); and AGDV, (100 μmol/L) for 45 minutes before the addition of SS RBC (2% Hct) and E-CM (100 μg/mL). This was followed by the addition of fluorescently labeled PBM. The transmigrated monocyte cells were counted at 2 hours. Data are expressed as mean ± SD for three different donors RBC.
Our previous studies37 showed that 15-HPETE induced the phosphorylation of PECAM-1 and, concomitant with it, increased the transendothelial migration of monocytes; thus we investigated whether the transendothelial migration of monocyte-like HL-60 cells induced by SS RBC was linked to PECAM-1 phosphorylation. As shown in Fig 4B, pretreatment of HUVEC with the PKC inhibitor GF-109203X (20 nmol/L)46 for 30 minutes, followed by incubation with SS RBC and E-CM, resulted in an approximate 60% decrease in the transendothelial migration of monocyte-like HL-60 cells at the 2-hour time point. As predicted, augmentation of PECAM-1 phosphorylation with the protein phosphatase inhibitor Calyculin A (2 nmol/L)47increased by ∼70% (P < .001) the transendothelial migration of monocyte-like HL-60 cells, relative to endothelial cells incubated with SS RBC and E-CM (Fig 4B). These studies indicate that there may be a direct or causal relationship between phosphorylation of PECAM-1 and transendothelial migration of monocytes.
Treatment of HUVEC with antioxidant probucol (50 μmol/L) reduced by ∼70% the migration of monocyte-like HL-60 cells (Fig 4C). As shown in Fig 4C, pretreatment of HUVEC with GSH-ethylester, which causes elevation of intracellular GSH levels,48 for 24 hours reduced by ∼90% the migration of monocyte-like HL-60 cells. However, treatment of HUVEC with BSO to prevent GSH synthesis48increased by 50% the migration of monocyte-like HL-60 cells induced by SS RBC plus E-CM (Fig 4C).
Effect of SS RBC on transendothelial migration of human PBM.
Because our studies showed that incubation of HUVEC with SS RBC in presence of E-CM increased transendothelial migration of monocyte-like HL-60 cells (a transformed cell line); it was of interest to examine whether freshly isolated monocytes from human peripheral blood exhibited similar migration characteristics. As shown in Fig 4D, incubation of HUVEC with SS RBC plus E-CM resulted in twofold increase in the migration of human PBM at 2-hour time point as was observed for monocyte-like HL-60 cells. Additionally, antibody to PECAM-1 inhibited the migration of monocytes by ∼80% (Fig 4D). However, control antibody to ICAM-2 had no affect (data not shown). The addition of KYRGDS, but not a variant peptide AGDV, blocked the transendothelial migration of human PBM by 70%.
Phosphorylation of PECAM-1 in HUVEC induced by SS RBC.
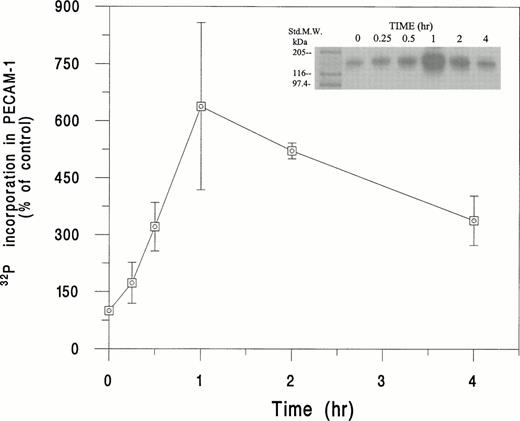
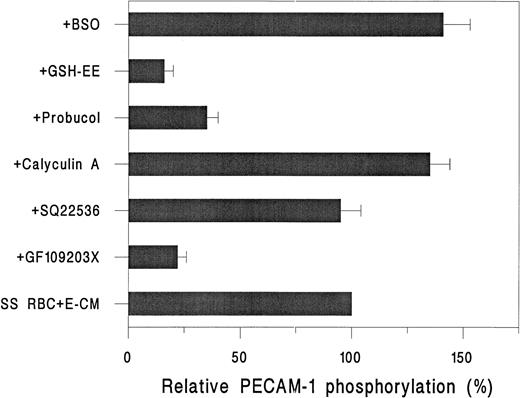
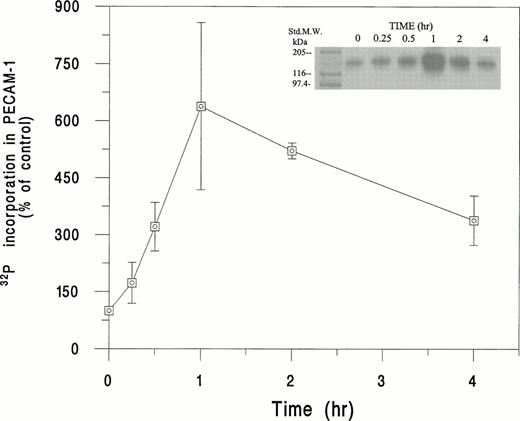
As shown in Fig 5, incubation of32P-labeled HUVEC with SS RBC plus E-CM resulted in a time-dependent increase in PECAM-1 phosphorylation. We observed approximately a sixfold (638% ± 220%) increase in the phosphorylation of PECAM-1 at 1 hour. At the 2-hour time point; the phosphorylation of PECAM-1 showed a fivefold increase above the basal level. Conversely, normal (AA) RBC, under the same conditions, showed no induction of PECAM-1 phosphorylation (data not shown). As shown in Fig 6, SS RBC-induced phosphorylation of PECAM-1 in HUVEC was approximately 75% inhibited by the PKC inhibitor GF-109203X,46 but not by the adenylate cyclase inhibitor SQ-22536.49 However, treatment of HUVEC with a protein phosphatase inhibitor, Calyculin A,47 in the presence of SS RBC and E-CM, increased PECAM-1 phosphorylation by ∼35% above the level induced by SS RBC plus E-CM (Fig 6). Furthermore, the phosphorylation of PECAM-1 induced by SS RBC + E-CM was ∼65% inhibited by antioxidant probucol, indicating the importance of oxidant stress in the phosphorylation of PECAM-1. Addition of GSH-ethyl ester, inhibited by ∼85% the SS RBC plus E-CM–induced phosphorylation. Conversely, inhibition of intracellular synthesis of GSH by pretreatment of HUVEC with BSO elevated PECAM-1 phosphorylation by ∼50%. These results indicate that redox state (GSH/GSSG levels) of endothelial cells in response to the interaction with SS RBC can affect the phosphorylation of PECAM-1.
Time course of phosphorylation of PECAM-1 in HUVEC in response to incubation with SS RBC. HUVEC were labeled with32P and incubated with SS RBC (2% Hct) for the indicated time period and then processed for immunoprecipitation with PECAM-1 antibody. The immunoprecipitate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and radioactivity quantitated in the gel lane corresponding to PECAM-1 (130 kD) by Ambis radiogel scanner as described in Materials and Methods. The data is presented as percent increase in the incorporation of 32P in PECAM-1, assigning the value of 100% for 32P incorporated into PECAM-1 in untreated HUVEC. Data are mean ± SD of n = 3, with each experiment run in duplicate for indicated time points, except for 1-hour period (n = 7). 32P incorporated into PECAM-1 was 638% ± 220% at 1-hour time point, with a range of 410% to 850%. The replicate data for the same donor sample showed less than 15% difference in the 32P incorporation into PECAM-1.
Time course of phosphorylation of PECAM-1 in HUVEC in response to incubation with SS RBC. HUVEC were labeled with32P and incubated with SS RBC (2% Hct) for the indicated time period and then processed for immunoprecipitation with PECAM-1 antibody. The immunoprecipitate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and radioactivity quantitated in the gel lane corresponding to PECAM-1 (130 kD) by Ambis radiogel scanner as described in Materials and Methods. The data is presented as percent increase in the incorporation of 32P in PECAM-1, assigning the value of 100% for 32P incorporated into PECAM-1 in untreated HUVEC. Data are mean ± SD of n = 3, with each experiment run in duplicate for indicated time points, except for 1-hour period (n = 7). 32P incorporated into PECAM-1 was 638% ± 220% at 1-hour time point, with a range of 410% to 850%. The replicate data for the same donor sample showed less than 15% difference in the 32P incorporation into PECAM-1.
Effect of inhibitors on the 32P incorporation in PECAM-1 in HUVEC on incubation with RBC. 32P-labeled HUVEC were incubated with inhibitors either for 45 minute (GF 109203X, 20 nmol/L; SQ 22536, 2 μmol/L; calyculin A, 2 nmol/L; probucol, 50 μmol/L; or for 24 hours (GSH-EE, 0.5 mmol/L and BSO, 100 μmol/L), followed by 1-hour incubation with SS RBC (2% Hct) and E-CM. Data are expressed as mean ± SD for three different SS RBC donors, in duplicate determinations, relative to untreated HUVEC (none).
Effect of inhibitors on the 32P incorporation in PECAM-1 in HUVEC on incubation with RBC. 32P-labeled HUVEC were incubated with inhibitors either for 45 minute (GF 109203X, 20 nmol/L; SQ 22536, 2 μmol/L; calyculin A, 2 nmol/L; probucol, 50 μmol/L; or for 24 hours (GSH-EE, 0.5 mmol/L and BSO, 100 μmol/L), followed by 1-hour incubation with SS RBC (2% Hct) and E-CM. Data are expressed as mean ± SD for three different SS RBC donors, in duplicate determinations, relative to untreated HUVEC (none).
DISCUSSION
Vascular occlusion leading to episodes of painful crises and damage to various end organs is the major cause of morbidity in adults and older children with SCD.50-52 It is recognized that abnormal adherence of SS RBC to endothelium is a key participant in the vaso-occlusive events,53 because studies showed that the extent of adherence of SS RBC to cultured endothelial cells correlated with the clinical severity of vaso-occlusive crises in SCD.5,6 However, SCD patients with identical biochemical defects in their β-globin genes show a wide variability in the frequency and clinical severity of vasoocclusive crises, and can remain asymptomatic for prolonged periods,51 indicating that additional factors must contribute to the pathophysiology of vaso-occlusion.8 Previous studies have shown that biochemical properties of sickle cells, including membrane characteristics, microvascular dynamics,4,54 tissue hypoxia,55 and perturbation of vascular endothelium,9 contribute to the pathogenesis of the vaso-occlusive process. We hypothesized that the adherence of SS RBC to endothelium may cause injury or activation of endothelium leading to the surface expression of CAMs that can lead to the increased adherence of PMN and monocytes to the damaged/activated endothelium through counter ligands, as well as to the additional adherence of SS reticulocytes through the α4β1 ligand to VCAM-1.19,20,55 The adhesion of SS RBC and leukocytes to injured/activated microvascular endothelium can adversely affect the blood flow in the capillary. Such flow conditions will favor the direct contact of SS RBC with neutrophils and concomitant attachment of PMN to SS RBC, promoting the risk of vessel occlusion. We have recently observed that such recognition of SS RBC by PMN leads to activation of PMN,56 which can directly damage the vascular endothelium through the generation and release of superoxide radicals. The injury or activation of vascular endothelium in vivo has been observed in patients with sickle cell anemia, wherein one observes the presence of increased number of activated circulating endothelial cells in blood samples.9 57
In this study, we examined the mechanism(s) by which the adherence of SS RBC to cultured endothelial cells, proximal sites of stasis of SS RBC in the vasculature, potentially augments the adherence of SS reticulocytes, and promotes the adherence and migration of monocytes across the endothelial cell monolayer. SS RBC, because of the presence of unstable SS hemoglobin and spontaneous auto-oxidation of iron in sickle heme13 generate excessive (two- to threefold) amounts of ROS (O2., OH-, and H2O2) (ROS) compared with control normal RBC.58 We show here that HUVEC incubated with SS RBC generates threefold increased amount of lipid peroxides (measured as thiobarbituric acid reactive substances, TBARS). However, HUVEC incubated with SS RBC plus vWf (E-CM) resulted in an additional twofold TBARS formation. The formation of TBARS by HUVEC is inhibited by the addition of free radical scavenging enzymes, superoxide dismutase, and catalase. Moreover, blocking the adherence of SS RBC to endothelial cells, reduced TBARS formation. We also measured the activation of the transcription factor NF-kB in HUVEC, another indicator of cellular oxidant stress.16 Our results show that the adherence/contact of SS RBC to endothelial cells, mediated by multimers of vWf, led to activation of NF-kB. This activation of NF-kB in HUVEC required the adhesion/contact of SS RBC and was also inhibited by antioxidants. These results suggest that adherence/contact of SS RBC mediated by multimers of vWf, localizes the oxidant stimulus generated by SS RBC to the membrane of endothelial cells and generates intracellular ROS in HUVEC to activate NF-kB. The intracellular generation of ROS (oxidative stress) could occur as a result of activation of NADH-oxidase associated with the plasma membrane of endothelial cells.59
We show that incubation of HUVEC with SS RBC, in the presence of multimers of vWf, results in a time-dependent increase in the surface expression of a subset of CAMs, ICAM-1, E-selectin, and VCAM-1 without affecting the surface expression of constitutively expressed ICAM-2. The augmented expression of VCAM-1, examined in this study, was observed on the adhesion of SS RBC to HUVEC but was not observed when vWf (E-CM) alone was added to HUVEC. Our studies show that the cellular oxidant stress generated in HUVEC induced by SS RBC adherence involves a glutathione redox step because agents that restore intracellular GSH levels prevent VCAM-1 expression, whereas an opposite effect occurs when intracellular GSH levels are reduced by preincubation of HUVEC with BSO, an inhibitor of GSH synthesis. Consequently, agents that restore intracellular levels of GSH levels may prevent VCAM-1 expression and thus could have beneficial effects on microvascular occlusion. Our results are consonant with previous studies wherein the transcription of VCAM-1 in human vascular endothelial cells, induced by cytokine IL-1β, has also been shown to be regulated through an antioxidant-sensitive mechanism.18 Moreover, our studies show that the addition of free radical scavenger enzymes (superoxide dismutase and catalase), abrogated SS RBC plus E-CM–induced VCAM-1 expression. Our studies indicate that ROS formed by SS RBC or SS RBC ligand-receptor interaction (juxtacrine intercellular signaling)60 and/or both result in the generation of reactive oxygen intermediates (oxidant stress) intracellularly in endothelial cells, which activate signaling pathways leading to the activation of redox-sensitive transcription factor NF-kB16.
Our studies show here, for the first time, that interaction of SS RBC with HUVEC, in the presence of vWf causes an increase in the migration of monocyte-like HL-60 cells. We used vitamin D3-differentiated human HL-60 cells (monocyte-like HL-60 cells), because this cell line was previously used as a reliable model of in vivo monocyte function and transendothelial migration studies.30,31 Similarly, the transendothelial migration of human PBM was augmented by the cellular oxidant stress generated by the adhesion of SS RBC to endothelial cells. The increase in the transendothelial migration of both monocyte-like HL-60 cells and PBM is inhibited by an antibody to PECAM-1, as has been previously observed for the transendothelial migration of monocytes induced by lipoxygenase metabolites.37 We show here that the adherence of SS RBC to HUVEC, mediated by vWf, leads to PECAM-1 phosphorylation. Both the SS RBC plus E-CM–induced phosphorylation of PECAM-1 and the transendothelial migration of monocyte-like HL-60 cells are inhibited by protein kinase C inhibitor, indicating the direct or causal involvement of PECAM-1 phosphorylation in mediating the flux of monocytes across the endothelial cell monolayer, as has been previously observed for the lipoxygenase metabolite-induced transendothelial migration of monocytes.37 The importance of PECAM-1 phosphorylation to transendothelial migration is further supported by findings that agents such as the phosphatase inhibitor Calyculin A, which increases the phosphorylation of PECAM-1, also augment monocyte migration over and above the level of SS RBC alone. As expected, antioxidants and modulators of intracellular GSH levels, which affect SS RBC-induced cellular signals, also affect phosphorylation of PECAM-1 and, concomitant with it, the transendothelial migration of monocytes.
In conclusion, our studies show that interaction of oxygenated SS RBC with HUVEC in the presence of multimers of vWf, generates reactive oxygen species (oxidant stress) in endothelial cells. The adherence/contact of SS RBC to endothelial cells helps in localizing the oxidant stimulus to the cell surface to initiate cellular signaling. As a consequence of the cellular signaling, activation of transcription factor NF-kB occurs in HUVEC leading to the binding of activated NF-kB to the consensus sites in the regulatory regions of DNA for several genes including a subset of CAMs, thereby bringing about the cell-surface expression of a subset of CAMs, ICAM-1, E-selectin, and VCAM-1. The increased expression of CAMs in response to oxidative stress is not unique to the adhesion of SS RBC to endothelium, but can occur in response to endotoxins or hypoxia, conditions eg, bacterial infection and tissue hypoxemia commonly associated with SCD. The increased surface expression of VCAM-1 in endothelial cells, as a result of the adherence of SS RBC, will promote additional adherence of SS reticulocytes expressing the α4β1 ligand via VCAM-1. These CAMs also act as receptors/adhesive agents for PMN and monocytes expressing the appropriate counter-receptors. The adhesion of PMN and monocytes at the sites of thrombotic occlusion by SS RBC or in small venules in which adhesion of SS RBC is maximal8 may cause further obstruction in the flow of blood and promote vasoocclusion. Furthermore, our studies show that the cellular oxidant stress, generated by the adhesion of SS RBC to HUVEC brings about increased migration of monocytes across the endothelial cell monolayer. In SCD, it is possible that the accumulation of monocytes may participate in the stroke syndrome in which arterial narrowing and thrombosis resemble the events observed in cerebrovascular atherosclerosis.
Thus the abnormal adherence of SS RBC to endothelial cells from large vessels can generate enhanced oxidant stress leading to increased adhesion and diapedesis of monocytes, as well as heightened adherence of SS reticulocytes. Although the studies presented have been conducted in endothelial cells derived from large vessels, we suspect that a similar phenomenon may also occur in microvascular endothelium in which the adhesion of SS RBC has been shown to be mediated by thrombospondin. Such studies should provide an insight into the process of obstruction or intermittent flow of blood observed in the microcirculation of patients with SCD. Further studies should be performed to determine whether density-fractionated less-dense (enriched in reticulocytes), or most-dense fraction of sickle blood, with and without deoxygenation, are involved in the generation of cellular oxidant stress and hence activation of endothelium.
ACKNOWLEDGMENT
We deeply appreciate the assistance of Pat Corley, RN, for obtaining the blood specimens from patients. The assistance of Anne Erwin, MS, in technical editing of the manuscript is greatly appreciated.
Supported by National Institute of Health, Heart, Lung, and Blood Institute Grant No. P60-HL-48484.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.